Introduction
Malignant melanoma is not uncommon, with incidence
in the USA increasing by 4% every year (1) and an annual 3.86% increase in the
thickest tumor (>4 mm) category (2). The vast majority of primary malignant
melanoma occurs in the skin, followed by the choroid layer of the
eyes, under the nail, the leptomeninges, oral cavity, nasal mucosa,
pharynx, esophagus, bronchus, vaginal or anorectal mucosa (3). Primary malignant melanoma of the
retroperitoneum is extremely rare, particularly in young patients.
No cases have been reported in the English literature thus far. In
this study, we report a case of primary retroperitoneal malignant
melanoma in a young female patient and briefly review the
literature.
Case report
An 18-year-old female presented to the Zhongnan
Hospital, China, on January 19th, 2006, with a six-month history of
a progressively enlarging mass in the right upper abdomen. The
patient had no history of any fever, vomiting or icterus. The
patient's general physical and chest examination were not
significant. Abdominal examination revealed a mildly tender lump in
the right hypochondrium of approximately 12×12 cm. The mass was
firm in consistency with an indistinct border. The patient's past
medical and personal histories were insignificant. All of the
laboratory investigations, including liver and renal function
tests, were within normal limits.
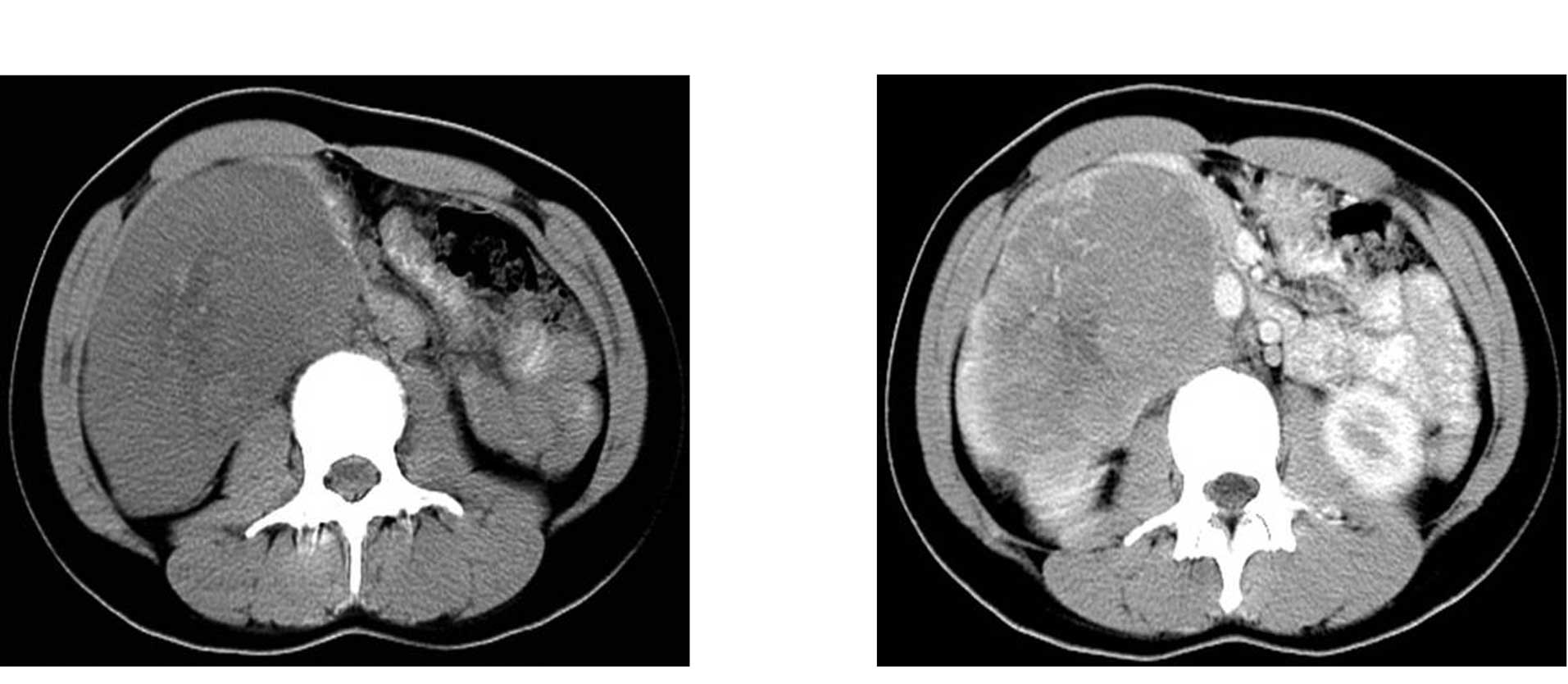
A computed tomography (CT) scan of the abdomen
showed an irregular heterogeneous soft-tissue density mass located
in the anterior pararenal space inferior to the right lobe of the
liver, with the right kidney slightly compressed and bowel loops
displaced medially. CT results showed that the mass measured
approximately 70×108 mm in the largest section with an indistinct
border and was not separable from the gastric sinus and gallbladder
(Fig. 1A). Post-contrast CT showed
a heterogeneously enhancing mass with marked peripheral
enhancement, and a number of tortuous and dilated vessels (Fig. 1B).
