Introduction
Small cell carcinoma of the uterine cervix (SCCUC)
is a rare gynecologic malignancy and constitutes less than 5% of
all invasive cervical carcinomas (1–4). The
histology and biologic behavior of SCCUC are similar to that of
small cell lung carcinoma (SCLC), which is highly aggressive. Due
to the high incidence of lymph vascular space involvement (LVSI),
lymph node metastases (LNM), and distant metastases, the prognosis
of SCCUC is poorer than that of other histological types of
cervical carcinoma (5,6). The 5-year survival rates for SCCUC
range from 31.6 to 46.6% for early-stage disease and from 0 to 14%
for advanced stage disease (1,2,7).
Previous studies showed that patients with SCCUC
treated with a modality similar to the standard treatment for
squamous cell carcinoma of the cervix have a poor prognosis
(7–9). It is thus imperative to identify
prognostic factors and optimal treatment strategies to improve
treatment outcome. Due to the rarity of SCCUC, it is difficult to
obtain comprehensive evidence-based information regarding
prognostic factors and optimal treatment modalities. Therefore, a
retrospective study of treatment experience is valuable to enhance
our understanding of SCCUC.
Thus, we pooled our cases with all of the reported
relevant cases in the literature and conducted a retrospective
study to obtain more information pertaining to treatment outcome
and prognostic factors in International Federation of Gynecology
and Obstetrics (FIGO) stage IB1-IIA patients with SCCUC.
Patients and methods
Patients
We searched the computerized hospital database of
patients treated for carcinoma of the uterine cervix between 1995
and 2008 at the Fudan University Shanghai Cancer Center, China, to
identify patients whose tumors were diagnosed as SCCUC. Only
patients who received radical surgery, with or without adjuvant
treatment for FIGO stage IB1-IIA SCCUC at our hospital were
included in this study. As primary treatment, patients underwent
radical hysterectomy and pelvic lymphadenectomy, with or without
para-aortic lymphadenectomy. Adjuvant treatment included
chemotherapy or chemotherapy plus pelvic or extended-field
radiotherapy. The follow-up data were updated on June 30, 2010.
Patient selection criteria and treatment
modalities
Histologic sections were reviewed with criteria for
the diagnosis of SCCUC (10). In
brief, the criteria included the presence of small cells with scant
cytoplasm, hyperchromatic nuclei with indistinct nucleoli and
nuclear molding, and numerous mitoses and extensive necrosis. In
addition, SCCUC had to be positive for at least one of the
neuro-endocrine markers.
To obtain a sufficient number of cases of this rare
disease for analysis, we also retrieved the relevant cases reported
in the English literature since 1990 through a search on PubMed.
Clinical and pathological variables included age, tumor size, FIGO
stage, tumor homology, lymph node status, depth of stromal
invasion, LVSI, types of chemotherapy and treatment modalities.
Adjuvant chemotherapy was divided into two categories: similar or
not similar to that of SCLC. The former category included VAC
(vincristine, adriamycin, and cyclophosphamide) and PE (platinum
and etoposide). The latter category included the single or multiple
administration of mitomycin, ifosfamide, cyclophosphamide,
paclitaxel and platinum.
Statistical analysis
The primary endpoints were any cancer- related death
and overall survival (OS), calculated from the date of diagnosis to
death, or censored at the last follow-up. Statistical analysis of
the pooled data from the combined patients was performed. OS was
estimated using the Kaplan-Meier method and log-rank test.
P<0.05 was considered to be statistically significant. The
statistical software package SPSS 13.0 (SPSS Inc. Chicago, IL, USA)
was used for all data analyses.
Results
Among the 5,127 patients with cervical carcinoma who
were treated at our hospital between 1995 and 2008, 24 patients
presented SCCUC, representing 0.5% of the total. There were 9
patients with stage IB1 SCCUC, 2 stage IB2, 7 stage IIA, 4 stage
IIB, and 2 stage IVB by FIGO staging. Only 14 patients with stage
IB1-IIA SCCUC met our criteria and were included in this study.
Characteristics of these patients are shown in Table I. Median age at diagnosis was 40
years (range 30–51). Median tumor size was 3.0 cm (range 2.0–8.0).
