Introduction
Lung cancer in the elderly is an increasingly common
problem. Elderly patients have more co-morbid diseases and tend to
tolerate toxic medical treatments more poorly than younger patients
(1). Recently, however, clinical
trials of platinum-based chemotherapy for selected elderly lung
cancer patients with favorable conditions have been conducted, and
have attracted attention to the utility of such therapy for the
elderly (2,3). Cisplatin is highly effective and has a
significant role in the treatment of lung cancer, but chronic
ototoxicity, neurotoxicity and particularly nephrotoxicity have
encouraged the development of several less toxic platinum
analogues, principally carboplatin (4). Carboplatin nephrotoxicity appears to
be less frequent and severe than cisplatin nephrotoxicity (4). In 1994, Thyss et al reported
Cockcroft and Gault-creatinine clearance (CG-CrCl) levels in 35
patients older than 80 years who received cisplatin-based
chemotherapy (5); however, there
has been no additional published information regarding CrCl levels
at which it is safe to perform platinum-based chemotherapy for lung
cancer patients, including elderly patients.
The first aim of this retrospective study was to
clarify whether measured-CrCl and CG-CrCl were capable of
appropriately detecting a decline in renal function. An additional
aim was to clarify a measured-CrCl level with which to discriminate
between lung cancer patients, including elderly ones, with or
without renal impairment.
Patients and methods
Patients
A total of 292 patients with newly diagnosed primary
lung cancer who were admitted to the Division of Respiratory
Medicine, Tsukuba Medical Center Hospital between 2007 and 2009,
were retrospectively analyzed. In this study, 59 of 292 consecutive
lung cancer patients whose creatinine clearance was measured over a
period of 24 h prior and subsequent to platinum-based chemotherapy
for lung cancer, were included. For each patient, the diagnosis of
lung cancer was confirmed with pathological and/or cytological
specimens. Pathological and/or cytological diagnosis was defined by
the WHO classification and patients were staged according to the
UICC TNM system (6). Age, height,
actual body weight and gender were recorded at the initial
visit.
CrCl
Urinary and serum creatinine, and BUN were measured
using the enzymatic method. Prior to commencement of the lung
cancer treatment, measured-CrCl was calculated 3 times in all
patients and averaged. Post-chemotherapeutic measured-CrCl was
evaluated 3 weeks after the completion of the therapy. For each
patient, the CrCl was also estimated using the Cockcroft and Gault
(CG) formula as follows: BSA = 0.007184 × Ht0.725 ×
Wt0.425; measured-CrCl = (1.73/BSA) ×
(UCr×Uvol)/(24×60×SCr); CG-CrCl = (1.73/BSA) × [(140-A)/(SCr+0.2)]
× (Wt/72) × 0.85 [in the case of a female patient (7)], where BSA indicates body surface area
(m2); Ht, height (cm); Wt, body weight (kg); UCr, urine
creatinine concentration (mg/dl) (using the enzymatic method);
Uvol, 24-h urine volume (ml/day); SCr, serum creatinine
concentration (mg/dl) (using the enzymatic method); and A, age
(years). We added 0.2 to an SCr value measured using an enzymatic
peroxidase-antiperoxidase method in order to render it equivalent
to the SCr value measured using the Jaffē method (8).
Statistical methods
Measured-CrCl exacerbation after platinum-based
chemotherapy was evaluated as [(pre-treatment measured-CrCl -
post-treatment measured-CrCl)/pre-treatment measured-CrCl],
expressed as a percentage. According to previous reports (9–11), we
defined a CrCl level of <60 ml/min as exacerbation.
Statistical significance between the 2 groups was
determined using the Mann-Whitney U test and Chi-square test.
Statistical significance between paired baseline and CrCl levels
was evaluated by the Wilcoxon signed rank test. To determine
whether age was a risk factor for decline of CrCl in platinum-based
chemotherapy, a multivariate logistic regression analysis was
performed. All statistical analyses were performed using SPSS 10.1
for Windows (SPSS, Chicago, IL, USA), and a probability value of
<0.05 was considered significant.
