Introduction
Prostate cancer (PC) is a major health problem,
accounting for a quarter of the new cancer cases diagnosed in adult
males in America each year, and accounting for approximately 9% of
cancer-related mortality in the same population (1). In the early stages, prostate cancer
cells depend on androgens for growth and survival, hence
androgen-ablation therapy at this time may be effective in causing
tumor regression. However, treatment options for advanced
hormone-refractory prostate cancers (HRPC) are still relatively
inefficient (2).
The role of Ca2+ is well established in
the majority of the cell signaling pathways involved in
carcinogenesis (3).
Calcium-permeable channels are potential candidates for involvement
in Ca2+ homeostasis in prostate cancer cells. One
transient receptor potential (TRP) superfamily of cation channels
is of particular interest. The human trpm8 gene, initially known as
trp-p8, has been shown to be mainly expressed in the prostate and
is overexpressed in prostate cancer (4). The precise physiological function of
the TRPM8 channel in normal and cancer prostate tissue remains
unknown. TRPM8 expression is markedly upregulated in PC and in
other tumors, suggesting a significant role in carcinogenesis
(4). It has been shown that
anti-androgen therapy greatly reduces the expression of TRPM8,
suggesting that TRPM8 is regulated by androgens (5). TRPM8 expression-silencing experiments
using small interfering RNA (siRNA) suggested that Ca2+
influx through this channel plays an essential role in cellular
Ca2+ homeostasis in prostate epithelial cells and is
involved in cell survival (6). Our
previous study revealed that PC-3 cells express an extremely low
level of TRPM8, and that overexpression of TRPM8 has a negative
effect on the proliferation and malignant progression of PC-3 cells
in vitro (7).
However, upon administration of anti-androgen
therapy, the prostate epithelial cells downregulate the expression
of androgen receptor (AR) and, consequently, that of TRPM8 mRNA.
Prostate cancer and metastasis then progress into an
androgen-independent (AI) stage, resulting in cancer relapse with a
more aggressive phenotype.
It is well known that angiogenesis is essential for
tumor progression and metastasis (8). In relation to PC, it has also been
suggested that the degree of tumor angiogenesis is correlated to
clinical stage (9). Various
endothelial growth factors have been shown to play crucial roles in
tumor angiogenesis. Vascular endothelial growth factor (VEGF) is
one of the most potent and specific angiogenic factors.
Immunohistochemical studies have revealed that PC cells produce
VEGF (10,11) and that VEGF expression correlates
with microvessel density (MVD) and tumor progression (12).
This study was designed to investigate the possible
effects of TRPM8 on the proliferation and angiogenesis of
androgen-independent cancer PC-3 cells in vivo.
Materials and methods
Cell culture
PC-3 cells were purchased from the American Type
Culture Collection (ATCC, Manassas, VA, USA). PC-3-m8 cells were
previously established in our laboratory. Cells were cultured as
previously described (7).
Animals
Thirty 5-week old male nude mice (weight range 15–18
g) were obtained from the Hubei Provincial Experimental Animal
Center, China. All animal study protocols were approved by
internationally accepted principles and the Guidelines for the Care
and Use of Laboratory Animals of Wuhan University.
Animal grouping
The animals were randomized into 3 groups: Group A
(PC-3 cell group), group B (PC-3-vector group) and group C
(PC-3-TRPM8 group).
Tumor models
PC-3, PC-3-vector and PC-3-TRPM8 cells growing
exponentially were each implanted into 10 male nude mice by
subcutaneous (SC) injection of 1×106 cells (in 200 μl
phosphate-buffered saline) into the right flank.
Observation on growing condition of
mice
The inoculated mice were fed in the Experimental
Animal Center of Wuhan University and monitored daily for clinical
signs. Tumor measurements were performed every three days and the
tumor volume was calculated according to the formula:
V=(π/6)(d1×d2)3/2 (13),
where d1 and d2 are perpendicular tumor diameters.
Sample collection
Twenty-eight days after the inoculation of cells,
each mouse was injected with 10% chloral hydrate for
hyperanesthesia. The mice were sacrificed by decapitation and
tumors were removed from the body. One section of the tumor was
fixed in formalin for paraffin embedding and one section was
snap-frozen in liquid nitrogen and stored at −80°C.