The positive staining for synaptophysin, chromogranin, and
neuron-specific enolase (NSE) was 85.7% (12/14), 78.6% (11/14), and
92.9% (13/14), respectively. Based on the preoperative histologic
examination, 11 patients (79%) were accurately diagnosed as SCCUC
and the remaining 3 patients were misdiagnosed as moderately
differentiated squamous cell carcinoma, poorly differentiated
carcinoma and poorly differentiated squamous cell carcinoma prior
to surgery. A total of 12 patients were pure SCCUC while 2 had
focal squamous cell carcinoma (n=1) or adenosquamous cell carcinoma
(n=1). Postoperative adjuvant chemotherapy was administered to 1
patient, and the remaining 13 patients received postoperative
chemotherapy and RT.
 | Table ICharacteristics and survivorship of 14
patients at the Shanghai Cancer Center. |
Table I
Characteristics and survivorship of 14
patients at the Shanghai Cancer Center.
| Characteristics | Survivorship, no.
(survived/total) |
|---|
| Median age, years
(range) | 40 (30–51) |
| Median tumor size, cm
(range) | 3.0 (2.0–8.0) |
| FIGO stage | |
| IB1 | 5/8 |
| IB2 | 0 |
| IIA | 2/6 |
| Histology, (No. of
patients) | |
| Pure | 5/12 |
| Mixed | 2/2 |
| DSI, (≥2/3 stromal
invasion) | |
| Yes | 3/10 |
| No | 4/4 |
| LVSI | |
| Yes | 3/8 |
| No | 4/6 |
| LNM | |
| Yes | 5/11 |
| No | 2/3 |
| Treatment
modalities | |
| SU+Chemo | 1/1 |
| SU+Chemo+RT | 6/13 |
| Chemotherapy
regimen | |
| Cs | 6/10 |
| Cns | 1/4 |
The median survival of the 14 patients was 45.8
