Introduction
Pancreatic cancer is one of the most aggressive
types of malignancy, with the majority of patients exhibiting
surgically unresectable disease at the time of diagnosis (1). Surgical resection is the only
potentially curative therapy, but even in resectable cases the
overall 5-year survival rate is only 15–20% (2–3).
Accordingly, surgical resection, as well as other forms of adjuvant
therapy are required for improving the prognosis of such
patients.
Since Neoptolemos et al reported the
significant effect of postoperative chemotherapy on survival time
after curative resection for pancreatic cancer (4), a number of studies have focused on
adjuvant postoperative chemotherapy for improving the outcome of
patients with pancreatic cancer (5–6).
Gemcitabine (GEM), a deoxycytidine analogue of arabinosylcytosine,
is one of the most promising chemotherapeutic agents to have
emerged in recent years. Oettle et al reported that adjuvant
chemotherapy with GEM was capable of prolonging not only
disease-free survival, but also overall survival following curative
resection for pancreatic cancer (7). That report, known as the CONKO-001
study, resulted in the adoption of GEM as a standard form of
adjuvant chemotherapy following resection of pancreatic cancer.
However, few reports have described suitable regimens for patients
who suffer relapse after adjuvant chemotherapy.
Thus, we retrospectively evaluated the efficacy and
safety of S-1, an oral fluoropyrimidine derivative (8), as a second-line chemotherapy for
patients who had suffered relapse after adjuvant chemotherapy with
GEM.
Patients and methods
Patients
Between 2001 and 2009, 51 patients with pancreatic
cancer treated at our institution suffered relapse after curative
resection and subsequent adjuvant chemotherapy with GEM. A group of
26 of these patients received S-1 orally twice daily after meals at
a dose of 80 mg/m2 for body surface areas for 14
consecutive days, followed by a 7-day rest (S-1 group). After the
disease was judged to be progressive, 10 patients underwent a
third-line chemotherapy. In total, 3 patients were administered
paclitaxel at 80 mg/m2; 4 patients returned to
chemotherapy with GEM (at 1000 or 800 mg/m2); 2 patients
were administered GEM and S-1 concurrently; and 2 patients
underwent the two-drug chemotherapy with CDDP and CPT-11. The
remaining 25 patients were not administered any other anticancer
drugs other than continuation of GEM (GEM/BSC group). If GEM was
continued after disease recurrence, it was administered at 1000
mg/m2 bi-weekly for as long as possible. Among the
latter 25 patients, 5 (20%) continued to receive GEM, and 20 (80%)
were not administered any other anticancer drugs. The differences
between the S-1 and GEM groups were analyzed with regard to patient
demographics, clinical characteristics, overall survival (OAS), and
survival after recurrence (SAR).
Statistical analysis
Demographic and clinical characteristics were
expressed as means, medians and ranges (continuous outcomes).
Groups were compared using the Wilcoxon rank-sum test for
continuous outcomes and the Fisher's exact test for categorical
outcomes. Survival distributions were estimated using the
Kaplan-Meier method, and groups were compared using the log-rank
test. Differences were considered to be significant at p<0.05.
The data were analyzed using the Stat View software program (Abacus
Concepts, Inc., Berkeley, California, USA).
Results
Patient characteristics
Patient characteristics in the S-1 and GEM/BSC
groups are shown in Table I. This
retrospective study included 51 patients (26 in the S-1 group and
25 in the GEM/BSC group). The following parameters were compared
between the groups: gender, age, final stage, T factor, N factor,
operative procedure employed, R0/R1 resection rate, and pattern of
recurrence. However, the two groups were statistically similar.
