Introduction
Oral verrucous carcinoma (OVC) is considered a rare,
low-grade and well-differentiated carcinoma, with less potential
for lymph node metastasis than other oral carcinomas. OVC is also
called ‘Ackerman’s tumor’ or ‘verrucous carcinoma of Ackerman’
since it was first reported and described by Ackerman in 1948
(1). Besides the oral cavity, OVC
is known to occur in the larynx, pyriform sinus, nasal cavity,
paranasal sinuses, skin and esophagus, but the oral cavity is the
most common site (2). Within the
oral cavity, the buccal mucosa and gingival are the most preferred
sites.
OVC, which accounts for 2.2–20% of all oral cancer,
is mainly found in elderly males, particularly in tobacco smokers
(3). OVC is regarded as a variant
of squamous cell carcinoma with specific clinical, pathological and
cytokinetic features, which also renders it different from squamous
cell carcinoma. Concerning the etiology of OVC, tobacco use is
believed to be markedly correlated with OVC, including inhaled and
smokeless tobacco (4). Opportunist
viral activity associated with human papillomavirus (HPV) has also
been shown to have a close correlation, which includes HPV-2, 18,
20, 27, 57 and 62 (5,6). In southern or southeastern Asia, betel
nut chewing is considered to be another significant cause of OVC
(7). Nonetheless, the
etiopathogenesis of OVC remains unclear, particularly its molecular
mechanisms.
αB-crystallin is a member of the family of small
heat shock proteins and acts as a molecular chaperone. The
essential biological function of αB-crystallin is reflected by the
conservation of its structure, from bacteria to humans (8). As with Hsp27, αB-crystallin is also
stress inducible. By binding non-naive, non-host proteins or
specific apoptosis-related proteins during cellular stress,
αB-crystallin protects cells from thermal shock, osmotic shock,
oxidative insults, ischemia or exposure to heavy metals, and
sustains cell homeostasis (9,10).
αB-crystallin may play a significant role in cancer, as its
overexpression has been reported in renal, hepatocellular and
gastric carcinoma, glioma, and lung and breast cancers (11–14).
In cancer, αB-crystallin has demonstrated anti-apoptotic properties
and is even considered to be a potential oncoprotein in a number of
types of human cancer (15).
Furthermore, it was found that αB-crystallin suppresses cell
apoptosis by inhibiting the activation of caspase-3, but this has
not been confirmed in human cancer (16).
Although overexpression of αB-crystallin has been
reported in head and neck cancers, this study, to the best of our
knowledge, is the first attempt to characterize expression patterns
of αB-crystallin in OVC and to explore the potential role of
αB-crystallin in oral cancer.
Materials and methods
Tissue specimens
A total of 17 OVCs, 15 oral squamous cell carcinomas
(OSCCs) and 15 samples of healthy oral mucosa were obtained at
Xiangya Hospital, Central South University, between 2002 and 2009.
All patients gave written consent under the protocol reviewed, and
the study was approved by the institutional review board of the
Xiangya Hospital, Central South University. The OVC group,
comprised 14 males and 3 females, with a mean age of 51.18 years
(range 23–79). The most frequent site of OVC was the gingiva,
observed in 9 patients (52.94%), followed by the buccal mucosa in 4
patients (23.52%). The OVC cases had no neck lymph node or distant
metastasis (Table I). In the OSCC
group, there were 9 males and 6 females. There were 4 patients with
lymph node metastasis and none with distant metastasis (Table I).
 | Table ILymph node metastasis and stage
category of OVC and OSCC. |
Table I
Lymph node metastasis and stage
category of OVC and OSCC.
| Lymph node
metastasis | Stage category |
|---|
|
|
|
|---|
| Positive No. (%) | Negative No. (%) | I–II No. (%) | III–IV No. (%) |
|---|
| OVC | 0 (0) | 17 (100) | 15 (88.2) | 2 (11.8) |
| OSCC | 4 (26.7) | 11 (73.3) | 9 (60.0) | 6 (40) |
OVC was only diagnosed when the characteristic
clinical and pathological features were present (2). OSCC was diagnosed by the 1997 WHO
criteria, and their clinical stage (TNM) was determined in terms of
the 2002 UICC criteria (17,18).