months. During a median follow-up of 25.4 months (range 6.8–46.4),
7 patients remained alive without disease, while 7 patients
succumbed to the disease. The 7 patients with recurrent disease
received postoperative chemotherapy and RT. Of the 7 patients, no
patient was found to have local recurrence alone, 5 (36%) had
distant metastases, and 2 (14%) had both at the time of recurrence.
The median time of recurrence was 14.2 months (range 4.6–30.4). Of
the 7 patients with recurrent disease, 5 (71%) had relapse within
24 months after diagnosis.
A total of 82 early-stage SCCUC patients who had
undergone SU with or without adjuvant therapy were identified by a
search on PubMed (3,4,9,11–30).
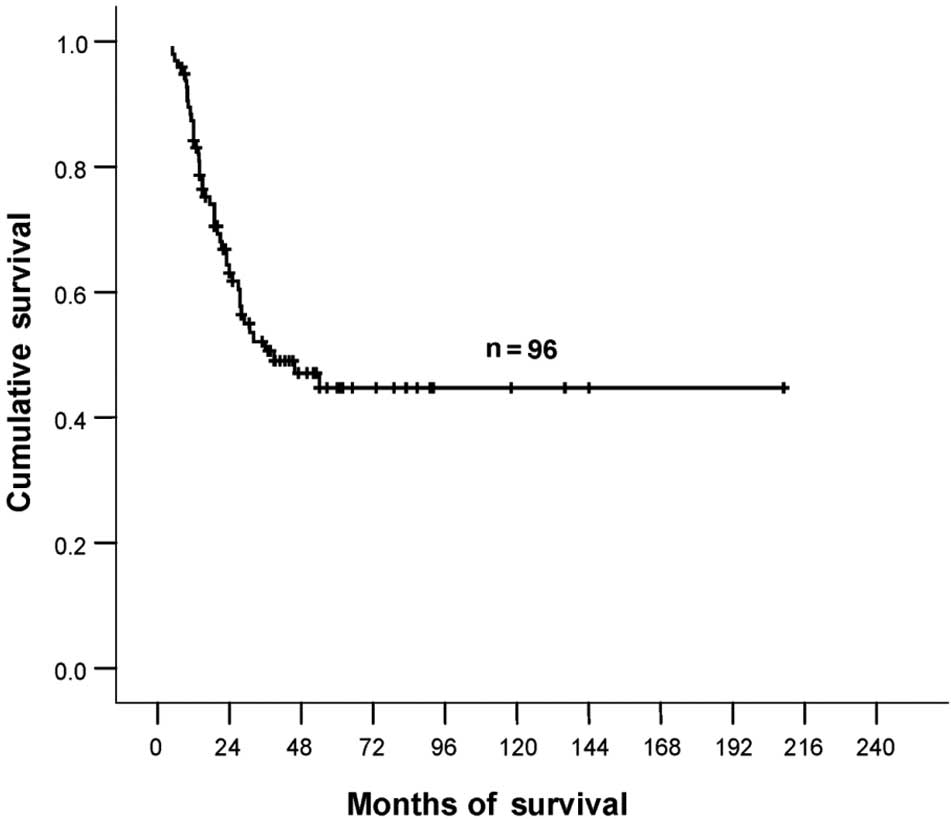
The total number of patients for analysis was 96. Median age was 40
years (range 20–67), and the median survival was 39.0 months (95%
CI, 14.5–63.5). The estimated 2- and 5-year survival rates were 62
and 45%, respectively (Fig. 1). For
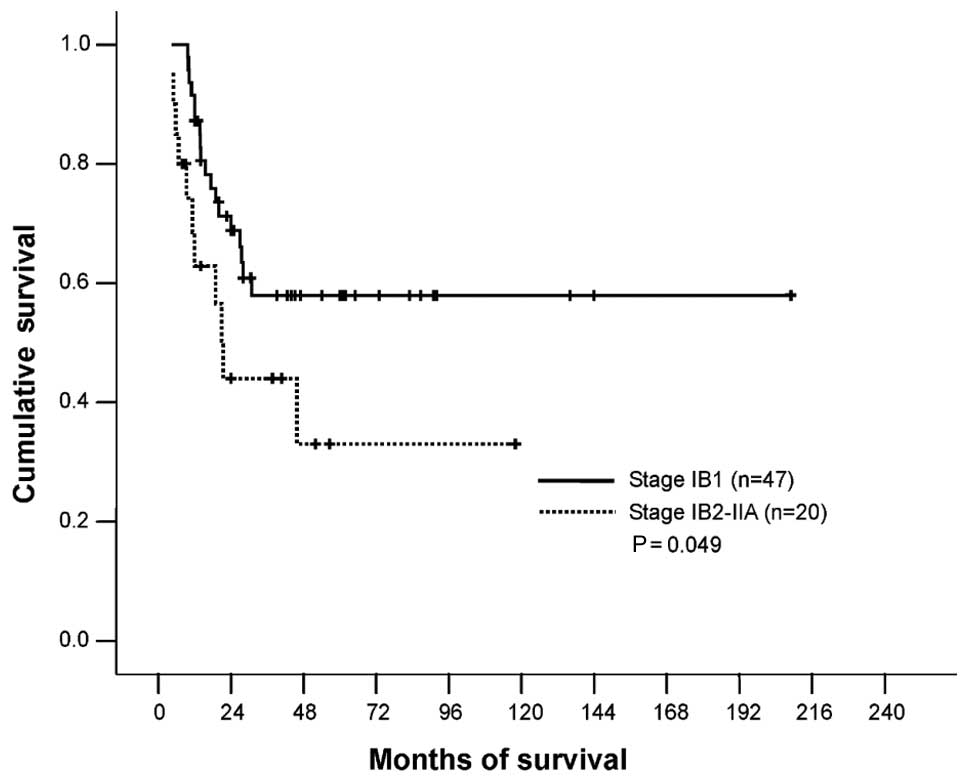
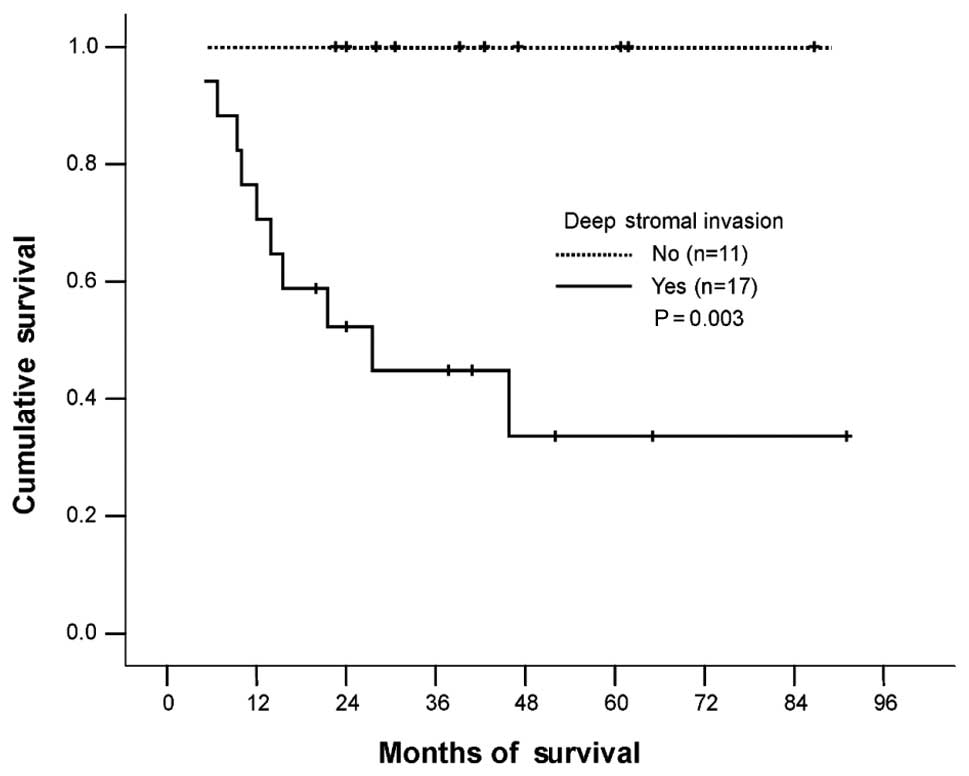
the 96 patients, the independent variables of stage IB1, absence of
LNM and inner 1/3 stromal invasion were found to have a
significant, favorable impact on survival (Table II, Figs. 2–4).
We observed that patients who received chemotherapy similar to that
of SCLC appeared to have more survival benefits than patients who
received other types of chemotherapy regimen. However, statistical
significance was not achieved (P=0.079). Treatment modalities were