The patient underwent total resection of the
retroperitoneal mass. Per-operatively, the mass, measuring 10×7×5
cm, was seen extending superiorly to the inferior border of the
liver, inferiorly to the level of the iliac crest, and crossing the
midline medially, with certain areas enclosing the abdominal aorta
and compressing the inferior vena cava. The mass was encapsulated,
with the capsule absent at certain areas with tortuous and dilated
vessels on its surface. The tumor was soft and homogeneously black
and fleshy on the cut surface, with a tendency to bleed.
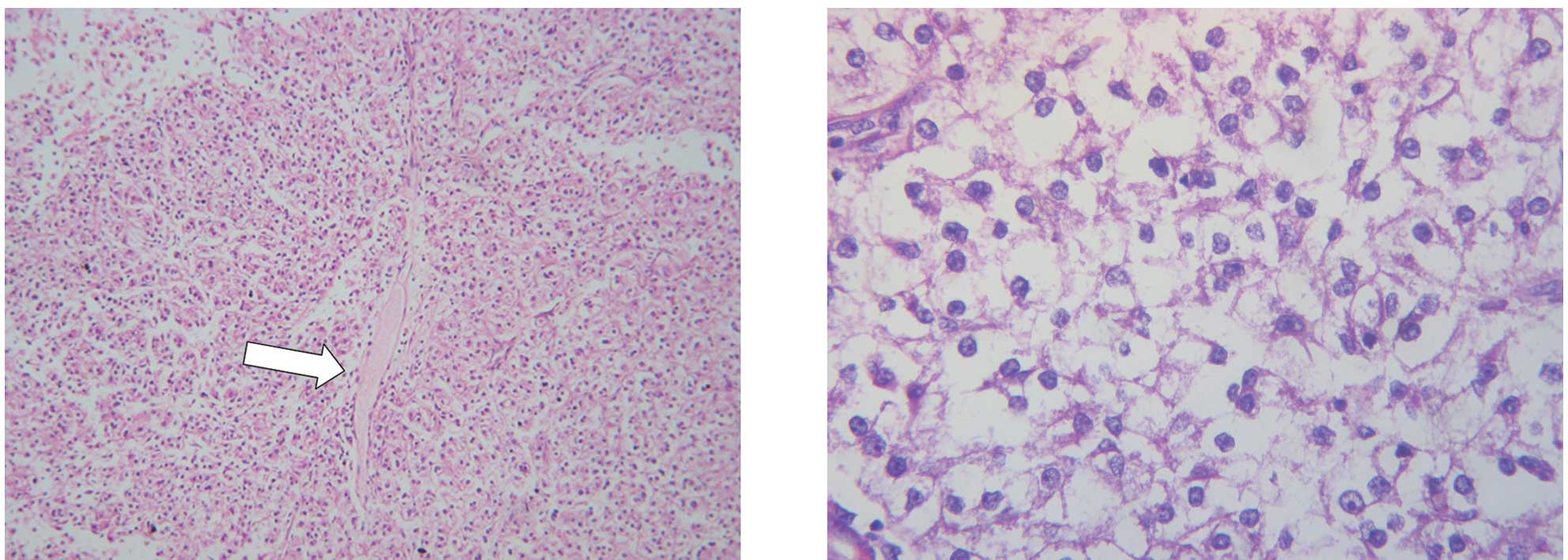
Histopathological analysis of the tissue biopsies
disclosed a dense proliferation of epithelioid, polygonal
neoplastic cells with abundant eosinophilic cytoplasm, round to