H&E staining assay
Paraffin-embedded tissues were cut into 4-μm slices
and deparaffinated in dimethylbenzene for 5–10 min. Then the
tissues were put into 100, 95, 85 and 70% alcohol for 2–5 min in
turn and finally washed with distilled water and immersed in
staining solution. Following hematoxylin staining for 5–15 min, the
excess stain solution on the slides was washed off, and color
separation with 0.5–1% hydrochloride alcohol was performed for
approximately 10 sec. After washing in running water for 15–30 min,
the tissues were stained by 0.1–0.5% eosin for 1–5 min. The tissues
were then dehydrated with 75, 85, 95 and 100% alcohol for 2–3 min
in turn prior to hyalinization with dimethylbenzene twice for
approximately 10 min in total. Finally, neutral gum was dropped
onto the slip, and then the slip was covered by a slide. As a
result, nuclei were stained blue and cytoplasm and collagen fibers
were stained various shades of red or pink.
Immunohistochemical assay
The streptavidin-peroxidase-biotin (SP) method was
used for immunohistochemistry. The slides were deparaffinized
conventionally and were immersed in 3% H2O2
for 10 min to block endogenous peroxidase. Following antigen
retrieval by microwave, newborn calf serum was added for blocking
for 10 min. The primary antibody (1:50) was then added for
incubation overnight (4°C) and secondary antibodies were added for
incubation for 20 min at room temperature. Then
streptavidin-biotin-peroxidase solution was used for incubation for
30 min and 3,3′-diaminobenzidine (DAB) was added to the chlorate
for 15 min. This was followed by hematoxylin staining, dehydration
and hyalinization, and the slip was then covered.
CD34 marked MVD test
CD34 is expressed in vascular endothelial cells,
tumor cytoplasm or membrane and is used as a specific marker of
vascular endothelial cells. By immunohistochemical staining it
reveals a distribution of brown or light brown solid bud-like or
cord-like blood vessels. Low magnification (x100) was used to
review the microvascular staining in each section and determine the
maximum microvascular staining regions, and then the vascular
endothelial cells or cell groups which appeared brown at high
magnification (x200) were counted. Each cell group counted as
independent micrangium on condition of an obvious distinction from
neighboring micrangium and tumorous cell. Five counts of micrangium
of each slide at high magnification vision were recorded, and the
average was taken as the MVD.
Detection of VEGF protein expression by
Western blotting
One hundred milligrams of tumor tissue was obtained.
The protein lysate was added according to quality/volume (w/v,
mg/μl) at a concentration of 1:5, fully homogenized, placed on ice
for 30 min and then centrifuged for 5 min at 260 × g. The total
protein content was measured using a bicinchoninic acid (BCA) kit.
The protein expression of VEGF and β-actin was assayed using
Western blot analysis using anti-VEGF-specific and
anti-β-actin-specific antibodies (Santa Cruz Biotechnology, Santa
Cruz, CA, USA).
Statistical analysis
Measurement data were shown as the mean ± SD. Groups
were compared with the one-way ANOVA analysis. The Spearman
coefficent was used to analyse the correlation between MVD and
VEGF. P<0.05 was considered to be statistically significant. All
of the data were analyzed with SPSS 17.0.
Results
Observation on mice growing conditions
and behavior
In group C, the response to stimuli, activity level,
and appetite of each mouse was similar to that in groups A and B,
and the body weight did not change significantly (data not
shown).
