Introduction
Keratinocyte growth factor (KGF), also known as
fibroblast growth factor (FGF)-7, was initially identified in a
human embryonic lung fibroblast cell line (1,2). KGF
is produced and secreted from various types of mesenchymal cells,
including fibroblasts, smooth muscle cells and endothelial cells
(2). KGF transcription is
up-regulated in mesenchymal cells by platelet-derived growth factor
(3,4) and only acts on KGF receptor (KGFR)
expressed on epithelial cells (5,6). The
KGFR is tyrosine kinase FGF receptor-2 (FGFR-2) IIIb, a spliced
variant of FGFR2 (7). KGF modulates
proliferation, differentiation, migration and adhesion to
extracellular matrices of epithelial cells (8,9). KGF
plays significant roles in the wound healing of skin, proliferation
of gut epithelium and angiogenesis in the rat cornea (10).
In the healthy human pancreas, KGF is localized in
the islet cells, whereas KGFR is present in the islet and ductal
cells (11). Previously, we
reported that KGF was strongly expressed in the cancer cells of 34%
of pancreatic ductal adenocarcinoma (PDAC) patients (12). KGF expression in the cancer cells
was significantly correlated with shorter survival (12). KGF was also strongly expressed in
the fibroblasts adjacent to cancer cells and the chronic
pancreatitis-like lesions adjacent to cancer cells in PDAC patients
(11–13). A small number of studies using
pancreatic cancer cell lines revealed that KGF stimulated
pancreatic cancer cell growth. Recombinant human KGF (rhKGF)
induced the growth of one of three PDAC cells, termed COLO 357
(14). rhKGF also induced the
growth of HPAF-II, a metastatic pancreatic cancer cell line
(15). These findings indicated
that endogenous and exogenous KGF may contribute to PDAC cell
growth and a poor prognosis. However, the signaling pathway
involved in KGF-induced pancreatic cancer cell growth is not fully
understood.
The aim of the present study was to examine whether
the mitogen-activated protein kinase (MAPK) pathway, including the
extracellular signal-regulated kinase (ERK), p38 and JNK pathways,
contributes to exogenous KGF-induced pancreatic cancer cell growth.
We report that KGF-induced activation of the ERK signaling pathway
plays a significant role in pancreatic cancer cell growth.
Materials and methods
Materials
The following were purchased from the indicated
suppliers: rhKGF from R&D Systems, Inc. (Westerville, OH, USA);
WST-8 cell counting kit from Wako Pure Chemical Industries (Osaka,
Japan); rabbit polyclonal anti-ERK-1 antibody, rabbit polyclonal
anti-phospho-p38 antibody, rabbit polyclonal anti-p38 antibody and
rabbit polyclonal anti-JNK antibody from Santa Cruz Biotechnology
(Santa Cruz, CA, USA); rabbit monoclonal anti-phospho-p44/42 MAPK
antibody and mouse monoclonal anti-phospho-SAPK/JNK antibody from
Cell Signaling Technology Inc. (Beverly, MA, USA); HRP-conjugated
goat anti-rabbit IgG secondary antibody and HRP-conjugated goat
anti-mouse IgG secondary antibody from American Qualex (San
Clemente, CA, USA); M-PER Mammalian Protein Extraction Reagent and
Super Signal West Dura Extended Duration Substrate from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA); Immobilon-P transfer
membrane from Millipore (Tokyo, Japan) and U0126 from Merck
Biosciences (Bad Soden, Germany). Other chemicals and reagents were
purchased from Sigma Chemical Corp. (St. Louis, MO, USA).
PDAC cell line
The human PDAC cell line MIA PaCa-2 cells were
obtained from the Cell Resource Center for Biomedical Research,
Institute of Development, Aging and Cancer, Tohoku University
(Sendai, Japan). The cells were grown in RPMI-1640 containing 10%
fetal bovine serum (FBS) at 37°C under a humidified 5%
CO2 atmosphere.