Disease-free survival periods for the two groups were estimated by
the Kaplan-Meier method. The median disease-free survival period
was 6.4 months in the S-1 group and 5.9 months in the GEM/BSC
group; the difference was not significant (p=0.6019).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| S-1 | GEM/BSC | p-value |
|---|
| Patients | 26 | 25 | |
| Gender
(male/female) | 14/12 | 14/11 | 0.903 |
| Age (years) | 63.8 (50–78) | 68.4 (48–81) | 0.091 |
| Pathological
stagea (I/II/III/IV) | 6/18/0/2 | 4/20/0/1 | 0.416 |
| T factor
(T1,2/T3,4) | 3/23 | 4/21 | 0.406 |
| N factor (N0/N1) | 9/17 | 14/11 | 0.125 |
| Operative procedure
(head/distal resection) | 20/6 | 18/7 | 0.938 |
| Resection status
(R0/R1) | 23/3 | 22/3 | 0.959 |
| Recurrence pattern
(liver met.b/local rec.c/disseminationd) | 6/16/4 | 8/13/4 | 0.188 |
| Median of
disease-free survival (months) | 6.40 | 5.86 | 0.602 |
Survival
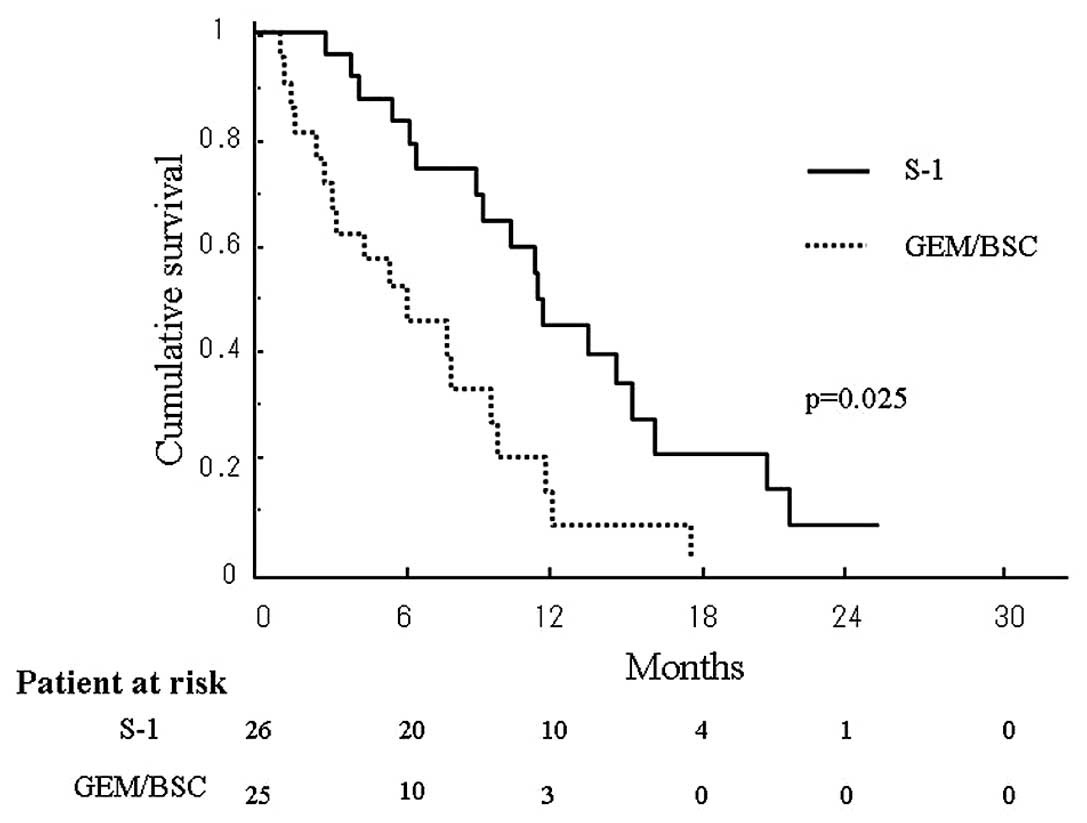
Survival periods after recurrence in the two groups
were compared using the Kaplan-Meier method (Fig. 1). The median survival period after
recurrence was 11.4 months in the S-1 group and 6.2 months in the
GEM/BSC group, with survival in the former being significantly
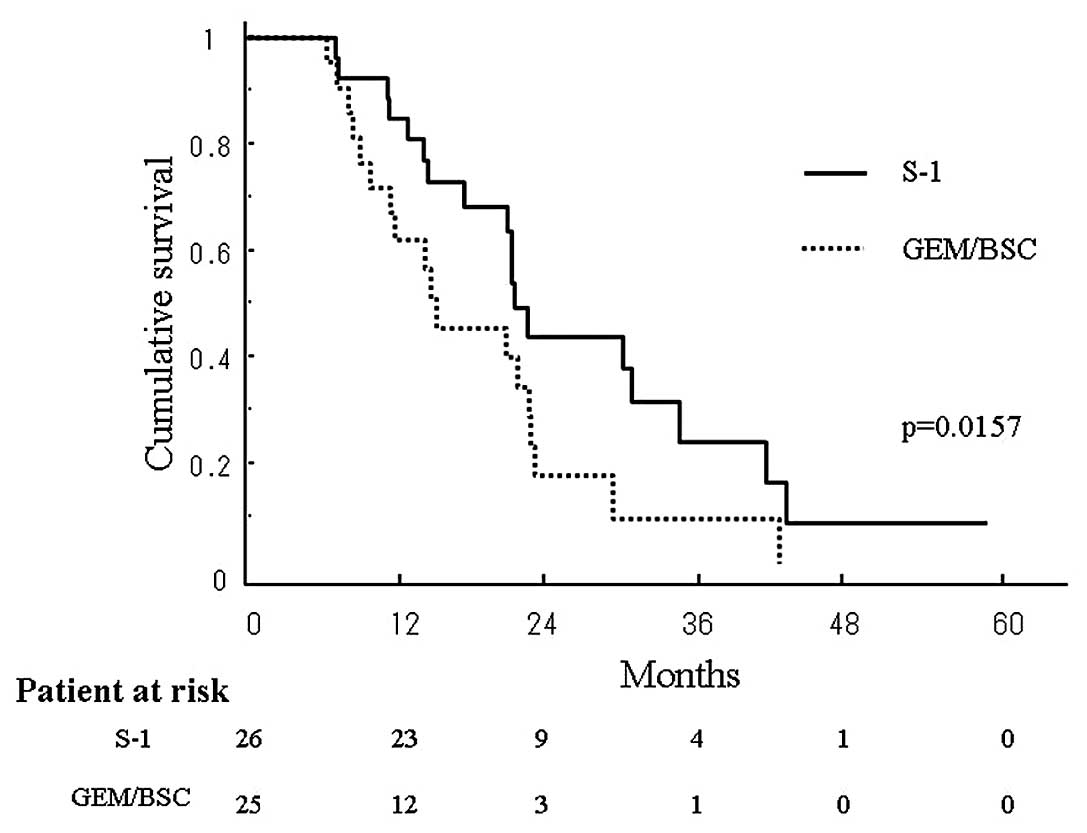
longer than that in the latter (p=0.025). The estimated OAS in the
S-1 and GEM/BSC groups at 3 years was 24.7 and 7.6%, respectively,
again being significantly longer in the former than in the latter
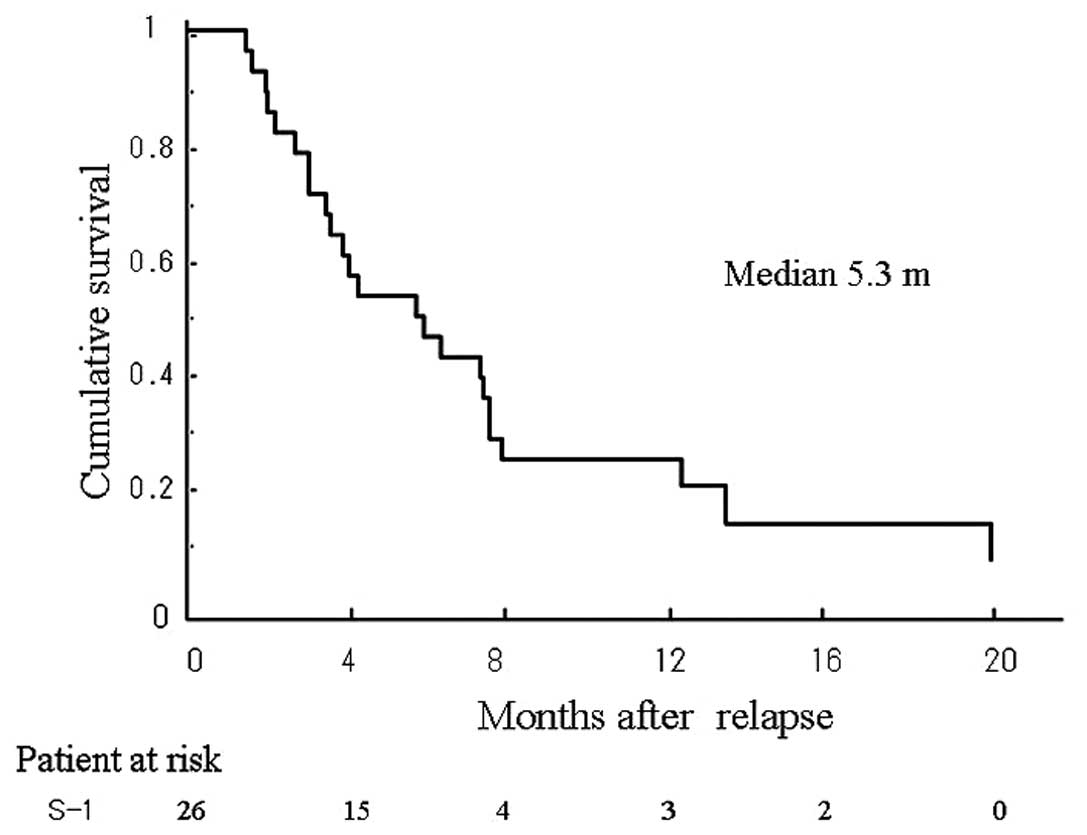
(p=0.0157) (Fig. 2). The median
period until progression and the 6-month progression-free survival
rate were 5.4 months and 38.5%, respectively (Fig. 3).