Results
Characteristics of patients
The clinicopathological characteristics of the lung
cancer patients are shown in Table
I. Of the 59 patients, 74.6% (n=44) were men. The median age
was 62 years (range, 41–81 years). There were 25 patients aged ≥65
years. The lung cancers comprised 35 non-small cell carcinomas and
24 small cell carcinomas. In total, 4 patients had stage IA-IIIA,
19 patients had stage IIIB, and 36 patients had stage IV
disease.
 | Table ICharacteristics of 59 patients with
lung cancer. |
Table I
Characteristics of 59 patients with
lung cancer.
| Age (years) | Median, 62; range,
41–81 |
| Gender | |
| Male | 44 (74.6%) |
| Female | 15 (25.4%) |
| Histology | |
| Adenocarcinoma | 29 (49.2%) |
| Small cell
carcinoma | 24 (40.7%) |
| Squamous cell
carcinoma | 6 (10.1%) |
| Clinical stage | |
| IA-IIIA | 4 (6.8%) |
| IIIB | 19 (32.2%) |
| IV | 36 (61.0%) |
| Chemotherapy | |
| Cisplatin-based | 31 (52.5%) |
|
Carboplatin-based | 28 (47.5%) |
| Number of courses of
chemotherapy | |
| 1–3 | 36 (61.0%) |
| 4–6 | 23 (39.0%) |
In total, 31 and 28 patients had cisplatin- and
carboplatin-based chemotherapy, respectively. Their median age was
59 and 66 years, respectively. The median number of cisplatin- and
carboplatin-based chemotherapy courses was 2 (range, 1–4) and 3
(range, 1–4) per patient, respectively.
Cisplatin-based chemotherapy
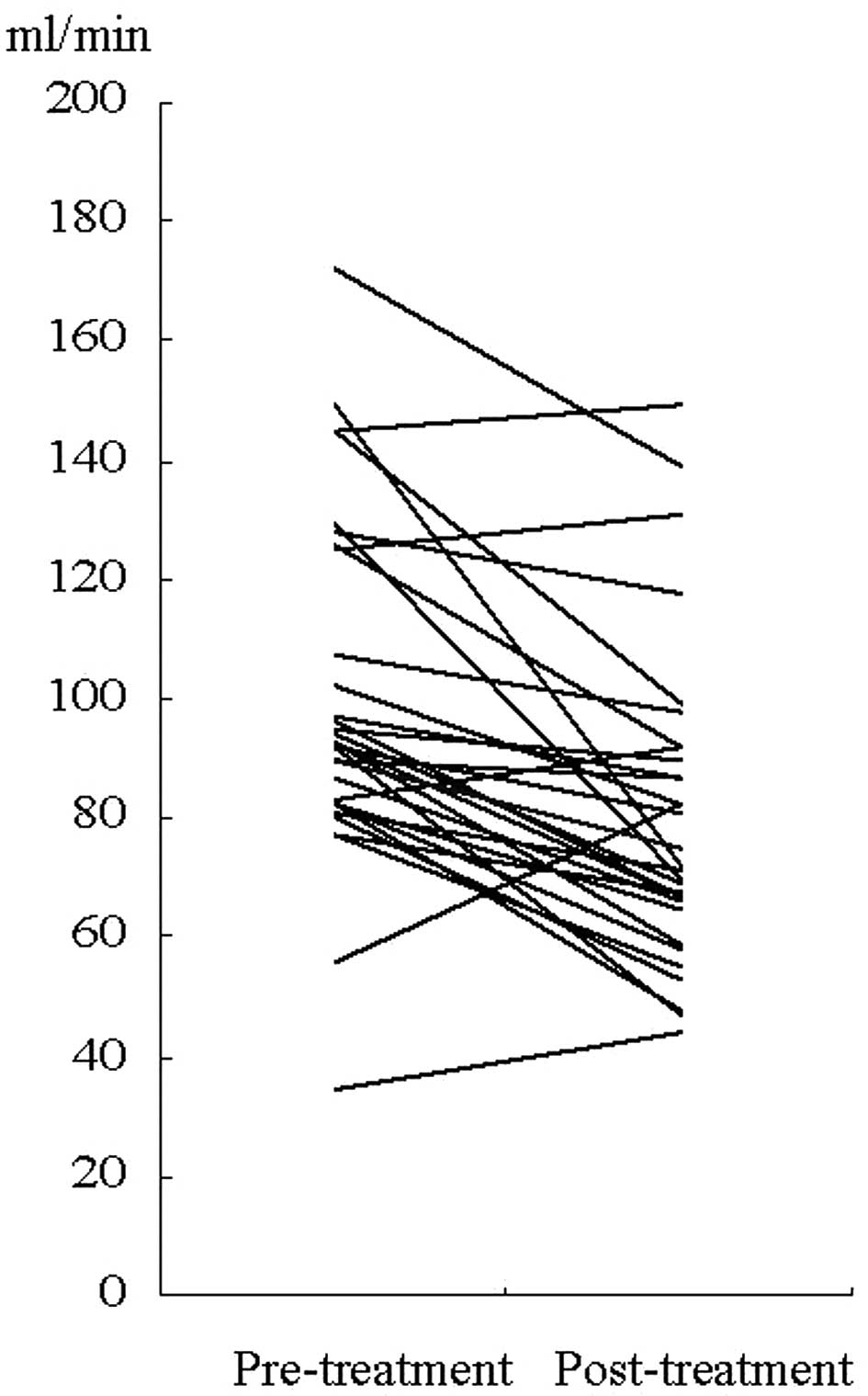
In the 31 patients treated with cisplatin-based