Effect of rhKGF on cell growth of PDAC
cells
MIA PaCa-2 cells (1×105 cells/well) were
plated in 6-well plates and grown in 2 ml of RPMI-1640 with 10% FBS
for 24 h. The cultured medium was then changed to serum-free medium
in the presence or absence of 10 or 100 ng/ml rhKGF for 48 h. The
cells were then incubated with WST-8 cell counting reagent for 4 h
at 37°C and the optical density of the culture solution in the
plate was measured using an ELISA plate reader (Bio-Rad Laboratory)
at 450 nm.
Western blot analysis
The MIA PaCa-2 cells were grown in RPMI-1640 with
10% FBS for 24 h and cultured with serum-free medium for 24 h. To
determine the effect of KGF on the signaling pathway for cell
proliferation, 10 or 100 ng/ml rhKGF was added to the culture
medium and cells were incubated for 30 min. The cells were then
solubilized in M-PER Mammalian Protein Extraction Reagent with
Protease Inhibitor Cocktail for Mammalian Tissues. The protein
concentration of the cell extract was measured by the Bradford
method. Equal concentrations of the cell extract of each sample
were subjected to SDS-PAGE under reducing conditions, and the
separated proteins were transferred to Immobilon-P transfer
membranes. The membranes were immersed for 2 h at room temperature
(RT) in 5% skim milk in Tris-buffered saline containing 0.05%
Tween-20, and incubated with an anti-phospho-p44/42 MAPK antibody
(dilution, 1:1000), anti-phospho-p38 antibody (dilution, 1:1000),
or anti-phospho-SAPK/JNK antibody (dilution, 1:1000) at 4°C
overnight. The membranes were washed and incubated with the
HRP-conjugated anti-rabbit IgG antibody (dilution, 1:4000) or
HRP-conjugated anti-mouse IgG antibody (dilution, 1:4000) for 60
min at RT. After washing, the blots were visualized using enhanced
chemiluminescence and detected with a ChemiDoc XRS system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). To ensure similar amounts
of protein in each sample, the same membranes were reprobed with
anti-ERK-1 antibody (dilution, 1:1000), anti-p38 antibody
(dilution, 1:1000) or anti-JNK antibody (dilution, 1:1000) and
developed with HRP-conjugated secondary antibody using Super Signal
West Dura Extended Duration Substrate. Three independent
experiments were performed.
Effect of inhibition of the ERK signaling
pathway on KGF- induced cell proliferation
MIA PaCa-2 cells (1×105 cells/) were
grown in RPMI-1640 with 10% FBS for 24 h, and then cultured with
serum-free medium for 24 h. Prior to KGF administration (100
ng/ml), the cells were cultured with U0126 (10 mM) for 30 min. The
cells were then cultured for 48 h and the cell growth rate was
measured as described above.
Statistical analysis
Data were expressed as the mean ± SE. Data between
the groups were compared using the Mann- Whitney U test. P<0.05
was considered to be significant in all analyses. Statistical
analysis was performed using StatView Ver. 5.0.1 software (SAS
Institute Inc, NC, USA).
Results
Cell growth assay of rhKGF-treated MIA
PaCa-2 cells
MIA PaCa-2 cells, which express KGFR and negligible
levels of KGF (KGFR-positive, KGF-negligible), were used to examine
the effect of exogenous KGF on pancreatic cancer cell growth
(12). After the addition of KGF,
the growth rates of the MIA PaCa-2 cells were significantly
increased compared to the untreated control cells. The growth
stimulatory effects of KGF on the MIA PaCa-2 cells occurred in a
dose-dependent manner at 10 and 100 ng/ml KGF (Fig. 1, p<0.01).