Normal oral mucosa (NM) specimens were obtained from healthy
volunteers. All tissue specimens were fixed in 4% buffered formalin
solution and paraffin-embedded sections were made for routine
pathological diagnosis and immunohistochemical staining.
Immunohistochemical staining for
αB-crystallin
Immunohistochemical staining was performed on 4 μm
serial sections following routine procedures of deparaffinization
and dehydration. The slides were incubated in 3%
H2O2 for 20 min to block endogenous
peroxidase activity, and were then boiled under pressure in citrate
buffer (pH 6.0, for 5 min) for antigen retrieval. The tissues were
then incubated with the αB-crystallin antibody (1:100 dilution,
sc-101437, mouse anti-αB-crystallin monoclonal antibody, Santa Cruz
Biotechnology, Santa Cruz, CA, USA) or actived-caspase-3 (p17)
antibody [1:50 dilution, BS7004, rabbit anti-caspase-3 (p17)
monoclonal antibody, Bioworld Technology, St. Louis Park, MN, USA]
at 4°C in a moist chamber overnight. Slides were then washed with
Tris-buffered saline (TBS) and incubated with Polymer Helper and
polyperoxidase-anti-mouse/rabbit IgG successively for approximately
20 min, respectively. The color was developed with DAB and
counterstained with hematoxylin (Polymer Detection System for
immunohistological staining, PV-9000, Zhongshan, Godbridge, China).
Negative controls were incubated with phosphate-buffered saline as
a replacement for the primary antibody.
Evaluation of staining
The expression level was scored based on the
percentage of positively stained cells and the intensity of
staining. A mean percentage of positive tumor cells was determined
by the examination of 200 cells in at least 10 areas, at a
magnification of ×400. Five categories were presented according to
the percentage of positive cells (PP): i) 0, <5%; ii) 1, 5–24%;
iii) 2, 25–49%; iv) 3, 50–75%; or v) 4, >75%. The intensity of
staining (SI) was scored as follows: i) 0, no -; ii) 1, weak +;
iii) 2, moderate ++; and iv) 3, intense +++. The final
immunoreactive score (IRS): IRS= SI × PP, was: i) −, 0; ii) +, 1–2;
iii) ++, 4–6; iv) +++; 8–12. The evaluation was performed by two
observers independently.