chemotherapy, post-chemotherapeutic measured-CrCl was lower than
pre-chemotherapeutic measured-CrCl (P=0.001, Wilcoxon signed rank
test) (Fig. 1). In CG-CrCl, a
statistically significant difference was also observed
(P=0.001).
In 9 patients aged ≥65 years, no statistical decline
was observed in measured-CrCl and CG-CrCl (P=0.005, and 0.065,
respectively). In 22 patients aged <65 years,
post-chemotherapeutic measured-CrCl was lower than
pre-chemotherapeutic measured-CrCl (P=0.001). In CG-CrCl, this
decline was also significant (P=0.001).
Carboplatin-based chemotherapy
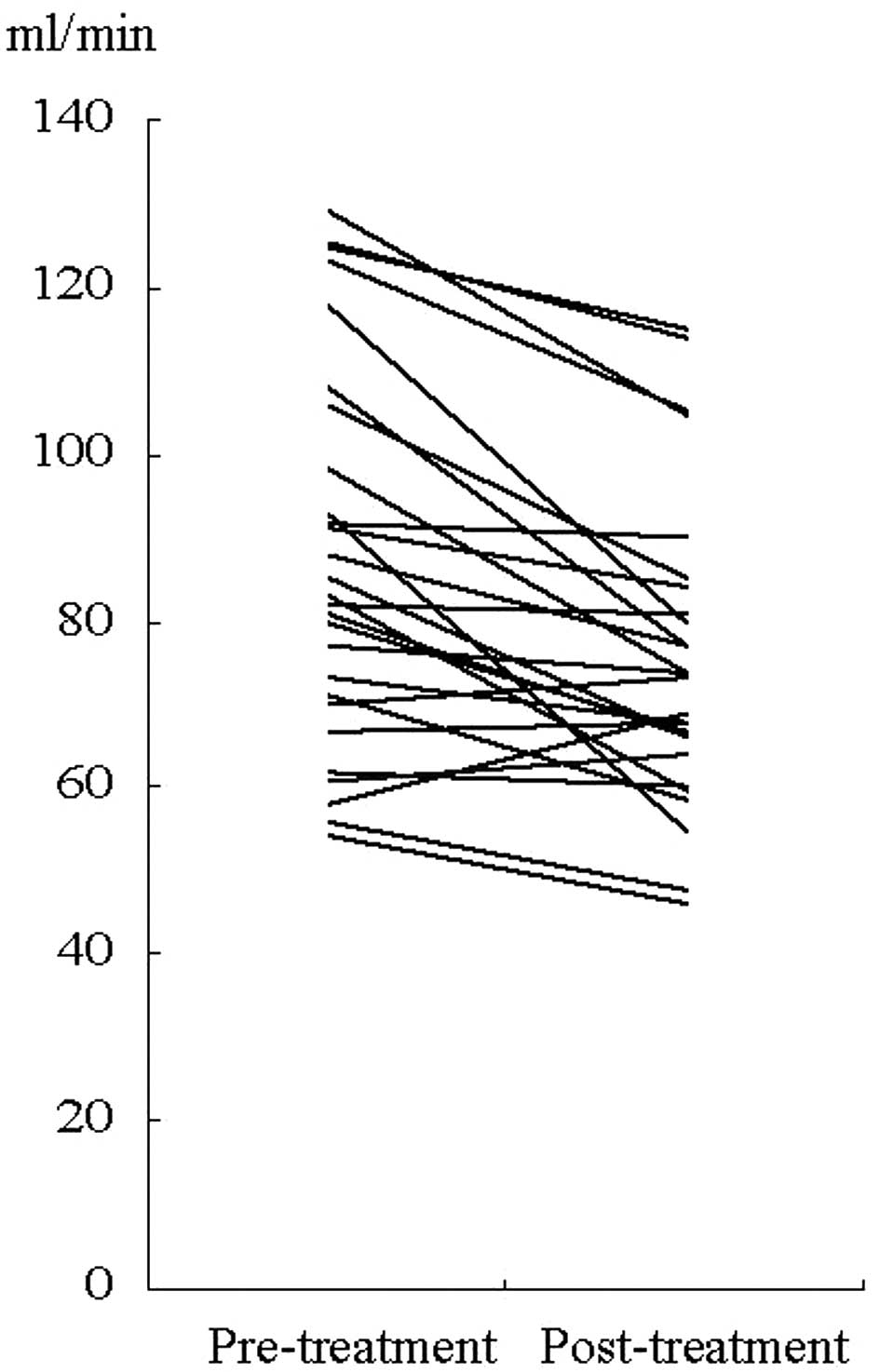
In 28 patients treated with carboplatin-based
chemotherapy, post-chemotherapeutic measured-CrCl was lower than
pre-chemotherapeutic measured- CrCl (P=0.001) (Fig. 2). In CG-CrCl, however, this decline
was not observed (P=0.065).
In 16 patients aged ≥65 years, the
post-chemotherapeutic measured-CrCl level was lower than the
pre-chemotherapeutic measured-CrCl level (P=0.009). In CG-CrCl,
however, no statistically significant difference was observed
between pre- and post-chemotherapy (P=0.278). In 12 patients aged
<65 years, the post-chemotherapeutic measured-CrCl level was
lower than the pre-chemotherapeutic measured-CrCl level (P=0.003).
In CG-CrCl, however, no statistically significant difference was
observed between pre- and post-chemotherapy (P=0.117).
Optimal pre-treatment cut-off