Effect of rhKGF on the MAPK signaling
pathway in MIA PaCa-2 cells
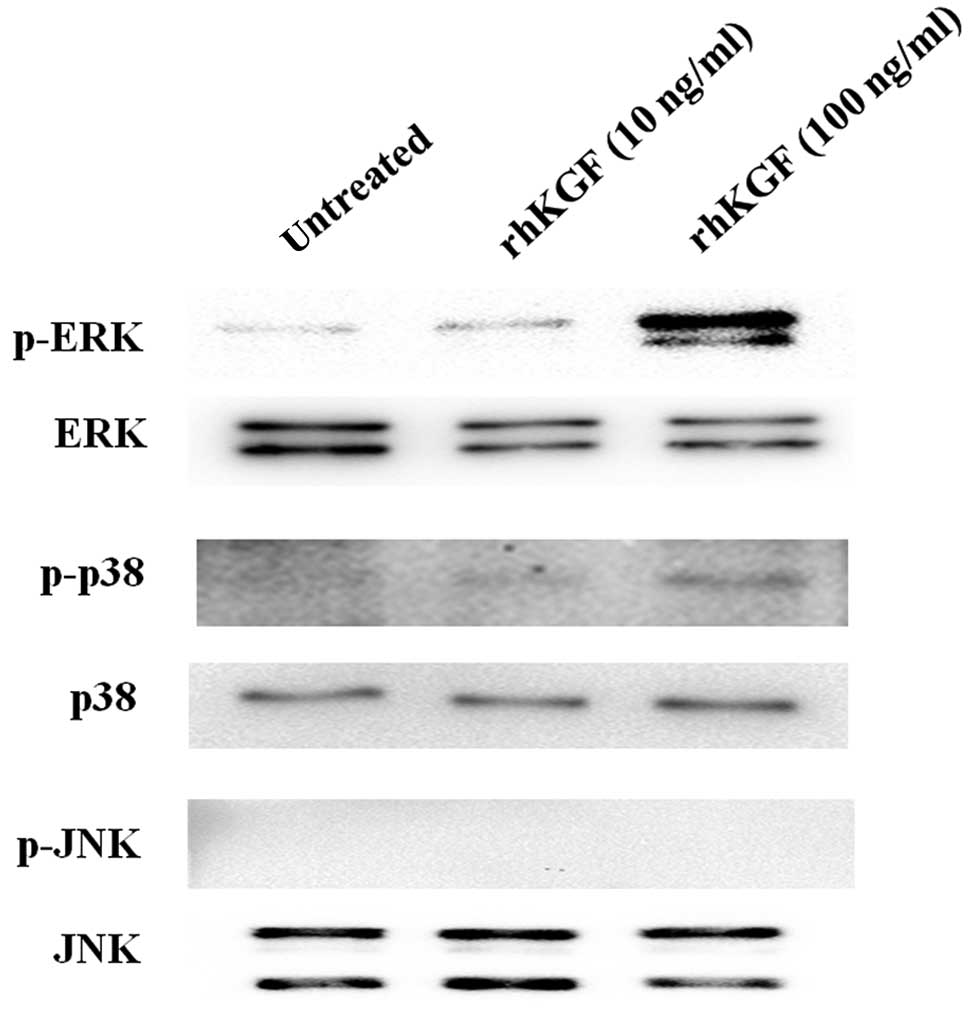
We then examined phosphorylation of the MAPK
signaling pathway, including the ERK, p38 and JNK pathways, to
determine the signaling pathway induced by exogenous KGF in MIA
PaCa-2 cells. The phosphorylated level of ERK in MIA PaCa-2 cells
was increased in a dose-dependent manner after the addition of
rhKGF (Fig. 2, top panel, p-ERK).
Conversely, p38 was moderately activated by the addition of 100
ng/ml rhKGF (Fig. 2, third panel
from top, p-p38), and JNK was not activated by the addition of
rhKGF (Fig. 2, fifth panel from
top, p-JNK).