Toxicity
The toxicity profiles are shown in Table II. Severe adverse events (grade
3/4) included leukopenia (3.8%), neutropenia (7.7%), anorexia
(3.8%), and fatigue (3.8%). No treatment-related death
occurred.
 | Table IIDrug-related adverse effects. |
Table II
Drug-related adverse effects.
| S1 group (n=26) |
|---|
|
|
|---|
| G1/2 (%) | G3/4 (%) |
|---|
| Hematological
toxicity |
| Leukopenia | 4 (15.4) | 1 (3.8) |
| Neutropenia | 3 (11.5) | 1 (3.8) |
| Anemia | 1 (0.4) | 0 (0.0) |
| Thrombopenia | 0 (0.0) | 0 (0.0) |
| Non-hematological
toxicity |
| Appetite loss | 2 (7.7) | 2 (7.7) |
| Diarrhea | 0 (0.0) | 0 (0.0) |
| Nausea | 1 (3.8) | 0 (0.0) |
| Vomiting | 3 (11.5) | 0 (0.0) |
| Fatigue | 3 (11.5) | 1 (3.8) |
Efficacy of S-1 in terms of recurrence
pattern
Among the 51 patients studied, 16 suffered relapse
with liver or lung metastasis, 10 developed peritoneal
dissemination, and 25 had local recurrence. The efficacy of S-1 in
terms of the various patterns of recurrence was evaluated (Table III). The median OAS of the
patients who developed lung or liver metastasis and peritoneal
dissemination was 10.5 and 13.5 months in the S-1 group and 11.6
and 8.7 months in the GEM/BSC group, respectively. A log-rank test
using the Kaplan-Meier method revealed significant difference
between the two groups. However, the median OAS of the patients who
developed local recurrence was 26.9 months in the S-1 group and
17.8 months in the GEM/BSC group (p=0.0469). This result indicates
that S-1 was capable of prolonging the OAS in patients who
developed local recurrence.
 | Table IIIEfficacy of S-1 in terms of recurrence
pattern. |
Table III
Efficacy of S-1 in terms of recurrence
pattern.
| S-1 MST (months) | GEM/BSC MST
(months) | p-value
(log-rank) |
|---|
| Liver metastasis | 10.5 | 11.6 | 0.796 |
| Peritoneal
dissemination | 13.5 | 8.7 | 0.152 |
| Local recurrence | 26.9 | 17.8 | 0.046 |
Discussion
In this retrospective study, we investigated the
efficacy and feasibility of S-1 as second-line chemotherapy after
adjuvant chemotherapy with GEM for patients with pancreatic cancer.
Our results show that the administration of S-1 as a second-line
chemotherapy was capable of prolonging not only the survival period
after relapse (median 11.4 vs. 6.2 months), but also the overall
survival period (median 20.9 vs. 13.7 months). Second-line
chemotherapy with S-1 combined with adjuvant chemotherapy using GEM
may therefore be an efficient and beneficial strategy for
pancreatic cancer patients.
Neoptolemos et al previously demonstrated
that adjuvant chemotherapy was potentially beneficial for patients
with pancreatic cancer, whereas adjuvant chemoradiotherapy had a
deleterious effect on survival (6).