also not related to survival. Treatment selection was related to
LNM and DSI (Table III).
Following stratification according to these variables, we did not
find any relationship between treatment and survival (data not
shown). With a median follow-up of 24.5 months (range 9-209), 49
patients exhibited recurrence. Among them, 45 patients succumbed to
the disease, and 4 patients were alive with disease. Detailed
relapse data were available in 32 patients (Table IV). Of these 32 patients, 2 (6%)
presented local, 24 (75%) distant, and 6 (19%) presented both local
and distant recurrence.
 | Table IIAnalysis of prognostic factors in 96
early-stage SCCUC patients. |
Table II
Analysis of prognostic factors in 96
early-stage SCCUC patients.
| Characteristics | Survivorship, no.
(survived/total) (%) | P-valuea |
|---|
| Age (years) |
| ≤40 vs. >40 | 27/55 (49) | 24/41 (59) | 0.199 |
| FIGO stage |
| IB1 vs.
IB2-IIA | 29/47 (62) | 9/20 (45) | 0.049 |
| Tumor size
(cm) |
| ≤4 vs. >4 | 29/50 (58) | 9/16 (56) | 0.343 |
| Histology |
| Pure vs.
Mixed | 31/53 (58) | 11/19 (58) | 0.880 |
| LVSI |
| No vs. Yes | 13/20 (65) | 17/36 (47) | 0.308 |
| LNM |
| No vs. Yes | 26/41 (63) | 13/30 (43) | 0.045 |
| DSI (≥2/3 stromal
invasion) |
| No vs. Yes | 11/11 (100) | 7/17 (41) | 0.003 |
| Chemotherapy
regimen |
| Cs vs. Cns | 34/55 (62) | 2/10 (20) | 0.079 |
| Treatment
modalities |
| SU vs.
SU+Chemo | 7/11 (64) | 19/33 (58) | 0.440 |
| SU vs.
SU+Chemo+RT | 7/11 (64) | 25/52 (48) | 0.250 |
| SU+Chemo vs.