Effect of inhibition of the ERK signaling
pathway on KGF-induced cell growth
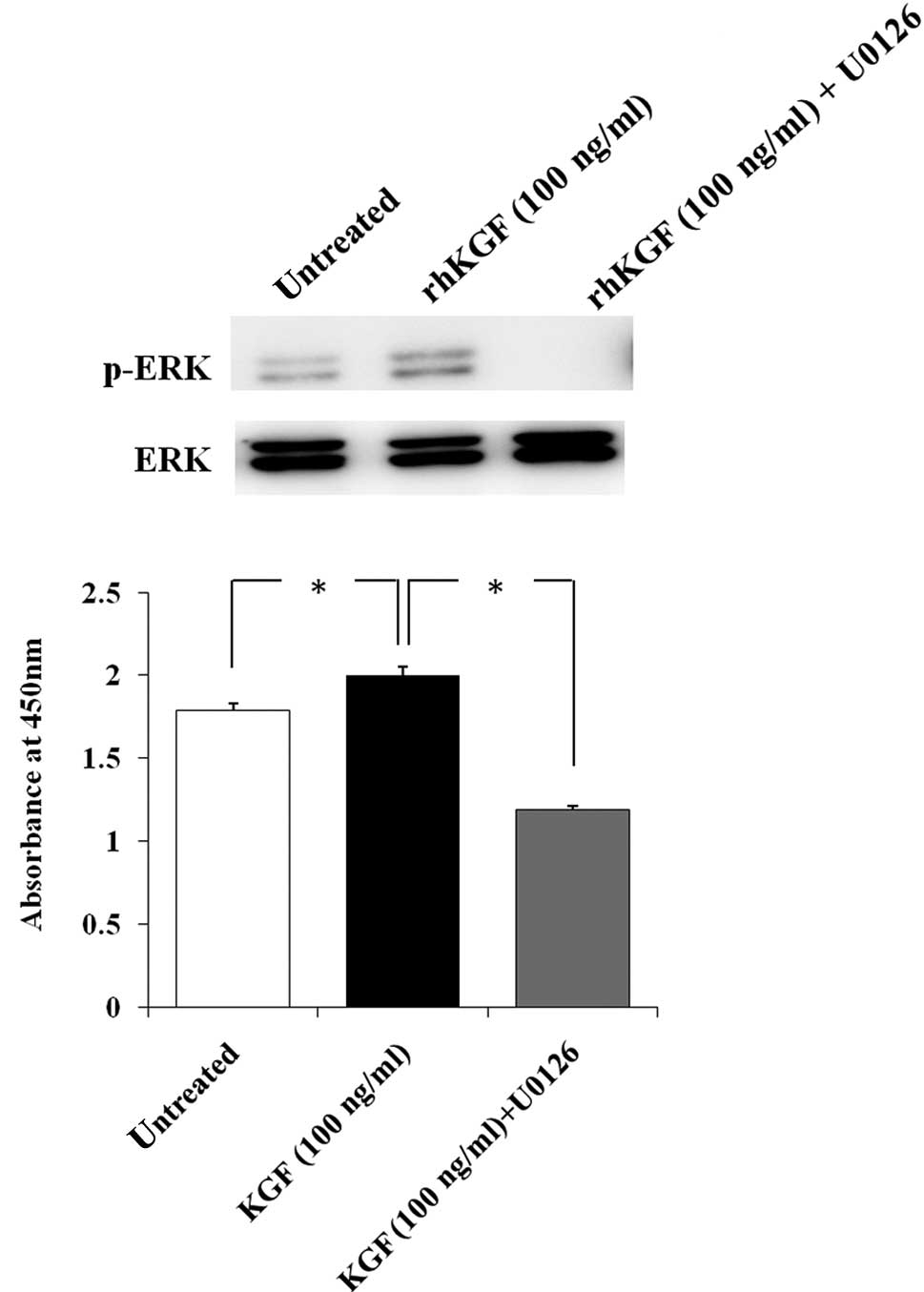
To determine whether ERK activation by rhKGF
stimulates MIA PaCa-2 cell growth, MIA PaCa-2 cells were
pre-treated with U0126 to selectively inhibit the ERK signaling
pathway. ERK activation by rhKGF was completely inhibited by
pre-treatment with U0126 (Fig. 3A).