Statistical analysis
Statistical significance was evaluated by the
Kruskal-Wallis test among the three groups of OVCs, OSCCs and NM;
the Mann-Whitney U test for comparison of every two groups of OVCs,
OSCCs and NM; the χ2 analysis and Fisher’s exact test
for analyzing the correlations with clinicopathological parameters.
The Spearman’s rank correlation test was used to analyze the
expression of αB-crystallin and activated caspase-3 in tumor cells.
P<0.05 was considered to be statistically significant and data
analysis was performed using SPSS software, version 16.0.
Results
Protein expression of αB-crystalllin
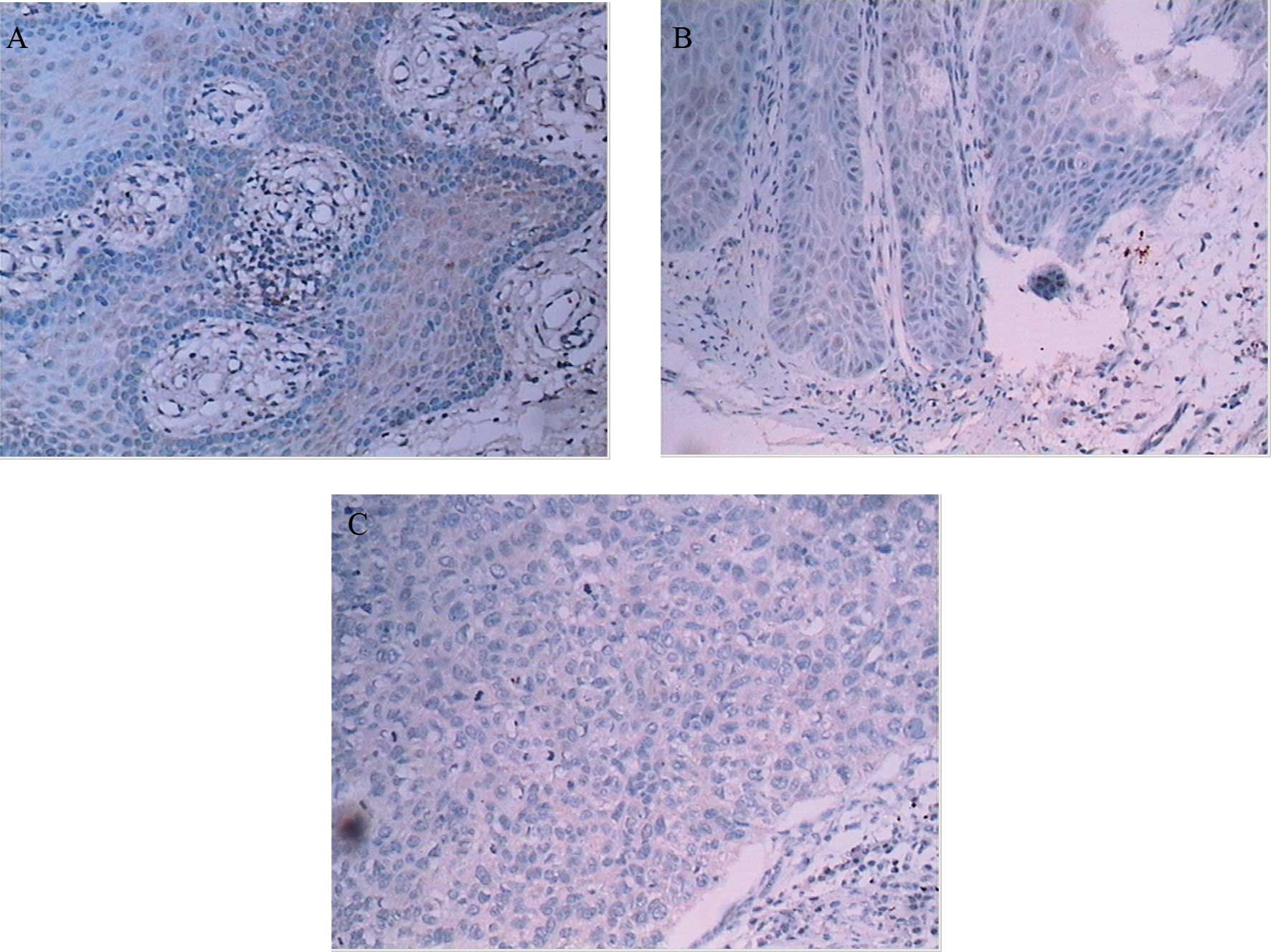
αB-crystallin was expressed predominantly in the
cytoplasm of tumor cells. In 17 OVC cases, there were 2 with
negative staining, 7 with weak staining, 6 with moderate staining
(Fig. 1B) and 2 with strong
staining. In all 15 OSCC cases, αB-crystallin was expressed and the
intensity of staining was demonstrated as moderate (10) or strong (5), respectively (Fig. 1C), whereas in NM, there were 5 cases
with negative staining (Fig. 1A)
and 10 with weak staining. Statistically significant differences
were shown between every two groups (Table II).
 | Table IIExpression of αB-crystallin in OVC,
OSCC and NM. |
Table II
Expression of αB-crystallin in OVC,
OSCC and NM.
| Group | αB-crystallin | Total | Z | P-value |
|---|
|
|---|
| Negative | Weak | Moderate | Strong |
|---|
| OVC | 2 | 7 | 6 | 2 | 17 | −2.118a | 0.044a |
| OSCC | 0 | 0 | 10 | 5 | 15 | −2.792b | 0.010b |
| NM | 5 | 10 | 0 | 0 | 15 | 4.268c | 0.000c |
| Total | 7 | 17 | 16 | 7 | 47 | | |
Using the χ2 test analysis, αB-crystallin
expression in OVC was compared with clinicopathological parameters.
No statistically significant correlation was found with age,
gender, site and clinical stage. However in OSCC, the expression of
αB-crystallin was correlated significantly with the clinical stage
of tumors [intensity of staining in stage I–II was weak (3) or moderate (6), while in stage III–IV staining was
moderate (1) and strong (5), χ2=10.557, p=0.032], but was
not significantly correlated with age, gender, site,
differentiation and nodal status.