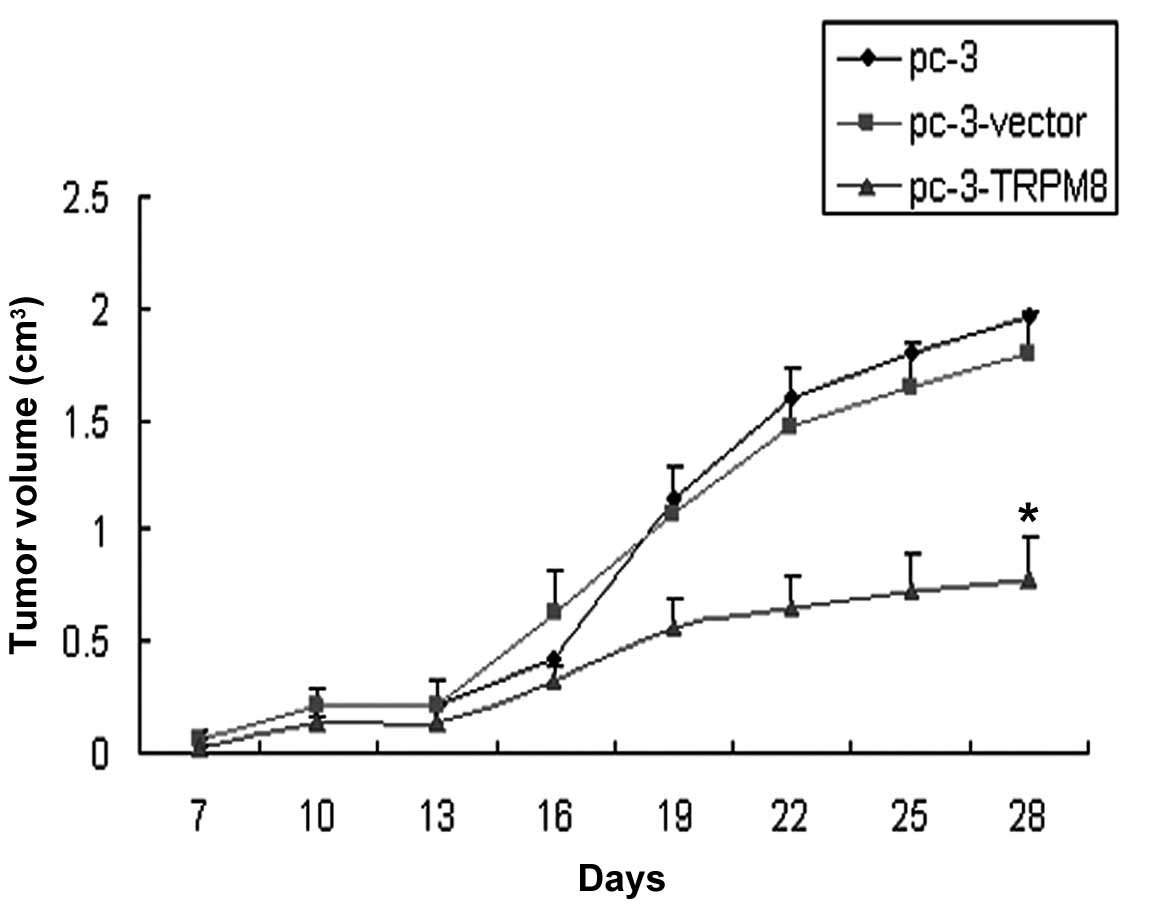
Observation on tumor growth
The tumor formation rate in each group was 100%. The
tumor growth was infiltrative with a round or oval shape. The tumor
volume in group C was less than that of groups A and B, and there
was a difference in the volume of the graft between group C and
group A or B (P=0.000 and P=0.000, respectively; Fig. 1).
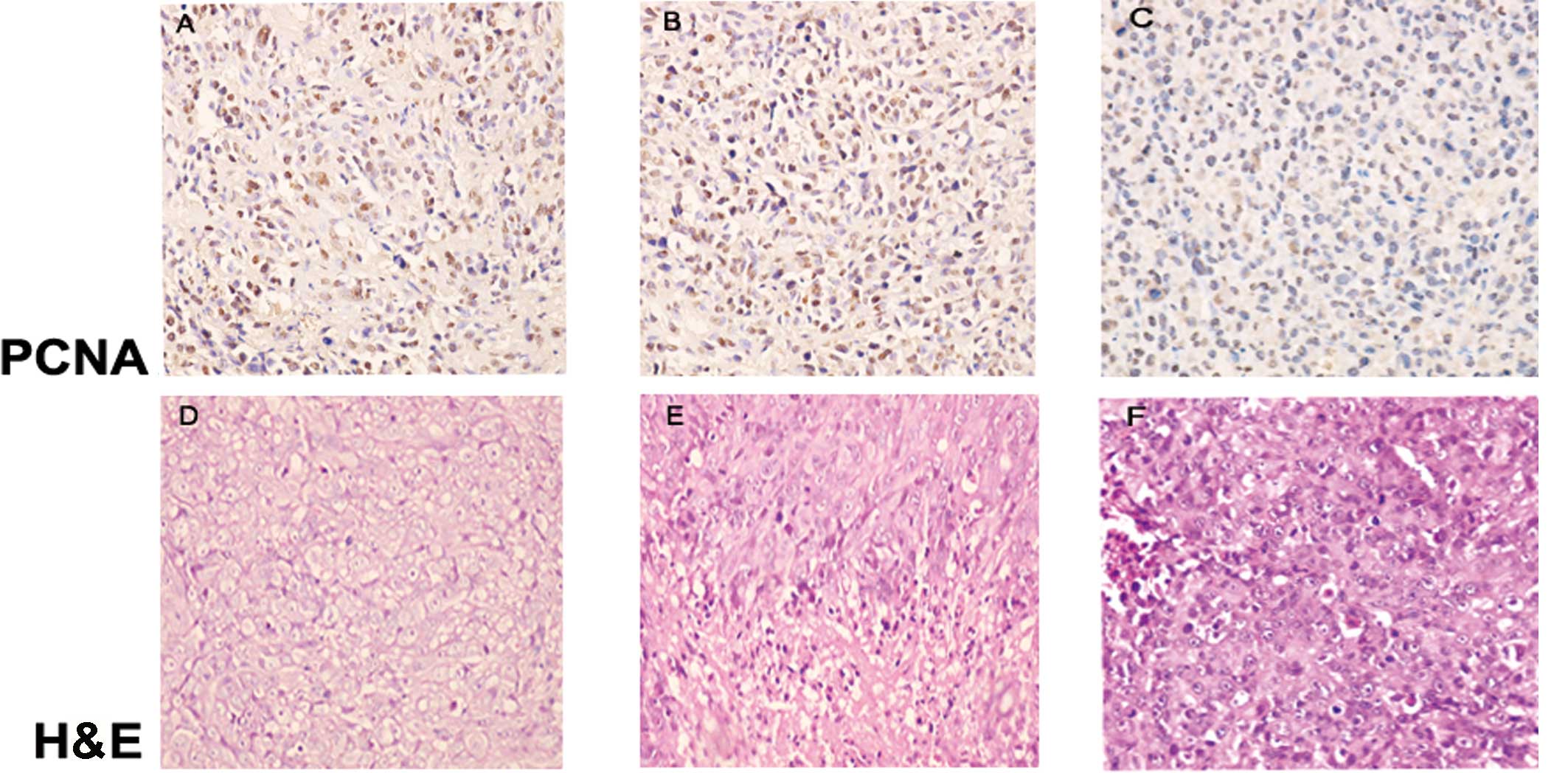
Histopathologic observation
Following H&E staining, the transplanted tumors
in each group revealed glands of unequal size, which integrated to
become lamellar, solid-like or acne-like accompanied with diffuse
infiltration when observed under a light microscope. Pathological
caryokinesis and low-degree differentiation implied a high degree
of malignancy. There was no difference in the Gleason score between
the three groups (P>0.05; Table
I).
 | Table IComparison of Gleason score in each
group (values are presented as the mean ± SD). |
Table I
Comparison of Gleason score in each
group (values are presented as the mean ± SD).
| Group | Gleason score |
|---|
| PC-3 | 8.7±0.48 |
| PC-3-vector | 8.7±0.48a |
| PC-3-TRPM8 | 8.4±0.52b |
MVD count of the different groups
To evaluate tumor neovascularization, we
immunostained tissue samples using a CD34 antibody (Abcam,
Cambridge, UK). CD34 is a cell surface sialomucin widely used as a
marker of most vascular endothelial cells, including those of
capillaries in the majority of tissues. In the present study, the
MVD in groups A and B was found to be higher than that in group C
(P=0.045 and P=0.041, respectively; Fig. 2G, H and I; Table II).
 | Table IIMVD values of tumor in each group
(values are presented as the mean ± SD). |
Table II
MVD values of tumor in each group
(values are presented as the mean ± SD).