SU+Chemo+RT | 19/33 (58) | 25/52 (48) | 0.573 |
 | Table IIIThe treatment relationship between
LNM and DSI. |
Table III
The treatment relationship between
LNM and DSI.
| LNM | DSI |
|---|
|
|
|
|---|
| Treatment
modality | No | Yes | No | Yes |
|---|
| SU | 8 | 0 | 3 | 0 |
| SU+Chemo | 20 | 5 | 3 | 2 |
| SU+Chemo+RT | 13 | 25 | 5 | 15 |
| P-value | 0.000 | 0.027 |
 | Table IVRecurrence patterns of 32 patients
with SCCUC. |
Table IV
Recurrence patterns of 32 patients
with SCCUC.
| Recurrence
patterns | No. of patients
(%) |
|---|
| Local | 2 (6) |
| Local +
distant | 6 (19) |
| Distant | 24 (75) |
| Multiple sites | 12 (37.5) |
| Single site | 12 (37.5) |
| Distant sites |
| Liver | 15 |
| Bone | 12 |
| Lung | 11 |
| Para-aortic
nodes | 7 |
| Brain | 6 |
| Breast | 2 |
| Pancreas | 2 |
| Inguinal
nodes | 2 |
| Mediastinal
nodes | 2 |
| Supraclavicular
nodes | 2 |
| Kidney | 1 |
| Pleural | 1 |
| Vertebrae | 1 |
Discussion
SCCUC is a rare and aggressive subtype of cervical
carcinoma. Our results revealed that the 5-year survival rate for
patients with FIGO IB1-IIA SCCUC was 45%, consistent with a
previous report of 46.6% (2). FIGO
stage, lymph node status, and depth of stromal invasion were
significant predictors of survival. Although 85 of 96 patients in
the current study had received chemotherapy, distant metastasis was
found to be the main recurrent pattern. Thus, SCCUC remains a
therapeutic challenge for clinicians.
Clinicopathological characteristics, such as large
tumor size, LNM, advanced stage, DSI, number of positive lymph
nodes, and pure small cell histology have been suggested as
possible poor prognostic factors (1,3,8,9,31).
As for early-stage SCCUC, only four studies exist concerning the
analysis of prognostic features (2,23,31,32).
FIGO stage (IB1 vs. IB2-IIA) (2,32) and
lymph node status (23,31,32)
are significant indicators for survival. In addition, postoperative
VAC or PE is a favorable regimen for improving survival (23,31).
We observed a favorable survival for patients who received VAC or
PE, but the difference was not statistically significant (P=0.079).
This finding may be due to the small number of patients who
received other chemotherapy regimens (Table II). In our current study, the
5-year survival rate for stages IB1 and IB2-IIA patients were 58
and 34%, respectively (P=0.049); for patients with and without LNM,
the rates were 33 and 60%, respectively (P=0.045). These findings
are consistent with those of previous reports (2,31). We
also observed that DSI was a poor prognostic factor. The 5-year
survival rate for patients with outer 2/3 stromal invasion was 34%,
while it was 100% for patients with inner 1/3 stromal invasion
(P=0.003).
However, determining the optimal treatment for
early-stage SCCUC remains a challenge. While the majority of
early-stage cervical carcinomas can be successfully managed with
SU, this conventional local treatment has yet to be revealed to be
successful in SCCUC. In the study by Sevin et al (9), where surgery with or without adjuvant
radiation was used for early-stage SCCUC, the 5-year disease-free
survival was 36.4%. Sheets et al (8) reported 14 patients with early-stage
SCCUC, all treated with surgery; in addition, 7 patients with
positive nodes or other high-risk features were also given adjuvant
radiation. During follow-up, 12 patients had died of disease and
the 2 survivors had recurrent disease. There are, however, some
cases successfully treated with surgery alone. Boruta et al
(23) described 3 early-stage
patients who were treated with surgery alone and were noted to have
no evidence of recurrent disease 56, 86 and 98 months after
treatment. The 3 patients had negative surgical margins and no
evidence of metastatic disease to their lymph nodes. In our current
study, there were 11 patients treated with surgery alone. With a
median follow up of 60 months, 7 patients survived without disease.