ovoid nuclei and prominent nucleoli, arranged in a uniform nested
to fascicular pattern of growth, separated by vascularized
fibrocollagenous septa (Fig. 2A and
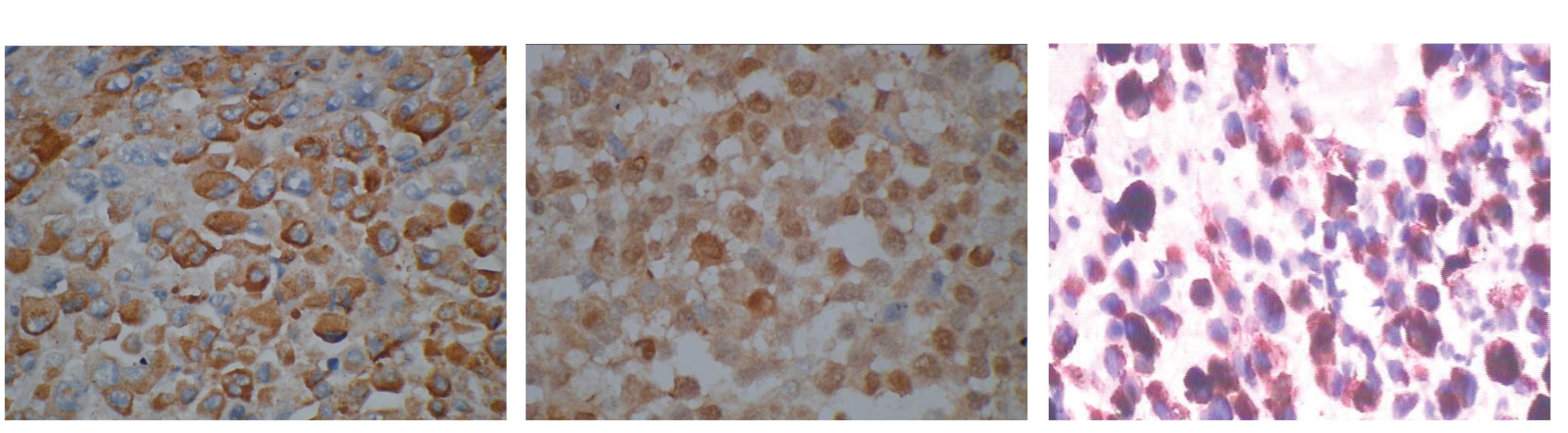
B). Focal tumor cells stained strongly positive by
immunohistochemistry for S-100, HMB-45 and melanin A, and mildly
positive for tyrosinase (Fig.
3A–C). A Fontana-Masson stain confirmed the presence of melanin
pigment.
A thorough laboratory and radiological investigation
was performed to locate any primary or secondary deposits in the
liver and brain; no other abnormalities were detected. A diagnosis
of primary retroperitoneal melanoma was then made following careful
dermatological and ophthalmological examination, which ruled out
the presence of cutaneous or choroidal melanoma.