| Group | MVD in tumor |
|---|
| PC-3 | 38.82±12.11 |
| PC-3-vector | 37.50± 9.97a |
| PC-3-TRPM8 | 29.66± 6.04b,c |
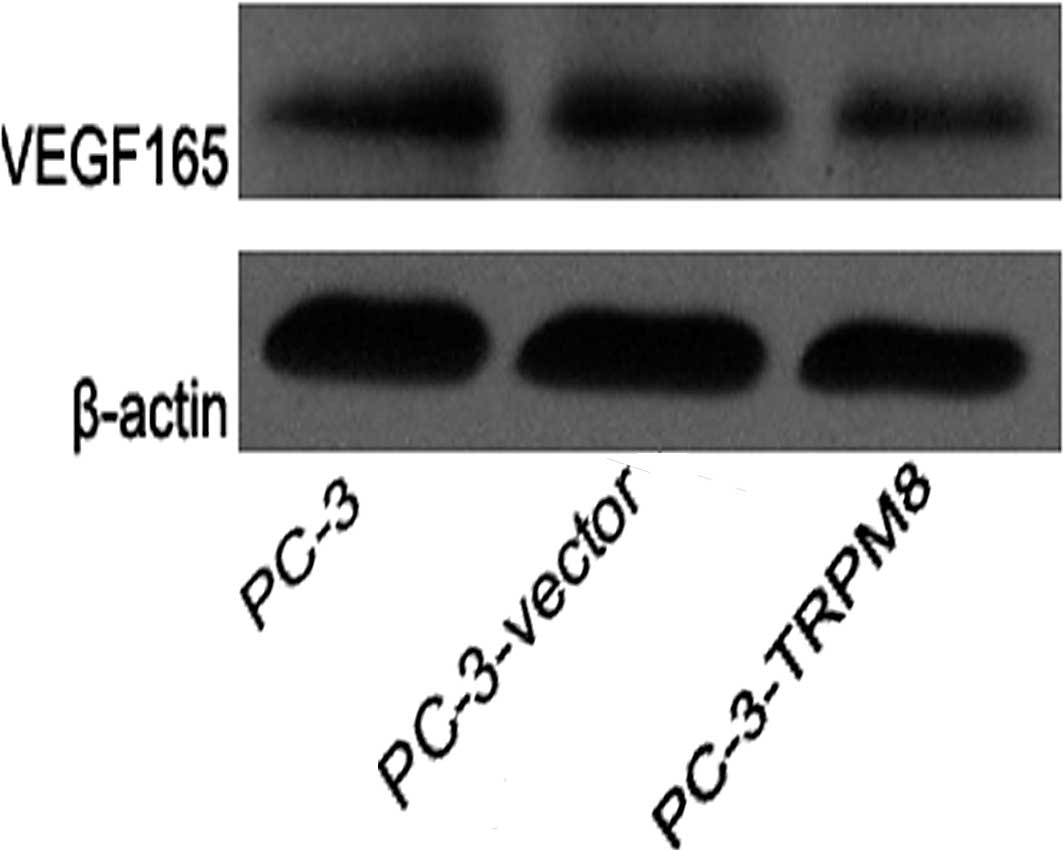
Expression of VEGF protein in each
group
All groups revealed VEGF protein expression. The
expression of VEGF protein in groups A and B was higher than that
in group C (P=0.000 and P=0.000, respectively; Fig. 3).
Relationship between MVD and VEGF protein
expression in each group
MVD and VEGF expression in tumor tissue had a
correlation coefficient of r=0.419 (P=0.021) for all three
groups.
Expression of focal adhesion kinase (FAK)
and proliferating cell nuclear antigen (PCNA) in each group
FAK is a non-receptor protein tyrosine kinase that
regulates adhesion-dependent cell signaling (14). In prostate cancer, FAK is known
primarily for its role in cell motility and cytoskeletal
rearrangement, as supported by in vivo and in vitro
evidence (15). PCNA is a nuclear
protein synthesized in the G1/S phase and plays a significant role
in DNA replication. PCNA expression is low in non-dividing cells,
but increases greatly in proliferating and transformed cells. In
the present study, anti-FAK-pY397-specific (Biosource, Camarillo,
CA, USA) and anti-PCNA-specific (Abcam) antibodies were used. The
expression of FAK and PCNA in groups A and B was higher than that
in group C (Fig. 2A, B, C, J, K and
L).
Discussion
Recent studies have focused on the role of TRPM8,
rendering it a novel molecular target potentially useful in the
diagnosis and treatment of PC. The channel is activated by voltage,
cold temperatures and cooling compounds, such as menthol and icilin
(16). Our previous results
indicated that the overexpression of TRPM8 has a negative effect on
the proliferation and malignant progression of PC-3 cells in
vitro (7). Similarly, Gkika
et al (17) have
demonstrated that PC-3 cells artificially overexpressing TRPM8 have
reduced motility, suggesting a possible connection between TRPM8
activity and reduced metastatic potential. In this study, the tumor
volume in group C was less than that in groups A and B, suggesting
that the overexpression of TRPM8 possibly has a negative effect on
the proliferation of PC-3 cells in vivo.