Protein expression of activated
caspase-3
Expression of caspase-3 was detected in the
cytoplasm of specimens. Compared to NM, expression of activated
caspase-3 was significantly reduced in OVC and OSCC (Table III, Fig. 2). In the OVC and OSCC cases,
however, there was no statistically significant difference for
their expression (Table III).
 | Table IIIExpression of activated caspase-3 in
OVC, OSCC and NM. |
Table III
Expression of activated caspase-3 in
OVC, OSCC and NM.
| Group | Activated
caspase-3 | Total | Z | P-value |
|---|
|
|---|
| Negative | Weak | Moderate | Strong |
|---|
| OVC | 11 | 6 | 0 | 0 | 17 | −1.448a | 0.202 |
| OSCC | 7 | 8 | 0 | 0 | 15 | −3.934b | 0.000 |
| NM | 1 | 7 | 7 | 0 | 15 | −2.983c | 0.040 |
| Total | 19 | 21 | 7 | 0 | 47 | | |
Using the χ2 test analysis, no
significant difference was found for the expression of activated
caspase-3 and clinicopathogical variables in OVCs and OSCCs samples
(p>0.05).
Correlation of two protein
expressions
In OVCs, the overexpression of αB-crystallin was
significantly correlated to the repression of activated caspase-3
statistically (p<0.05, Table
IV). In OSCCs, while no significant correlation between the two
proteins was found, a trend towards increasing αB-crystallin
expression with decreasing activated caspase-3 expression was noted
(Table V).
 | Table IVCorrelation analysis between
αB-crystallin and activated caspase-3 in OVC. |
Table IV
Correlation analysis between
αB-crystallin and activated caspase-3 in OVC.
| αB-crystallin | r | P-value |
|---|
|
|---|
| Negative | Weak | Moderate | Strong |
|---|
| Activated
caspase-3 | | | | | −0.547 | 0.023 |
| Negative | 0 | 4 | 5 | 2 | | |
| Weak | 2 | 3 | 1 | 0 | | |
| Moderate | 0 | 0 | 0 | 0 | | |
| Strong | 0 | 0 | 0 | 0 | | |
| Total | 2 | 7 | 6 | 2 | | |
 | Table VCorrelation analysis between
αB-crystallin and activated caspase-3 in OSCC. |
Table V
Correlation analysis between
αB-crystallin and activated caspase-3 in OSCC.
| αB-crystallin | r | P-value |
|---|
|
|---|
| Negative | Weak | Moderate | Strong |
|---|
| Activated
caspase-3 | | | | | −0.363 | 0.183 |
| Negative | 0 | 0 | 4 | 2 | | |
| Weak | 0 | 2 | 3 | 3 | | |
| Moderate | 0 | 1 | 0 | 0 | | |
| Strong | 0 | 0 | 0 | 0 | | |
| Total | 0 | 3 | 7 | 5 | | |
Discussion
αB-crystallin was originally found in the human
lens, which contributed to the transparency of the lens as a
component of crystallin. Later, it was gradually found to exist in
various other tissues including the heart, brain, kidney and
muscle, but the expression in non-lens tissue was low (19). Overexpression of αB-crystallin has
been detected in a number of types of human cancer, including
hepatocellular carcinoma, glioma, lung, gastric and breast cancer.
The expression in cancer has been found to be correlated with lymph
node metastasis, low rates of survival and poor prognosis of
patients, and αB-crystallin was even considered a novel oncoprotein
in basal-like breast carcinoma (11–15).
In the current study of OVC and OSCC, we also found the
overexpression of αB-crystallin. The intensity of
immunohistochemistry staining increases from NM to OVC and OSCC
(Fig. 1). The expression of
αB-crystallin in stage III–IV was found to be significantly higher
than in stage I–II, and the stronger staining site in OVC was in
the pushing border, which may reflect the maximum proliferative
activities of this tumor. These findings indicated that
αB-crystallin may play a significant role in tumor progression.
When comparing the OVC cases with OSCC cases, we
found that the staining of αB-crystallin in OVC was significantly
lower than in OSCC. This may partially show that OVC behaves in a
gentler manner than OSCC (20). In
this study, there were no patients with lymph node metastasis in
the OVC group, whereas there were 4 patients (26.7%) in the OSCC
group. In the OVC group, only 2 patients (11.8%) were stage III–IV,
but in the OSCC group, 40% of patients were stage III–IV. The
results indicated that OVC is different from OSCC and there may be
different pathological mechanisms between the two tumors (20,21).