We then performed the cell growth assay under the same conditions
and found the cell growth of rhKGF-treated MIA PaCa-2 cells to be
significantly decreased after 48 h when ERK activation was
inhibited (Fig. 3B, p<0.05).
Discussion
Exogenous KGF has been reported to induce cancer
cell proliferation in several types of cancer. Exogenous KGF
stimulated the growth of 5 of 35 human solid tumor cell lines,
including two lung cell lines, one breast, one stomach and one
colorectal cancer cell line (16,17).
Following KGF treatment, ER-positive breast cancer cells exhibited
a rapid increase in proliferation and motility and increased
metastatic potential via the activated ERK signaling pathway
(18–20). In the present study, rhKGF directly
induced the cell growth of PDAC cells in a dose-dependent manner,
which is consistent with other cancer cells.
To clarify the intracellular signaling pathway in
rhKGF involved in PDAC cell growth, we examined the activation of
three MAPK pathways. Exogenous KGF markedly activated the ERK
signaling pathway in a dose-dependent manner. In contrast, p38 was
only activated at high concentrations of rhKGF, while JNK was not
activated even at a high concentration of rhKGF. These findings
indicate that rhKGF mainly stimulates the ERK signaling pathway of
the MAPK pathway, and the ERK pathway contributes to KGF-induced
PDAC cell growth. We then examined the inhibitory effect of the ERK
signaling pathway on KGF-induced cell growth using a specific
inhibitor. Inhibition of the ERK signaling pathway clearly
decreased the KGF-induced cell growth of MIA PaCa-2 cells.
Therefore, the ERK signaling pathway is considered a significant
pathway in KGF-induced PDAC cell growth. The cell growth rate of
MIA PaCa-2 cells when the ERK signaling pathway was inhibited was
lower than that of the untreated MIA PaCa-2 cells. It has been
reported that MIA PaCa-2 cells produce several growth factors
including FGF-2 (21), and these
growth factors induced cell growth to activate the ERK signaling
pathway (22,23). Thus, treatment with the ERK
inhibitor may suppress the autocrine-loops of these growth factors,
leading to lower growth rates compared to the untreated cells.
In conclusion, the ERK signaling pathway plays a
significant role in KGF-induced cell growth in PDAC cells. These
findings indicate that KGF may directly contribute to the growth of
a certain type of PDAC cell, which express KGFR, to activate the
ERK signaling pathway, and may be involved in the progression of
pancreatic cancer.
Acknowledgements
The authors would like to thank Mr. Kiyoshi Teduka
and Ms. Taeko Suzuki (Departments of Pathology, Integrative
Oncological Pathology, Nippon Medical School) for their excellent
technical assistance. This work was supported by a Grant-in-Aid for
Young Scientists from the Japan Society for the Promotion of
Science to T.Y. (B, No. 22790675) and Y.M. (A, No. 22689038),
Grant-in Aid for Challenging Exploratory Research from the Japan
Society for the Promotion of Science to Y.M. (No. 23650604) and
Grant-in Aid for Scientific Research from the Japan Society for the
Promotion of Science to T.I. (C, No. 22591531) and Z.N. (C, No.
23590477).
References
|
1
|
Rubin JS, Osada H, Finch PW, Taylor WG,
Rudikoff S and Aaronson SA: Purification and characterization of a
newly identified growth factor specific for epithelial cells. Proc
Natl Acad Sci USA. 86:802–806. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Finch PW, Rubin JS, Miki T, Ron D and
Aaronson SA: Human KGF is FGF-related with properties of a
paracrine effector of epithelial cell growth. Science. 245:752–755.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brauchle M, Angermeyer K, Hubner G and
Werner S: Large induction of keratinocyte growth factor expression
by serum growth factors and pro-inflammatory cytokines in cultured
fibroblasts. Oncogene. 9:3199–3204. 1994.PubMed/NCBI
|
|
4
|
Katoh M: Cancer genomics and genetics of
FGFR2 (Review). Int J Oncol. 33:233–237. 2008.