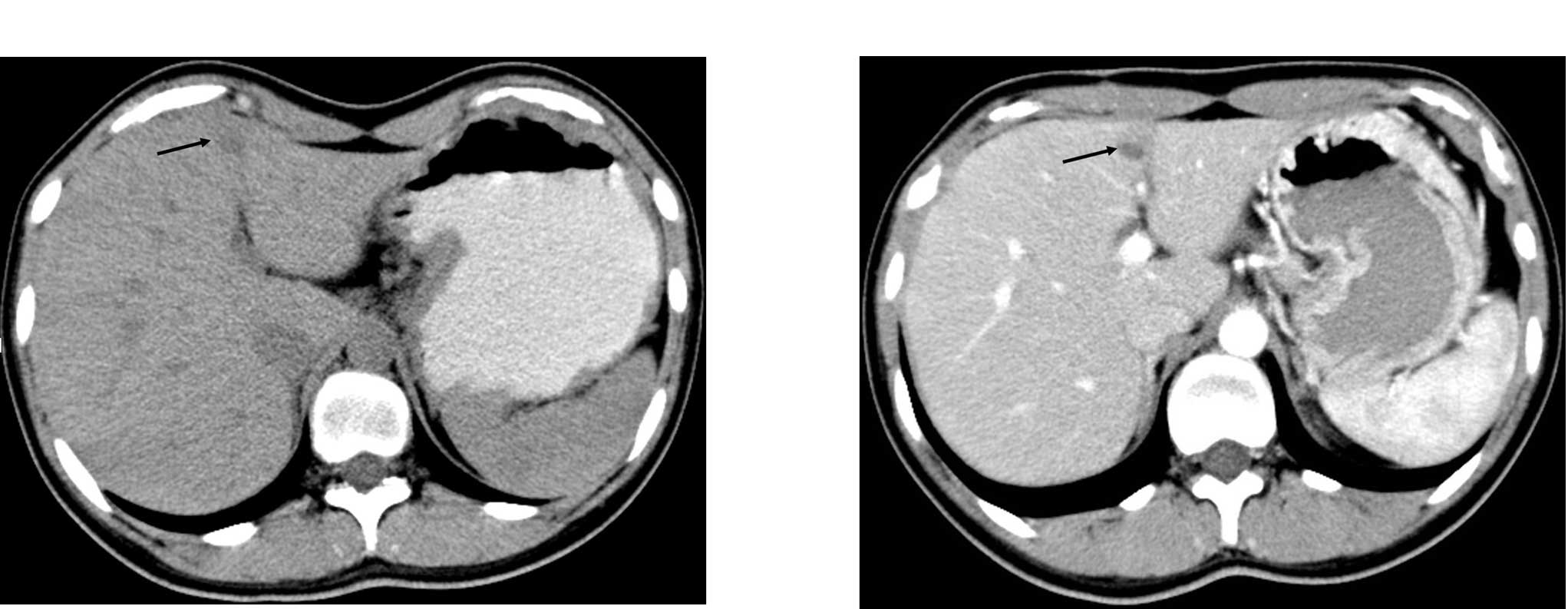
The immediate postoperative course of the patient
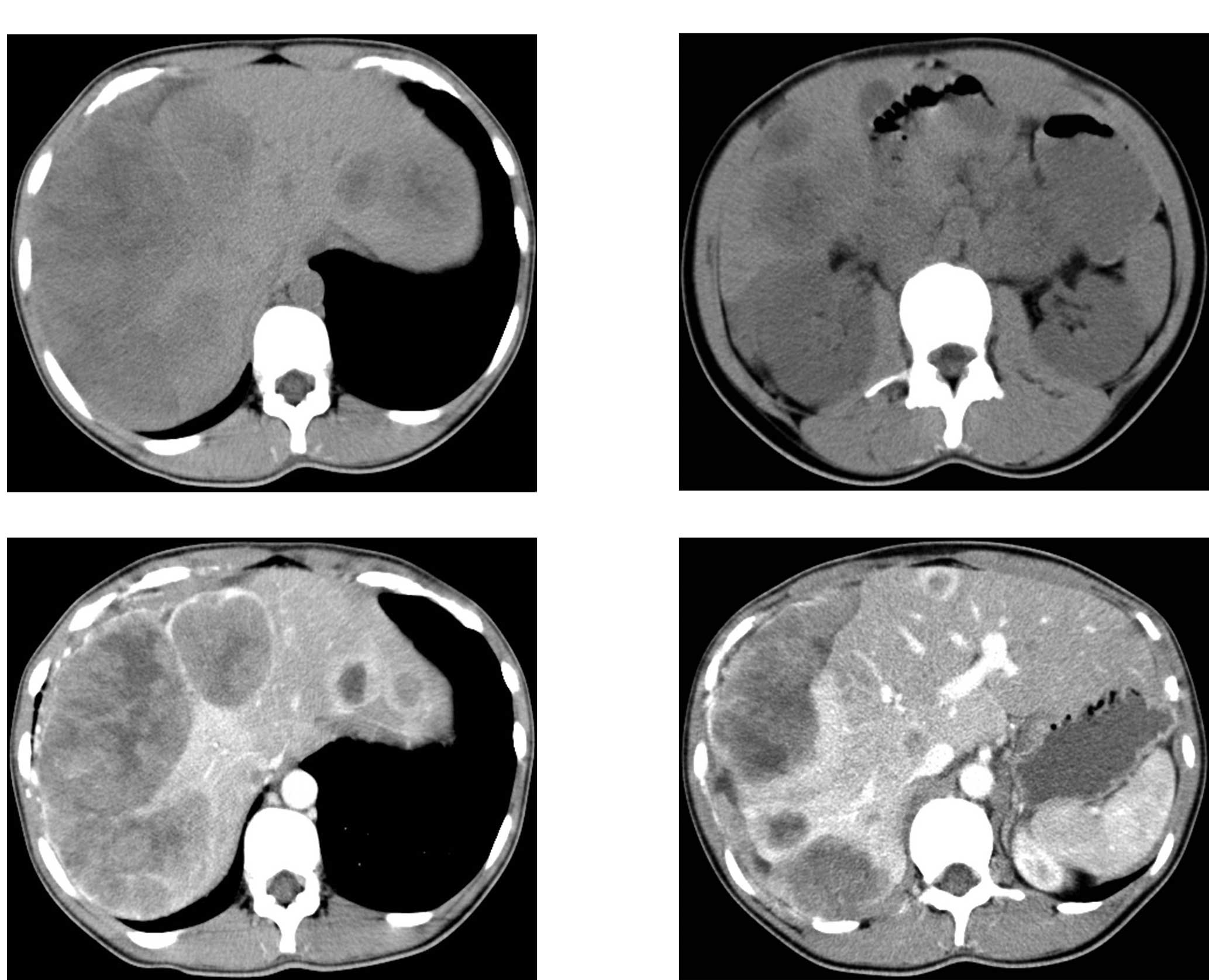
was uneventful and she was discharged. A follow-up CT examination
performed nine months later showed three metastatic nodular lesions
in the liver (Fig. 4A and B).
Although regular chemotherapy (6 cycles of DDBT regimen:
dacarbazine, DTIC; cisplatin, DDP; carmustine, BCUN; and tamoxifen,
TAM) was administered, the metastasis progressed, as shown on the
abdominal CT taken on February 20th, 2008 (Fig. 5A–D). The patient succumbed to the
disease in December 2008, with a survival interval from the initial
diagnosis of approximately 23 months.
Discussion
Accounting for 3% of all primary cutaneous
malignancies (4), malignant
melanoma primarily presents as a cutaneous lesion, with the overall
mean age of presentation in the 6th decade, with a slight
predilection in men (5). However,
less than 10% of all cases arise from non-cutaneous regions
(3), which may be caused by unknown
cytokines excreted from cells of the skin when exposed to
ultraviolet rays, or by unknown injurious factors (6).
Malignant melanoma primarily occurring in the
retroperitoneum at a young age has not been previously reported in
the English literature. Our patient was extensively investigated at
the time of diagnosis. However, no other sites showed evidence of
tumor or nevus, thus confirming the retroperitoneum as the primary
site of involvement. Melanoma is thought to arise where melanocytes
reside, including the sympathetic chain, which originates from the
neural ectoderm, as well as the autonomic ganglion cells (7). A few cases of malignant melanoma have
been reported to occur in the posterior mediastinum, derived from
the melanocytes scattered in the sympathetic chain and autonomic
nerve plexuses (7–9). We postulate that the tumor of the
current patient also originated from the sympathetic chain or
autonomic nerve plexuses of the retroperitoneum, which potentially
connected to its counterpart in the mediastinum.
Radiological examination is useful in the diagnosis
of malignant melanoma. CT is useful as a common tool to detect
focal retroperitoneal lesions, to rule out metastasis, to guide
percutaneous biopsies and to demonstrate response to therapy. CT is
employed to evaluate the margin, size and density of a tumor mass,
and particularly the pattern of enhancement, as malignant melanoma
is usually immersed in blood and surrounded by blood vessels, which
cause prominent enhancement and tortuous vessels on post-contrast
CT, as was evident in this patient. The MRI findings are variable,
but relatively specific, depending on the type of melanoma, the
content of melanin within the tumor regions and the presence or
absence of intratumoral hemorrhage (10–12).