Tani et al have reported that adjuvant chemotherapy was an
independent factor affecting long-term survival in patients with
locally advanced pancreatic cancer who had undergone surgery
(10). Oettle et al have
shown that adjuvant chemotherapy with GEM for pancreatic cancer
patients was significantly effective for prolonging disease-free
survival (7), and their subsequent
study revealed that it was also capable of prolonging OAS (9). In their study, Ueno et al have
shown that GEM prolonged disease-free survival in patients who had
undergone macroscopically curative resection of pancreatic cancer
(8). Since these reports were
published, adjuvant chemotherapy with GEM has been the standard
treatment in Japan for patients following resection of pancreatic
cancer. However, few reports have described the optimal regimens
for patients who suffer relapse after adjuvant chemotherapy. In the
present study, we retrospectively evaluated the efficacy and safety
of S-1, an oral fluoropyrimidine derivative, as second-line
chemotherapy for patients suffering disease relapse after adjuvant
chemotherapy with GEM.
S-1 is an oral anticancer drug consisting of
tegafur, a prodrug of 5-FU, and two biochemical modulators,
5-chloro-2,4-dihydroxypyridine and potassium oxonate (11). S-1 has been shown clinically to
exert potent antitumor activity against various solid tumors
(12–15). Okusaka et al have reported
that S-1 is a promising agent for advanced pancreatic cancer, with
a response rate of 37.5% and an MST of 9.2 months (16) In our present study, the MST after
recurrence was prolonged for up to 11.4 months by S-1
administration. The median progression-free survival time after
administration of S-1 was estimated to be 5.4 months. Results show
that second-line chemotherapy with S-1 was capable of maintaining
progression-free survival for approximately 6 months, but also
extended survival for an additional 6 months. This may have been
due to the fact that the toxicity of S-1 was sufficiently mild to
allow the introduction of third-line chemotherapy.
In general, S-1 should be administered orally for 28
conse-cutive days, followed by a 14-day rest. However, the
incidence of adverse reactions tended to be high (83.2%), and 20.3%
of all adverse reactions were reported to be of grade 3 or more
severe (12,16). Therefore, certain previous reports
have proposed that S-1 should be administered for 2 weeks, followed
by a 1-week rest, rather than for 4 weeks followed by a 2-week
rest. Tsukuda et al have reported that, in patients with
advanced head and neck cancer, a 2-week administration of S-1
followed by a 1-week rest was safer and more tolerable than 4-week
administration followed by a 2-week rest (18). With regard to the administration of
S-1 for advanced or recurrent gastric cancer, Kimura et al
have reported that the rate of adverse reactions was 77% in the
2-week regimen, compared with 93% for the 4-week regimen. They also
reported that the total 6-month compliance for S-1 was much more
favorable for the 2-week regimen than for the 4-week regimen. These
authors concluded that the 2-week regimen may mitigate adverse
reactions and prolong the medication period (19). In the present study, S-1 was
administered orally for 14 consecutive days, followed by a 7-day
rest (2-week regimen). Neither hematological nor non-hematological
adverse events were frequent. Severe adverse effects (grade 3/4)
were almost not evident, and the medication time was therefore
prolonged. This may have contributed to prolonging not only
progression-free but also overall survival.
S-1 administration was not capable of prolonging the
OAS of patients who had suffered relapse in the form of either
peritoneal dissemination or liver or lung metastasis, and was
effective only for local recurrence. S-1 administration allowed
patients who had suffered local recurrence to survive longer than
those who continued with GEM, or received best supportive care. In
a phase II study report, Okusaka et al stated that S-1
administration was effective against metastatic pancreatic cancer.
In their study, although 90% of patients had liver metastasis, a
relatively long MST (9.3 months) was observed (16). In the present study, as only a small
number of patients developed relapse in the form of liver
metastasis, the effectiveness of S-1 may not have reached a
significant level.
In conclusion, following not only major surgical
treatment, but also cancer relapse, patients experience a
relatively severe condition. S-1, an oral anticancer drug, is
capable of maintaining a reasonable quality of life under such
conditions (20). Since this study
revealed a promising anticancer effect of S-1 and a significantly
long survival time, S-1 is a potentially beneficial drug for
second-line chemotherapy following adjuvant chemotherapy with GEM
in patients with pancreatic cancer.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics. CA Cancer J Clin.
58:71–96. 2008.
|
|
2
|
Bradley EL: Long-term survival after
pancreatoduodenectomy for ductal adenocarcinoma: the emperor has no
clothes? Pancreas. 37:349–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yeo CJ, Cameron JL, Lillemoe KD, et al:
Pancreatico-duodenectomy for cancer of the head of pancreas: 201
patients. Ann Surg. 221:721–733. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neoptolemos JP, Dunn AA, Stocken DD, et
al: Adjuvant chemoradiotherapy and chemotherapy in resectable
pancreatic cancer: a randomized controlled trial. Lancet.