|
|
5
|
Ornitz DM, Xu J, Colvin JS, et al:
Receptor specificity of the fibroblast growth factor family. J Biol
Chem. 271:15292–15297. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Elghazi L, Cras-Meneur C, Czernichow P and
Scharfmann R: Role for FGFR2IIIb-mediated signals in controlling
pancreatic endocrine progenitor cell proliferation. Proc Natl Acad
Sci USA. 99:3884–3889. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miki T, Bottaro DP, Fleming TP, et al:
Determination of ligand-binding specificity by alternative
splicing: two distinct growth factor receptors encoded by a single
gene. Proc Natl Acad Sci USA. 89:246–250. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Putnins EE, Firth JD, Lohachitranont A,
Uitto VJ and Larjava H: Keratinocyte growth factor (KGF) promotes
keratinocyte cell attachment and migration on collagen and
fibronectin. Cell Adhes Commun. 7:211–221. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ornitz DM and Itoh N: Fibroblast growth
factors. Genome Biol. 2:Reviews30052001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gillis P, Savla U, Volpert OV, et al:
Keratinocyte growth factor induces angiogenesis and protects
endothelial barrier function. J Cell Sci. 112:2049–2057.
1999.PubMed/NCBI
|
|
11
|
Ishiwata T, Friess H, Buchler MW, Lopez ME
and Korc M: Characterization of keratinocyte growth factor and
receptor expression in human pancreatic cancer. Am J Pathol.
153:213–222. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho K, Ishiwata T, Uchida E, et al:
Enhanced expression of keratinocyte growth factor and its receptor
correlates with venous invasion in pancreatic cancer. Am J Pathol.
170:1964–1974. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kornmann M, Beger HG and Korc M: Role of
fibroblast growth factors and their receptors in pancreatic cancer
and chronic pancreatitis. Pancreas. 17:169–175. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siddiqi I, Funatomi H, Kobrin MS, Friess
H, Buchler MW and Korc M: Increased expression of keratinocyte
growth factor in human pancreatic cancer. Biochem Biophys Res
Commun. 215:309–315. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zang XP, Lerner M, Brackett D and Pento
JT: Influence of KGF on the progression of pancreatic cancer.
Anticancer Res. 29:3417–3420. 2009.PubMed/NCBI
|
|
16
|
Oelmann E, Haghgu S, Kulimova E, et al:
Influence of keratinocyte growth factor on clonal growth of
epithelial tumor cells, lymphoma and leukemia cells and on
sensitivity of tumor cells towards 5-fluorouracil in vitro.
Int J Oncol. 25:1001–1012. 2004.PubMed/NCBI
|
|
17
|
Finch PW and Rubin JS: Keratinocyte growth
factor expression and activity in cancer: implications for use in
patients with solid tumors. J Natl Cancer Inst. 98:812–824. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zang XP and Pento JT: Keratinocyte growth
factor-induced motility of breast cancer cells. Clin Exp
Metastasis. 18:573–580. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zang XP, Bullen EC, Manjeshwar S, Jupe ER,
Howard EW and Pento JT: Enhanced motility of KGF-transfected breast
cancer cells. Anticancer Res. 26:961–966. 2006.PubMed/NCBI
|
|
20
|
Zang XP, Siwak DR, Nguyen TX, Tari AM and
Pento JT: KGF-induced motility of breast cancer cells is dependent
on Grb2 and Erk1,2. Clin Exp Metastasis. 21:437–443. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beauchamp RD, Lyons RM, Yang EY, Coffey RJ
Jr and Moses HL: Expression of and response to growth regulatory
peptides by two human pancreatic carcinoma cell lines. Pancreas.
5:369–380. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grose R and Dickson C: Fibroblast growth
factor signaling in tumorigenesis. Cytokine Growth Factor Rev.
16:179–186. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Korc M and Friesel RE: The role of
fibroblast growth factors in tumor growth. Curr Cancer Drug
Targets. 9:639–651. 2009. View Article : Google Scholar : PubMed/NCBI
|