Isiklar et al (11) divided
melanoma into four patterns: i) melanotic pattern, the typical type
of melanoma, comprising high signal intensity on T1-weighted images
and low signal intensity on T2-weighted images; ii) amelanotic
pattern, another commonly described type, which is hypointense or
isointense on T1-weighted images and hyperintense or isointense on
T2-weighted images; iii) mixed pattern, which presents between a
melanotic and amelanotic pattern; and iv) hemorrhagic pattern,
which has a signal intensity that is related to an associated
hematoma. An MRI scan, which would have added diagnostic value in
this particular case, was not performed.
Histopathological and immunohistochemical analyses
are indispensable to the diagnosis of melanoma. Pathologically,
tumor cells may be classified into four types: i) epithelioid-cell
pattern, consisting of enlarged round cells, with large round to
oval, hyperchromatic to vesicular nuclei, eosinophilic nuclear
inclusions, prominent eosinophilic nucleoli and a variable amount
of eosinophilic to clear-appearing cytoplasm, which are always
arranged in compact nests or cords, as shown in this patient; ii)
spindle-cell pattern, consisting of a fascicular to storiform
growth pattern composed of elongated to oval cells characterized by
cellular pleomorphism, multinucleated cells and increased mitotic
activity. The nuclei are large, vesicular to hyperchromatic with
prominent nucleoli, and have a variable amount of eosinophlic
cytoplasm; iii) lymphoma-like pattern, composed of cells with
round, eccentrically located, vesicular to basophilic appearing
nuclei and eosinophilic cytoplasm; and iv) pleomorphic pattern
(13,14).
Extra-cutaneous melanomas exhibit the same
immunohistochemical and ultrastructural features as their cutaneous
counterparts. S-100 protein, HMB-45, melanin A (also knownas
MART-1), vimentin and occasionally anti-tyrosinase antibody are
used as immunohistochemical tools in the diagnosis of melanoma
(14–18). S-100 has the highest sensitivity for
the differentiation of melanocytes. However, as a protein affecting
the trafficking of intracellular calcium, S-100 is also found in a
number of other tissues, including nerve sheath tumors and gliomas,
neuroendocrine cells, certain histiocytic proliferations,
Langerhans cells and, rarely, in poorly differentiated carcinomas.
S-100 is thus preferentially used as a screening tool (14,17).
HMB-45 and melanin A are the two most common ‘melanocyte-specific’
monoclonal antibodies used in the diagnosis of melanoma, as they
are specific to the inner membrane proteins present on
premelanosomes, noted exclusively in cells that show any type of
melanocytic differentiation. The sensitivity of HMB-45 for melanoma
is reported to reach up to 93–100%, with a similar
distribution as melanin A (15,16,18).
Vimentin is uniformly present in malignant melanoma with a reported
sensitivity of 93% (14). Although
less widely used, anti-tyrosinase antibodies are highly specific
and sensitive for melanocytic neoplasms, having sufficient
maturation with a sensitivity of approximately 86% (14). The presence of melanin pigment is
favorable to the diagnosis of malignant melanoma, but it is not
essential, as approximately 50% of cases are negative for the
melanin pigment.
When considering the differentiation of malignant
melanoma, metastasis from a cutaneous melanoma is the most likely
of the differential diagnoses. Approximately 90% of melanoma
patients present with secondary lesions, of which 45% have
radiological evidence, and in 10% of cases, the primary site is
unknown (19). Thus, extensive
investigation is required when facing a single lesion of melanoma.
Other differential diagnoses include pigmented extra-adrenal
paraganglioma (20,21), melanotic Schwannoma (22) and other neuroectodermal neoplasms
(23–25), which may be excluded by negative
immuno-histochemical stains for neuroendocrine markers, e.g., NSE,
chromogranin and synaptophysin (26).