In tumors, the overexpression of αB-crystallin may
play a significant role in preventing tumor cell apoptosis. For
cell apoptosis, there are two main pathways: mitochondrial and
death receptor pathways. In the initial stages of the two pathways,
the cell responds to a variety of stimuli, exogenous and
endogenous, by releasing apoptosis-related factors, which then
activate/recruit procaspase-8 and -9, respectively. Then by
multiple steps, active caspase-8 and -9 initiate the proteolytic
activation of procaspase-3: first, caspase-8 or -9 cleaves
procaspase-3 at an aspartate residue between its large and small
subunits to generate a p24 intermediate (the prodomain and the
large subunit) and the p12 small subunit; second, the p24
intermediate generates the p20 and p17 forms of the large subunit
by autoproteolysis. Two p17/p12s form the active caspase-3 as
heterodimers, and then active caspase-3 induces apoptosis of the
cell by proteolyzing key cell targets (22). Caspase-3 is thus the central
molecule in the two pathways. Kamradt et al found that
αB-crystallin is capable of inhibiting the autoproteolyzing
maturation of caspase-3 by directly binding to the p24
intermediate, resulting in the negative regulation of cytochrome-C
and caspase-8-dependent apoptosis (23). These authors also observed a similar
course in myogenic differentiation. In the study by Dimberg et
al, it was found that the activated caspase-3 expression was
significantly elevated when the authors knocked down the
αB-crystallin gene (24).
In this study, we used the specific antibody of p17
generated in the activation of caspase-3 to detect the activated
caspase-3. Similarly to Dimberg et al, we also found that in
OVC the increase in expression of αB-crystallin coincides with the
decrease in expression of activated caspase-3, and we then
confirmed that the expression of αB-crystallin was significantly
reversely correlated with the expression of activated caspase-3
statistically. This result indicates that in OVC, αB-crystallin may
function as an anti-apoptosis protein by inhibiting the activation
of caspase-3, and may play a significant role in the carcinogenesis
of OVC.
Although in OSCC, the statistical significance of
the two protein expressions was not found, we observed that with
the increasing expression of αB-crystallin, the expression of
activated caspase-3 was reduced (Table
V). This indicates that more samples need to be accumulated for
further study.
In conclusion, αB-crystallin is overexpressed in OVC
and may play a role in anti-apoptosis via inhibition of the
activation of caspase-3 in OVC.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 30872895) and the Key Science and
Technology Project of the Hunan Provincial Science and Technology
Department, P.R. China (2008FJ2011).
References
|
1
|
Ackerman LV: Verrucous carcinoma of the
oral cavity. Surgery. 23:670–678. 1948.PubMed/NCBI
|
|
2
|
Spiro RH: Verrucous carcinoma, then and
now. Am J Surg. 176:393–397. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Addante RR and McKenna SJ: Verrucous
carcinoma. Oral Maxillofac Surg Clin North Am. 18:513–519. 2006.
View Article : Google Scholar
|
|
4
|
Wray A and McGuirt WF: Smokeless tobacco
usage associated with oral carcinoma. Incidence, treatment,
outcome. Arch Otolaryngol Head Neck Surg. 119:929–933. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fliss DM, Noble-Topham SE, McLachlin M,
Freeman JL, Noyek AM, van Nostrand AW and Hartwick RW: Laryngeal
verrucous carcinoma: a clinicopathologic study and detection of
human papillomavirus using polymerase chain reaction. Laryngoscope.
104:146–152. 1994.
|
|
6
|
Mitsuishi T, Ohara K, Kawashima M,
Kobayashi S and Kawana S: Prevalence of human papillomavirus DNA
sequences in verrucous carcinoma of the lip: genomic and
therapeutic approaches. Cancer Lett. 222:139–143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walvekar RR, Chaukar DA, Deshpande MS, Pai
PS, Chaturvedi P, Kakade A, Kane SV and D’Cruz AK: Verrucous
carcinoma of the oral cavity: A clinical and pathological study of
101 cases. Oral Oncol. 45:47–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horwitz J: α-crystallin. Exp Eye Res.