Among them, 6 patients presented stage IB1 disease with tumor size
ranging from 0.8 to 3.0 cm; LNM was negative for all 6 patients,
and the remaining 1 stage IB patient had no detailed pathologic
information. Based on these retrospective reviews, we believe that
SU alone should be limited to patients with stage IB1 disease,
small tumors and favorable features, otherwise multimodality
treatment should be considered.
For early-stage SCCUC, most clinicians favor the use
of SU with adjuvant multimodality treatment due to its poor
prognosis. Recent studies, including the current one, have revealed
the high incidence of distant metastases even in early-stage
patients (3,31–33),
indicating the need for systemic chemotherapy. Since the natural
history of SCCUC is akin to that of SCLC, Pazdur et al
(34) first recommended in 1981
that the chemotherapy regimen used for SCCUC patients be similar to
that of SCLC. Zivanovic et al (33) reported that in patients with
early-stage disease the addition of systemic platinum and
etoposide-based chemotherapy appears to have a protective effect on
the development of distant metastases. Of the 5 early-stage
patients without chemotherapy as part of their initial treatment,
all developed distant metastases within 2 years of diagnosis. This
finding is in contrast to 6 patients who were treated with adjuvant
platinum and etoposide-based combination therapy. In that group
only 1 patient developed systemic disease (P=0.015). Two
meta-analyses reported by Chang et al (31) (40 patients) and Boruta et al
(23) (34 patients) revealed that
for early-stage patients, the postoperative adjuvant chemotherapy
using a VAC or PE regimen offered a more favorable survival than
other chemotherapeutic regimens. In the current study, adjuvant VAC
or PE chemotherapy tended to favor survival, but the difference was
not statistically significant (P=0.079). Similarly, other studies
were also unable to prove any statistically significant benefit to
using adjuvant chemotherapy (1,3).
Despite the propensity for early distant metastases, localized
control should be emphasized. Viswanathan et al (3) reported 2 patients who exhibited
recurrence following surgery and adjuvant chemotherapy; their first
site of recurrence was in the pelvis. Neither patient received
adjuvant RT. However, Sheets et al (8) proposed that these tumors may be
radioresistant as 5 of 7 patients treated with surgery and adjuvant
RT experienced pelvic failures. Sevin et al (9) reported similar results. Of the 5
patients receiving postoperative pelvic radiation who succumbed to
their disease, 4 patients exhibited pelvic recurrences and 3 had
distant metastases. However, the authors argued that radiation may
be beneficial since, in their study, 2 patients with LNM and LVSI
who received postoperative adjuvant radiation were cured, whereas
the remaining 2 patients, who did not receive postoperative
radiation, developed pelvic recurrences.
The above discussion indicates that it is impossible
to provide an optimal treatment protocol due to the different
results and the limited number of patients. To the best of our
knowledge, no studies exist that have compared SU alone with SU
plus adjuvant treatment in early-stage SCCUC. In the present study,
patients with early-stage SCCUC who were treated with SU alone, or
SU plus adjuvant chemotherapy (SU+Chemo), or SU plus adjuvant
chemotherapy and RT (SU+Chemo+RT) were examined. The number of
patients in the three groups was 11, 33 and 52, respectively. We
observed that there were no differences in survival in the three
groups, indicating that the current multimodality treatment did not
improve survival compared with surgery alone for women with
early-stage SCCUC. Given this, further studies are required to
develop novel therapies for this aggressive cancer. For instance,
SCCUC is a human papillomavirus (HPV)-associated neoplasm. Thus, it
remains to be determined whether this approach has any survival
benefit. In addition, there are ongoing trials evaluating targeted
agents such as gefitinib, sorafenib, bevacizumab, and thalidomide
in SCLC (35). These findings may
stimulate the search for, and design of, clinical trials to test
targeted therapies for the treatment of SCCUC.
The present study has certain limitations. This is a
retrospective analysis and some clinicopathologic information,
especially the depth of stromal invasion, was lacking. There is
also a paucity of information for some reports regarding
postoperative multimodality treatment including sequence, frequency
and type of chemotherapeutic agents, which prevent us from making a
more detailed analysis. Although it is one of the largest series
reported thus far, given these limitations, these findings should
be regarded as being preliminary to a large-scale study.