Melanoma is a lethal disease, with a clear character
and an unpredictable clinical course. Complete resection of the
lesion is the principal treatment for melanoma, which although only
palliative, does not prevent further metastasis. The role of
adjuvant chemotherapy has yet to be established, with no clear
evidence of improved survival. However, in patients with multiple
metastasis, it is always the main management modality. In recent
years, immunotherapy and molecular-targeted therapy have played a
more pivotal role in the treatment of malignant melanoma (27–30).
The prognosis of malignant melanoma is dismal, particularly
extra-cutaneous lesions, which are more aggressive than their
cutaneous counterparts. The median survival for primary mucosal
melanoma was reported to be from 23 to 48 months after the initial
lesions were resected (31–33). The survival interval of our patient
was 23 months, suggesting a relatively worse prognosis in a primary
melanoma involving the retroperitoneum.
In conclusion, we have followed up and reported a
pathologically confirmed case of primary malignant melanoma located
in the retroperitoneum, which is extremely rare due to its atypical
location and the relatively young age of presentation.
References
|
1
|
Rager EL, Bridgeford EP and Ollila DW:
Cutaneous melanoma: Update on prevention, screening, diagnosis, and
treatment. Am Fam Physician. 72:269–276. 2005.PubMed/NCBI
|
|
2
|
Linos E, Swetter SM, Cockburn MG, Colditz
GA and Clarke CA: Increasing burden of melanoma in the United
States. J Invest Dermatol. 129:1666–1674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang AE, Karnell LH and Menck HR: The
National Cancer Data Base report on cutaneous and noncutaneous
melanoma: a summary of 84,836 cases from the past decade. The
American College of Surgeons Commission on Cancer and the American
Cancer Society. Cancer. 83:1664–1678. 1998. View Article : Google Scholar
|
|
4
|
Capizzi P and Donohue J: Metastatic
melanoma of the gastrointestinal tract: a review of the literature.
Compr Ther. 20:20–23. 1994.PubMed/NCBI
|
|
5
|
Poos HPAM, Kruijff S, Bastiaannet E, Van
Ginker RJ and Hoekstra HJ: Therapeutic groin dissection for
melanoma: Risk factors for short term morbidity. Eur J Surg Oncol.
35:877–883. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamaguchi T, Shionki Y, Koide K and
Kurioka H: A case of primary malignant melanoma of the esophagus
and analysis of 193 patients in Japan. Nippon Shokakibyo Gnkkal
Zasshi. 101:1087–1094. 2004.PubMed/NCBI
|
|
7
|
Karuppiah SV and Buchan KG: Primary
malignant melanoma: a rare cause of mediastinal mass. Jpn J Thorac
Cardiovasc Surg. 54:396–398. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lau CL, Bentley RC, Gockerman JP, Que LG
and D'Amico TA: Malignant melanoma presenting as a mediastinal
mass. Ann Thorac Surg. 67:851–852. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shishido M, Nagao N and Miyamoto K:
Mediastinal amelanotic melanoma presenting as superior vena cava
syndrome. Nihon Kyobu Shikkan Gakkai Zasshi (Japanese Journal of
Thoracic). 35:240–244. 1997.PubMed/NCBI
|
|
10
|
Lemke AJ, Hosten N, Bornfeld N, et al:
Uveal melanoma: correlation of histopathologic and radiologic
findings by using thin-section MR imaging with a surface coil.
Radiology. 210:775–783. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Isiklar I, Leeds NE, Fuller GN and Kumar
AJ: Intracranial metastatic melanoma: correlation between MR
imaging characteristics and melanin content. AJR Am J Roentgenol.
165:1503–1512. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Escott EJ: A variety of appearances of
malignant melanoma in the head: A review. Radiographics.
21:625–639. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wenig BM: Laryngeal mucosal malignant
melanoma: a clinicopathologic, immunohistochemical, and
ultrastructural study of four patients and a review of the
literature. Cancer. 75:1568–1577. 1995. View Article : Google Scholar
|
|
14
|
Chute DJ, Cousar JB and Mills SE:
Anorectal malignant melanoma morphologic and immunohistochemical
features. Am J Clin Pathol. 126:93–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jungbluth AA, Busam KJ, Cerald WL, et al:
A103: An anti-melan-a monoclonal antibody for the detection of
malignant melanoma in paraffin-embedded tissues. Am J Surg Pat hol.