76:145–153. 2003.
|
|
9
|
Inagaki N, Hayashi T, Arimura T, Koga Y,
Takahashi M, Shibata H, Teraoka K, Chikamori T, Yamashina A and
Kimura A: αB-crystallin mutation in dilated cardiomyopathy. Biochem
Biophys Res Commun. 342:379–386. 2006.
|
|
10
|
Kumarapeli AR, Su H, Huang W, Tang M,
Zheng H, Horak KM, Li M and Wang X: αB-crystallin suppresses
pressure overload cardiac hypertrophy. Circ Res. 103:1473–1482.
2008.
|
|
11
|
Cherneva R, Petrov D, Georgiev O, Slavova
Y, Toncheva D, Stamenova M and Trifonova N: Expression profile of
the small heat-shock protein α-B-crystallin in operated-on
non-small-cell lung cancer patients: clinical implication. Eur J
Cardiothorac Surg. 37:44–50. 2010.
|
|
12
|
Holcakova J, Hernychova L, Bouchal P,
Brozkova K, Zaloudik J, Valik D, Nenutil R and Vojtesek B:
Identification of αB-crystallin, a biomarker of renal cell
carcinoma by SELDI-TOF MS. Int J Biol Markers. 23:48–53. 2008.
|
|
13
|
Tang Q, Liu YF, Zhu XJ, Li YH, Zhu J,
Zhang JP, Feng ZQ and Guan XH: Expression and prognostic
significance of the αB-crystallin gene in human hepatocellular
carcinoma. Hum Pathol. 40:300–305. 2009.
|
|
14
|
Chin D, Boyle GM, Williams RM, Ferguson K,
Pandeya N, Pedley J, Campbell CM, Theile DR, Parsons PG and Coman
WB: αB-crystallin, a new independent marker for poor prognosis in
head and neck cancer. Laryngoscope. 115:1239–1242. 2005.
|
|
15
|
Moyano JV, Evans JR, Chen F, Lu M, Werner
ME, Yehiely F, Diaz LK, Turbin D, Karaca G, Wiley E, Nielsen TO,
Perou CM and Cryns VL: αB-crystallin is a novel oncoprotein that
predicts poor clinical outcome in breast cancer. J Clin Invest.
16:261–270. 2006.
|
|
16
|
Shin JH, Kim SW, Lim CM, Jeong JY, Piao CS
and Lee JK: αB-crystallin suppresses oxidative stress-induced
astrocyte apoptosis by inhibiting caspase-3 activation. Neurosci
Res. 64:355–61. 2009.
|
|
17
|
Pindborg JJ, Reichart PA and Smith CJ:
Histological Typing of Cancer and Precancer of the Oral Mucosa. 2nd
edition. Springer-Verlag; Berlin: 1997, View Article : Google Scholar
|
|
18
|
Greene FL: American Cancer Society: AJCC
cancer staging manual. 6th edn. New York: American Joint Committee
on Cancer, Springer-Verlag; 2002
|
|
19
|
Masilamoni JG, Jesudason EP, Baben B,
Jebaraj CE, Dhandayuthapani S and Jayakumar R: Molecular chaperone
α-crystallin prevents detrimental effects of neuroinflammation.
Biochim Biophys Acta. 1762:284–293. 2006.
|
|
20
|
Tang Z, Xie X, Li J, Liu X, Yao Z and Zhao
S: A clinical study on oral verrucous carcinoma phenotypes. Chin J
Dent Res. 8:57–61. 2005.
|
|
21
|
Tang ZG, Zhao SP, Zhang L and Li XL: Gene
expression profile changes in oral verrucous carcinoma and oral
squamous cell carcinoma. Zhonghua Kou Qiang Yi Xue Za Zhi.
42:229–230. 2007.PubMed/NCBI
|
|
22
|
Hengartner MO: Apoptosis and the shape of
death. Dev Genet. 21:245–248. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kamradt MC, Chen F, Sam S and Cryns VL:
The small heat shock protein αB-crystallin negatively regulates
apoptosis during myogenic differentiation by inhibiting caspase-3
activation. J Biol Chem. 277:38731–38736. 2002.
|
|
24
|
Dimberg A, Rylova S, Dieterich LC, Olsson
AK, Schiller P, Wikner C, Bohman S, Botling J, Lukinius A,
Wawrousek EF and Claesson-Welsh L: αB-crystallin promotes tumor
angiogenesis by increasing vascular survival during tube
morphogenesis. Blood. 111:2015–2023. 2008.
|