In conclusion, our results indicate that FIGO stage,
LNM, and DSI are prognostic factors. Additionally, SU alone should
be limited to patients with stage IB1 disease, small tumors and
favorable characteristics. Since the current multimodality
treatment is not associated with improved survival for patients
with early-stage SCCUC, newer combined therapeutic protocols and
newer effective multi-agent chemotherapy should be evaluated.
References
|
1
|
Chan JK, Loizzi V, Burger RA, Rutgers J
and Monk BJ: Prognostic factors in neuroendocrine small cell
cervical carcinoma: A multivariate analysis. Cancer. 97:568–574.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee JM, Lee KB, Nam JH, et al: Prognostic
factors in FIGO stage IB-IIA small cell neuroendocrine carcinoma of
the uterine cervix treated surgically: Results of a multi-center
retrospective Korean study. Ann Oncol. 19:321–326. 2008. View Article : Google Scholar
|
|
3
|
Viswanathan AN, Deavers MT, Jhingran A,
Ramirez PT, Levenback C and Eifel PJ: Small cell neuroendocrine
carcinoma of the cervix: outcome and patterns of recurrence.
Gynecol Oncol. 93:27–33. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsunoda S, Jobo T, Arai M, et al:
Small-cell carcinoma of the uterine cervix: A clincopathologic
study of 11 cases. Int J Gynecol Cancer. 15:295–300. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen J, MacDonald OK and Gaffney DK:
Incidence, mortality, and prognostic factors of small cell
carcinoma of the cervix. Obstet Gynecol. 111:1394–1402. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SW, Nam JH, Kim DY, Kim JH, Kim KR,
Kim YM and Kim YT: Unfavorable prognosis of small cell
neuroendocrine carcinoma of the uterine cervix: a retrospective
matched case-control study. Int J Gynecol Cancer. 20:411–416. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abeler VM, Holm R, Nesland JM and Kjorstad
KE: Small cell carcinoma of the cervix: a clinicopathologic study
of 26 patients. Cancer. 73:672–677. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sheets EE, Berman ML, Hrountas CK, Liao SY
and DiSaia PJ: Surgically treated, early-stage neuroendocrine
small-cell cervical carcinoma. Obstet Gynecol. 71:10–14. 1998.
|
|
9
|
Sevin BU, Method MW, Nadji M, Lu Y and
Averette HA: Efficacy of radical hysterectomy as treatment for
patients with small cell carcinoma of the cervix. Cancer.
77:1489–1493. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Albores-Saavedra J, Gersell D, Gilks CB,
et al: Terminology of endocrine tumors of the uterine cervix:
results of a workshop sponsored by the College of American
Pathologists and the National Cancer Institute. Arch Pathol Lab
Med. 121:34–39. 1997.
|
|
11
|
O’Hanlan KA, Goldberg GL, Jones JG,
Runowicz CD, Ehrlich L and Rodriguez-Rodriguez L: Adjuvant therapy
for neuroendocrine small cell carcinoma of the cervix: review of
the literature. Gynecol Oncol. 43:167–172. 1991.PubMed/NCBI
|
|
12
|
Morris M, Gershenson DM, Eifel P, et al:
Treatment of small cell carcinoma of the cervix with cisplatin,
doxorubicin, and etoposide. Gynecol Oncol. 47:62–65. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoskins PJ, Wong F, Swenerton KD, et al:
Small cell carcinoma of the cervix treated with concurrent
radiotherapy, cisplatin, and etoposide. Gynecol Oncol. 56:218–225.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim YB, Barbuto D, Lagasse LD and Karlan
BY: Successful treatment of neuroendocrine small cell carcinoma of
the cervix metastatic to regional lymph nodes. Gynecol Oncol.
62:411–414. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang PH, Liu YC, Lai CR, Chao HT, Yuan CC
and Yu KJ: Small cell carcinoma of the cervix: analysis of clinical
and pathological findings. Eur J Gynaecol Oncol. 19:189–192.
1998.PubMed/NCBI
|
|
16
|
Sykes AJ, Shanks JH and Davidson SE: Small
cell carcinoma of the uterine cervix: a clinicopathological review.