22:595–602. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Busm KJ, Chen YT, Old LJ, et al:
Expression of melan-A (MART1) in benign melanocytic nevi and
primary cutaneous malignant melancma. Am J Surg Pathol. 22:976–982.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Busam KJ, Iversen K, Coplan KC and
Jungbluth AA: Analysis of microphthalmiat ranscription factor
expression in normal tissues and tumors, and comparison of it s
expression with S-100 protein, gp100, and tyrosinase in
desmorlastic malignant melanoma. Am J Surg Pathol. 25:197–204.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De-Vries TJ, Smeets M, de-Graaf R and
Hou-Jensen K: Expression of gp100, MART-1, tyrosinase, and S100 in
paraffin-embedd primary melanomas and locoregional, lymph node, and
visceral metastases: implications for diagnosis and immunotherapy.
A study conducted by the EORTC Melanoma Cooperative Group. J
Pathol. 193:13–20. 2001. View Article : Google Scholar
|
|
19
|
Greco CS, Soffietti R, Bradac GB and
Boldorini R: Primitive cerebral melanoma: case report and review of
the literature. Surg Neurol. 55:163–168. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lack EE, Kim H and Reed K: Pigmented
(‘black’) extraadrenal paraganglioma. Am J Surg Pathol. 22:265–269.
1998.
|
|
21
|
Moran CA, Albores-Saaverda J, Wenig BM and
Mena H: Pigmented extraadrenal papraganglions. A clinicopathologic
and immunohistochemical study of five cases. Cancer. 79:398–402.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marco V, Sirvent J, Alvarez Moro J, Clavel
M, Muntal MT and Bauza A: Malignant melanotic schwannoma
fine-needle aspiration biopsy findings. Diagn Cytopathol.
18:284–286. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsumine A, Kusuzaki K, Nakamura T, et
al: Differentiation between neurofibromas and malignant peripheral
nerve sheath tumors in neuro-fibromatosis 1 evaluated by MRI. J
Cancer Res Clin Oncol. 135:891–900. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li CS, Huang GS, Wu HD, et al:
Differentiation of soft tissue benign and malignant peripheral
nerve sheath tumors with magnetic resonance imaging. Clin Imaging.
32:121–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stoll G, Bendszus M, Perez J and Pham M:
Magnetic resonance imaging of the peripheral nervous system. J
Neurol. 256:1043–1051. 2009. View Article : Google Scholar
|
|
26
|
Vlodavsky E, Ben-Izhak O, Best LA and
Kerner H: Primary Malignant Melanoma of the Anterior Mediastinum in
a Child. Am J Surg Pathol. 24:747–749. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garbe C, Eigentler T, Keilholz U,
Hauschild A and Kirkwood J: Systematic Review of Medical Treatment
in Melanoma: Current Status and Future Prospects. Oncologist.
16:5–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ko JM and Fisher DE: A new era: melanoma
genetics and therapeutics. J Pathol. 223:241–250. 2011.PubMed/NCBI
|
|
29
|
Kim CJ, Dessureault S, Gabrilovich D,
Reintgen DS and Slingluff CL: Immunotherapy for melanoma. Cancer
Control. 9:22–30. 2010.
|
|
30
|
Sivendran S, Glodny B, Pan M, Merad M and
Saenger Y: Melanoma immunotherapy. Mt Sinai J Med. 77:620–642.
2010. View Article : Google Scholar
|
|
31
|
Schuchter LM, Green R and Fraker D:
Primary and metastatic diseases in malignant melanoma of the
gastrointestinal tract. Curr Opin Oncol. 12:181–185. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim W, Baek JM, Suh YJ, Jeon HM and Kim
JA: Ileal malignant melanoma presenting as a mass with aneurysmal
dilatation: a case report. J Korean Med Sci. 19:297–301. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Berger AC, Buell JF, Venzon D, Baker AR
and Libutti SK: Management of symptomatic malignant melanoma of the
gastrointestinal tract. Ann Surg Oncol. 6:155–160. 1998. View Article : Google Scholar : PubMed/NCBI
|