358:1576–1585. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ueno H, Kosuge T, Matsuyama Y, Yamamoto J,
Nakao A, Egawa S, Doi R, Monden M, Hotori T, Tanaka M, Shimada M
and Kanemitsu K: A randomised phase III trial comparing gemcitabine
with surgery-only in patients with resected pancreatic cancer.
Japanese Study Group of Adjuvant Therapy for pancreatic cancer. Br
J Cancer. 101:908–915. 2009. View Article : Google Scholar
|
|
6
|
Neoptolemos P, Stocken DD, Friess H, et
al: A randomized trial of chemoradiation therapy and chemotherapy
after resection of pancreatic cancer. N Engl J Med. 350:1200–1210.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oettle H, Post S, Neuhaus P, et al:
Adjuvant chemotherapy with gemcitabine vs observation in patients
undergoing curative-intent resection of pancreatic cancer: a
randomized controlled trial. JAMA. 297:267–277. 2007. View Article : Google Scholar
|
|
8
|
Ueno H, Okusaka T, Ikeda M, Takezako Y and
Morizane C: Phase II study of S-1 in patients with advanced biliary
tract cancer. Br J Cancer. 91:1769–1774. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neuhaus P, Riess H, Post S, et al:
CONKO-001: Final results of the randomized, prospective,
multicenter phase III trial of adjuvant chemotherapy with
gemcitabine versus observation in patients with resected pancreatic
cancer (PC). J Clin Oncol. 26:2142008.(suppl; abstr LBA4504).
|
|
10
|
Tani M, Kawai M, Terasawa H, Ina S, et al:
Prognostic factor for long-term survival in patients with locally
invasive pancreatic cancer. J Hepatobiliary Pancreat Surg.
14:545–550. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shirasaka T, Shimamoto Y, Ohshimo H, et
al: Development of a novel form of an oral 5-fluorouracil
derivative (S-1) directed to the potentiation of the tumor
selective cytotoxity of 5-fluorouracil by two biochemical
modulators. Anticancer Drugs. 7:548–557. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakata Y, Ohtsu A, Horikkoshi N, Sugimachi
K, Mitachi Y and Taguchi T: Late phase II study of novel oral
fluoropyrimidine anticancer drug S-1 (1M tegafur-0.4M gimestat-1M
otastat potassium) in advanced gastric cancer patients. Eur J
Cancer. 34:1715–1720. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saeki T, Takashima S, Sano M, et al: A
phase II study of S-1 Cooperative Study breast cancer – a Japanese
trial by the S-1 Cooperative Study Group, Breast Cancer Working
Group. Breast Cancer. 11:194–202. 2004.
|
|
14
|
Fukushima M, Satake H, Uchida J, et al:
Preclinical antitumor efficacy of S-1: A new oral formulation of
5-fluorouracil on human tumor xenografts. Int J Oncol. 13:693–698.
1998.PubMed/NCBI
|
|
15
|
Ueno H, Okusaka T, Ikeda M, Takezako Y and
Morizane C: An early phase II study of S-1 in patients with
metastatic pancreatic cancer. Oncology. 68:171–178. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okusaka T, Funakoshi A, Furuse J, Boku N,
Yamao K, Ohkawa S and Saito H: A late phase II study of S-1 for
metastatic pancreatic cancer. Cancer Chemother Pharmacol.
61:615–621. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sugimachi K, Maehara Y, Horikoshi N, et
al: An early phase II study of oral S-1, a newly developed
5-fluorouracil derivatives for advanced and recurrent
gastrointestinal cancers. Oncology. 57:202–210. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsukuda M, Kida A, Kono N, Yoshihara T,
Hasegawa Y and Sugita M; Chemotherapy Study Group of Head and Neck
Cancer. Randomized scheduling feasibility study of S-1 for adjuvant
chemotherapy in advanced head and neck cancer. Br J Cancer.
93:884–889. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kimura Y, Kikkawa N, Iijima S, et al: A
new regimen for S-1 therapy aiming at adverse reaction mitigation
and prolonged medication by introducing a 1-week drug-free interval
after each 2-week dosing session: efficacy and feasibility in
clinical practice. Gastric Cancer. 6:34–39. 2003. View Article : Google Scholar
|
|
20
|
O’Neill VJ and Twelves CJ: Oral cancer
treatment: developments in chemotherapy and beyond. Br J Cancer.
87:933–937. 2002.
|