It is well established that the growth and
dissemination of solid tumors is dependent on angiogenesis
(18). Human VEGF mRNA is
transcribed from eight exons of a single gene and is alternatively
spliced into at least six mRNAs, which give rise to the mature
proteins of 121, 145, 165, 183, 189 and 206 amino acids.
VEGF121 and VEGF165 are the best
characterized and are the most abundant in normal tissues,
including blood vessels. As with most tumors, prostate tumors
overexpress VEGF, thereby promoting the development of tumor
neovascularization (19). Certain
studies using immunohistochemistry have reported an increased
expression of total hVEGF protein in human prostate tumors, when
compared with normal tissue or preinvasive prostate lesions
(20). Our results revealed protein
expression of VEGF165 in each group, but
VEGF121 was not detected. This lack of detection may be
because VEGF165 has a greater molecular weight and is
expressed more widely. Furthermore, in this study, the VEGF
expression level of group C was lower than that of groups A and B
(P=0.00 and P=0.00, respectively).
MVD is a prognostic marker for various tumors,
including prostate cancer (21). In
prostate cancer, MVD is correlated with the development of
metastases, clinical stage and overall patient survival (22). In addition, the progression of
prostate cancer into the AI state has been shown to be associated
with increased angiogenesis (23);
thus, antiangiogenic therapy may be a possible means of improving
treatment for patients with HRPC. MVD is a quantitative description
of angiogenesis. In this study, the MVD of group C was
significantly decreased compared to that of groups A and B (P=0.045
and P=0.041, respectively; Fig. 2G, H
and I; Table II), which,
coupled with the results of the expression of VEGF, indicated that
TRPM8 may have a negative effect on the angiogenesis of PC-3 cells
in vivo.
PCNA is a nuclear protein and plays a significant
role in DNA replication. The expression level of PCNA is closely
correlated to the cell state, which means that the level of PCNA
expression correlates with the degree of malignancy, invasion and
metastasis in cancer cells. Thus, PCNA is a significant evaluative
marker for tumor growth and prognosis (24). FAK is a non-receptor protein
tyrosine kinase that regulates adhesion-dependent cell signaling
(14). FAK expression is increased
in prostate cancer cell lines (25), and an increased expression
correlates with enhanced motility and tumorigenicity (26). Our previous study indicated that
overexpression of TRPM8, through inactivation of FAK, reduced the
motility of PC-3 cells in vitro (7). In this study, we have further shown
that TRPM8 inhibits the expression of FAK in vivo. The
results of this study have shown that the expression of FAK and
PCNA in group C was lower than that in groups A and B (Fig. 2J, K and L). Coupled with the results
of tumor volume in each group, the TRPM8 channel may mediate the
repair and synthesis of DNA, and may, through inactivation of FAK,
have a negative effect on proliferation. However, there was no
difference in the Gleason score of each group, and the reason for
this remains to be determined, but possibly lies in the limited
time taken for the graft to grow.
In conclusion, this study demonstrates that the
overexpression of TRPM8 had a negative effect on the proliferation
and angiogenesis progression of PC-3 cells in vivo.
Therefore, for patients in the AI stage, although there is
currently no successful therapy, the activation of the existing
channels or the overexpression of the channel may serve as a
potential alternative treatment and should be further
investigated.
Acknowledgements
This work was supported by the Fundamental Research
Funds for the Central Universities (no. 20103030101000213).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2
|
Martel CL, Gumerlock PH, Meyers FJ and
Lara PN: Current strategies in the management of hormone refractory
prostate cancer. Cancer Treat Rev. 29:171–187. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berridge MJ, Lipp P and Bootman MD: The
versatility and universality of calcium signalling. Nat Rev Mol
Cell Biol. 1:11–21. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsavaler L, Shapero MH, Morkowski S and
Laus R: Trp-p8, a novel prostate-specific gene, is up-regulated in
prostate cancer and other malignancies and shares high homology
with transient receptor potential calcium channel proteins. Cancer
Res. 61:3760–3769. 2001.