measured-CrCl level
To obtain the optimal pre-treatment cut-off
measured-CrCl level with which to discriminate between patients
becoming post-treatment CrCl level <60 ml/min as exacerbation,
patients were divided into groups based on whether their
post-treatment measured-CrCl levels were < or >60 ml/min and
subdivided into groups with pre-treatment measured-CrCl levels of
70, 80, 90 and 100 ml/min. As shown in Table II, a statistically significant
difference was observed when patients were divided into groups at
pre-treatment measured-CrCl levels of 90 and 100 ml/min. These
comprised 31 of 59 patients including 8 of 25 elderly patients with
≥90 ml/min levels of pre-treatment measured-CrCl. Among these, only
2 younger and 1 patient aged ≥65 years, who were treated with
cisplatin-based chemotherapy, developed a post-treatment
measured-CrCl level of <60 ml/min. However, none of the elderly
patients treated with carboplatin-based chemotherapy, developed a
CrCl level of <60 ml/min. When the cut-off level was set at 100
ml/min, no patients exhibited a post-treatment measured CrCl of
<60 ml/min, although only 17 of 59 patients (3 of 25 patients
aged ≥65 years) exhibited such favorable pre-treatment
measured-CrCl levels.
 | Table IIOptimal pre-treatment cut-off level of
measured-CrCl (ml/min) in 59 patients with lung cancer. |
Table II
Optimal pre-treatment cut-off level of
measured-CrCl (ml/min) in 59 patients with lung cancer.