Int J Oncol. 14:381–386. 1999.PubMed/NCBI
|
|
17
|
Lim FK, Chong SM and Sethi V: Small cell
neuroendocrine carcinoma of the cervix with involvement of multiple
pelvic nodes: a successfully treated case by multimodal approach.
Gynecol Oncol. 72:246–249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang TC, Hsueh S, Lai CH, et al: Phase II
trial of neoadjuvant chemotherapy in early-stage small cell
cervical cancer. Anticancer Drugs. 10:641–646. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Delaloge S, Pautier P, Kerbrat P, et al:
Neuroendocrine small cell carcinoma of the uterine cervix: what
disease? what treatment? Report of ten cases and a review of the
literature. Clin Oncol. 12:357–362. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Collinet P, Lanvin D, Declerck D,
Chevalier-Place A, Leblanc E and Querleu D: Neuroendocrine tumors
of the uterine cervix clinicopathologic study of five patients. Eur
J Obstet Gynecol Reprod Biol. 91:51–57. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Straughn JM Jr, Richter HE, Conner MG,
Meleth S and Barnes MN: Predictors of outcome in small cell
carcinoma of the cervix: a case series. Gynecol Oncol. 83:216–220.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohwada M, Suzuki M, Hironaka M, Irie T and
Sato I: Neuroendocrine small cell carcinoma of the uterine cervix
showing polypoid growth and complicated by pregnancy. Gynecol
Oncol. 81:117–119. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boruta DM, Schorge JO, Duska LA, Crum CP,
Castrillon DH and Sheets EE: Multimodality therapy in early-stage
neuroendocrine carcinoma of the uterine cervix. Gynecol Oncol.
81:82–87. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim Y, Ha HJ, Kim JS, et al: Significance
of cytologic smears in the diagnosis of small cell carcinoma of the
uterine cervix. Acta Cytol. 46:637–644. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trinh XB, Bogers JJ, van Marck EA and
Tjalma WA: Treatment policy of neuroendocrine small cell cancer of
the cervix. Eur J Gynaecol Oncol. 25:40–44. 2004.PubMed/NCBI
|
|
26
|
Masumoto N, Fujii T, Ishikawa M, et al:
P16 overexpression and human papillomavirus infection in small cell
carcinoma of the uterine cervix. Human Pathol. 34:778–783. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Petru E, Pasterk C, Reich O, Obermair A,
Winter R and Breitenecker G: Small-cell carcinoma of the uterus and
the vagina: experience with ten patients. Arch Gynecol Obstet.
271:316–319. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Korcum AF, Aksu G, Bozcuk H, Pestereli E
and Simsek T: Small cell carcinoma of the cervix: a case report.
Arch Gynecol Obstet. 277:367–370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim MJ, Kim NR, Cho HY, Lee SP and Ha SY:
Differential diagnostic features of small cell carcinoma in the
uterine cervix. Diagn Cytopathol. 36:618–623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Puig F, Rodrigo C, Munoz G and Lanzon R:
Small cell neuroendocrine carcinoma of the cervix: report of two
cases. Eur J Gynaecol Oncol. 30:321–322. 2009.PubMed/NCBI
|
|
31
|
Chang TC, Lai CH, Tseng CJ, Hsueh S, Huang
KG and Chou HH: Prognostic factors in surgically treated small cell
cervical carcinoma followed by adjuvant chemotherapy. Cancer.
83:712–718. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang CY, Chen YL, Chu TC, Cheng WF, Hsieh
CY and Lin MC: Prognostic factors in women with early stage small
cell carcinoma of the uterine cervix. Oncol Res. 18:279–286. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zivanovic O, Leitao MM Jr, Park KJ, et al:
Small cell neuroendocrine carcinoma of the cervix: analysis of
outcome, recurrence pattern and the impact of platinum-based
combination chemotherapy. Gynecol Oncol. 112:590–593. 2009.
View Article : Google Scholar
|
|
34
|
Pazdur R, Bonomi P, Slayton R, et al:
Neuroendocrine carcinoma of the cervix: implications for staging
and therapy. Gynecol Oncol. 12:120–128. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rossi A, Maione P, Palazzolo G, et al: New
targeted therapies and small-cell lung cancer. Clin Lung Cancer.
9:271–279. 2008. View Article : Google Scholar : PubMed/NCBI
|