|
|
5
|
Henshall SM, Afar DE, Hiller J, et al:
Survival analysis of genome-wide gene expression profiles of
prostate cancers identifies new prognostic targets of disease
relapse. Cancer Res. 63:4196–4203. 2003.PubMed/NCBI
|
|
6
|
Zhang L and Barritt GJ: Evidence that
TRPM8 is an androgen-dependent Ca2+ channel required for
the survival of prostate cancer cells. Cancer Res. 64:8365–8373.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang ZH, Wang XH, Wang HP and Hu LQ:
Effects of TRPM8 on the proliferation and motility of prostate
cancer PC-3 cells. Asian J Androl. 11:157–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brawer MK, Deering RE, Brown M, Preston SD
and Bigler SA: Predictors of pathologic stage in prostatic
carcinoma. The role of neovascularity. Cancer. 73:678–687. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrer FA, Miller LJ, Andrawis RI, et al:
Vascular endothelial growth factor (VEGF) expression in human
prostate cancer: in situ and in vitro expression of VEGF by human
prostate cancer cells. J Urol. 157:2329–2333. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jackson MW, Bentel JM and Tilley WD:
Vascular endothelial growth factor (VEGF) expression in prostate
cancer and benign prostatic hyperplasia. J Urol. 157:2323–2328.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Borre M, Nerstrom B and Overgaard J:
Association between immunohistochemical expression of vascular
endothelial growth factor (VEGF), VEGF-expressing
neuroendocrine-differentiated tumor cells, and outcome in prostate
cancer patients subjected to watchful waiting. Clin Cancer Res.
6:1882–1890. 2000.
|
|
13
|
Warri AM, Huovinen RL, Laine AM,
Martikainen PM and Harkonen PL: Apoptosis in toremifene-induced
growth inhibition of human breast cancer cells in vivo and in
vitro. J Natl Cancer Inst. 85:1412–1418. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Parsons JT, Slack-Davis J, Tilghman R and
Roberts WG: Focal adhesion kinase: targeting adhesion signaling
pathways for therapeutic intervention. Clin Cancer Res. 14:627–632.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang YM, Kung HJ and Evans CP:
Nonreceptor tyrosine kinases in prostate cancer. Neoplasia.
9:90–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Voets T, Owsianik G and Nilius B: Trpm8.
Handb Exp Pharmacol. 329–344. 2007. View Article : Google Scholar
|
|
17
|
Gkika D, Flourakis M, Lemonnier L and
Prevarskaya N: PSA reduces prostate cancer cell motility by
stimulating TRPM8 activity and plasma membrane expression.
Oncogene. 29:4611–4616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: an imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferrer FA, Miller LJ, Lindquist R, et al:
Expression of vascular endothelial growth factor receptors in human
prostate cancer. Urology. 54:567–572. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mazzucchelli R, Montironi R, Santinelli A,
Lucarini G, Pugnaloni A and Biagini G: Vascular endothelial growth
factor expression and capillary architecture in high-grade PIN and
prostate cancer in untreated and androgen-ablated patients.
Prostate. 45:72–79. 2000. View Article : Google Scholar
|
|
21
|
Hollingsworth HC, Kohn EC, Steinberg SM,
Rothenberg ML and Merino MJ: Tumor angiogenesis in advanced stage
ovarian carcinoma. Am J Pathol. 147:33–41. 1995.PubMed/NCBI
|
|
22
|
Weidner N, Carroll PR, Flax J, Blumenfeld
W and Folkman J: Tumor angiogenesis correlates with metastasis in
invasive prostate carcinoma. Am J Pathol. 143:401–409.
1993.PubMed/NCBI
|
|
23
|
Kosaka T, Miyajima A, Takayama E, et al:
Angiotensin II type 1 receptor antagonist as an angiogenic
inhibitor in prostate cancer. Prostate. 67:41–49. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka S, Haruma K, Tatsuta S, et al:
Proliferating cell nuclear antigen expression correlates with the
metastatic potential of submucosal invasive colorectal carcinoma.
Oncology. 52:134–139. 1995. View Article : Google Scholar
|
|
25
|
Lacoste J, Aprikian AG and Chevalier S:
Focal adhesion kinase is required for bombesin-induced prostate
cancer cell motility. Mol Cell Endocrinol. 235:51–61. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Slack JK, Adams RB, Rovin JD, Bissonette
EA, Stoker CE and Parsons JT: Alterations in the focal adhesion
kinase/Src signal transduction pathway correlate with increased
migratory capacity of prostate carcinoma cells. Oncogene.
20:1152–1163. 2001. View Article : Google Scholar : PubMed/NCBI
|