| Post-treatment
measured-Cr-Cl (ml/min) | Pre-treatment
measured-Cr-Cl levels (ml/min) |
|---|
|
|---|
| <60 (ml/min) | >60 (ml/min) | P-value |
|---|
| >70 | 9 | 41 | |
| <70 | 4 | 5 | 0.0969 |
| >80 | 7 | 37 | |
| <80 | 6 | 9 | 0.0733 |
| >90 | 3 | 28 | |
| <90 | 10 | 18 | 0.0262 |
| >100 | 0 | 17 | |
| <100 | 13 | 29 | 0.0121 |
Discussion
As an early detection method and curative therapy
have yet to be established, lung cancer patients have a high
possibility of receiving chemotherapy at the time of the first
presentation or at recurrence. Since the discovery of the
anti-neoplastic effects of platinum-based compounds, cisplatin and,
later, carboplatin have developed into commonly used anticancer
agents (4). Although the proportion
of elderly patients aged ≥80 years continues to increase, intensive
chemotherapy yields a clinical benefit to elderly lung cancer
patients. Recently, Quoix reported that treatment with
platinum-based doublet chemotherapy resulted in better survival for
elderly patients with non-small cell lung cancer than standard
single-agent therapy (12).
However, there has been no additional published information
regarding the CrCl level at which chemotherapy can be safely
administered to lung cancer patients, including elderly patients.
It is well known that renal function decreases with aging and that
morphological changes, e.g., decrease of kidney weight, appearance
of sclerotic glomeruli (13) and
intimal proliferation in the renal artery are causes of renal
dysfunction (14). An assessment of
renal function is desirable when determining the dosage of drugs
with a narrow therapeutic index and those that are renally
excreted, in particular cytotoxic chemotherapeutic agents. Serum
creatinine concentration remains the most widely used index of
renal function in clinical practice (15). In the elderly, however, serum
creatinine is not always beneficial as a marker of renal function
since creatinine production is low due to decreased muscle mass
(16,17). The glomerular filtration rate (GFR)
is generally used as an index of renal function and can be
accurately measured through the renal clearance of either cold
(inulin, iohexol) or radiolabeled (51Cr-EDTA, 99mTc-DTPA) exogenous
filtration markers (18–21). Nonetheless, these methods are seldom
available in clinical practice because they are invasive and
expensive and require the use of radioelements for isotopic
clearance determination. Instead of these methods, indirect methods
are used for bedside renal function estimates, all of which are
based on CrCl.
In the present study we showed two significant
points. Firstly, our results clearly revealed a decline in renal
function even in the case of carboplatin-based chemotherapy for the
elderly and younger patients, although it is well known that renal
function should be closely observed in the case of cisplatin-based
chemotherapy. Not only for the elderly but also for the younger
patients, therefore, evaluation of renal function is essential in
order to avoid excessive dosage of cisplatin or carboplatin. In
addition, we should note that this decline of renal function may
not be detected when the CG formula is used. In selected elderly
patients, it may be possible to conduct clinical trials of
platinum-based chemotherapy. Secondly, we found that patients with
favorable renal condition whose pre-treatment measured-CrCl was
>90 ml/min were capable of undergoing cisplatin-based
chemotherapy even though they were aged ≥65 years. However, as 1
elderly patent with a pre-treatment measured-CrCl level of 92
ml/min fell to 47 ml/min, it is crucial that cisplatin-based
chemotherapy is administered with care even in the case of patients
with favorable renal condition, particularly the elderly.
Despite the current study’s novel findings, it had
several limitations. Firstly, GFR was not determined using the
isotopic reference method. Such methods are difficult to use in
elderly patients with lung cancer, as they are invasive and seldom
readily available in oncological practice. The use of the isotopic
method in the elderly patients in our study would have induced a
marked selection bias. Secondly, this was a retrospective study and
our findings were obtained from a limited number of patients
treated with cisplatin- and carboplatin-based chemotherapy. It may
be possible to conduct clinical trials of platinum-based
chemotherapy for selected elderly patients with favorable
conditions. Nevertheless, our results indicate that it is of note
to report the management in clinical practice of unselected groups
of elderly lung cancer patients.
In conclusion, in oncological practice, estimation
of GFR at the bedside is crucial, since renal insufficiency is
directly related to increased chemotherapeutic complications. It is
necessary to pay close attention to decline in renal function in
platinum-based chemotherapy, even in the case of carboplatin-based
therapy. Patients with favorable renal condition whose
pre-treatment measured-CrCl >90 ml/min may be capable of
undergoing cisplatin-based chemotherapy; however, careful
evaluation of renal function is essential, particularly in elderly
patients.
References
|
1
|
Satoh H, Kurishima K, Nakamura R, et al:
Lung cancer in patients aged 80 years and over. Lung Cancer.
65:112–118. 2009.PubMed/NCBI
|
|
2
|
Vamvakas L, Saloustros E, Karampeazis A
and Georgoulias V: Advanced non-small-cell lung cancer in the
elderly. Clin Lung Cancer. 10:158–167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lang K, Marciniak MD, Faries D, et al:
Trends and predictors of first-line chemotherapy use among elderly
patients with advanced non-small cell lung cancer in the United
States. Lung Cancer. 63:264–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanborn RE: Cisplatin versus carboplatin
in NSCLC: is there one ‘best’ answer? Curr Treat Options Oncol.
9:326–342. 2008.
|
|
5
|
Thyss A, Saudes L, Otto J, Creisson A,
Gaspard MH, Dassonville O, et al: Renal tolerance of cisplatin in
patients more than 80 years old. J Clin Oncol. 12:2121–2125.
1994.PubMed/NCBI
|
|
6
|
Lababede O, Meziane M and Rice T: Seventh
edition of the cancer staging manual and stage grouping of lung
cancer: quick reference chart and diagrams. Chest. 139:183–189.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cockcroft DW and Gault MH: Prediction of
creatinine clearance from plasma creatinine. Nephron. 16:31–41.
1976. View Article : Google Scholar
|
|
8
|
Horio M and Orita Y: Comparison of Jaffē
rate assay and enzymatic method for the measurement of creatinine
clearance. Jap J Nephrol. 38:296–299. 1996.
|
|
9
|
Sweileh WM, Sawalha AF, Zyoud SH, Al-Jabi1
SW and Shraim NY: Prevalence of reduced renal function among
diabetic hypertensive patients. Int J Physiol Pathophysiol
Pharmacol. 1:41–47. 2009.PubMed/NCBI
|
|
10
|
Wu MC, Lee WJ, Tschen SM, Lin SY, Lee IT,
Jeng CY, et al: Predictors of mortality in hospitalized diabetic
patients: A 7-year prospective study. Diabetes Res Clin Pract.
80:449–454. 2008.PubMed/NCBI
|
|
11
|
Briguori C, Colombo A, Violante A,
Balestrieri P, Manganelli F, Paolo Elia P, et al: Standard vs
double dose of N-acetylcysteine to prevent contrast agent
associated nephrotoxicity. Eur Heart J. 25:206–211. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Quoix E: Carboplatin-based Doublet
Effective for Elderly Patients with NSCLC. In: Plenary Session at
ASCO’s 2010 Annual Meeting (Abstract 2);
|
|
13
|
Kaplan C, Pasternack B, Shah H and Gallo
G: Age-related incidence of sclerotic glomeruli in human kidneys.
Am J Pathol. 80:227–234. 1975.PubMed/NCBI
|
|
14
|
Tauchi H, Tsuboi K and Sato K: Histology
and experimental pathology of senile atrophy of the kidney. Nagoya
Med J. 4:71–97. 1958.
|
|
15
|
Levey AS: Measurement of renal function in
chronic renal disease. Kidney Int. 38:167–184. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beck LH: Changes in renal function with
aging. Clinics in Geriatric Medicine. 11:199–209. 1998.
|
|
17
|
Herig PJ and Carlson RE: Plasma creatinine
and renal function in the elderly. JAMA. 248:311982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Millward MJ, Webster LK, Toner GC, et al:
Carboplatin dosing based on measurement of renal function -
experience at the Peter MacCallum Cancer Institute. Aust N Z J Med.
26:372–379. 1996. View Article : Google Scholar
|
|
19
|
Peters AM: Quantification of renal
haemodynamics with radionucleotides. Eur J Nucl Med. 18:274–286.
1991. View Article : Google Scholar
|
|
20
|
Fawdry RM, Gruenewald SM, Collins LT and
Roberts AJ: Comparative assessment of the techniques for estimation
of glomerular filtration rate with 99mTc-DTPA. Eur J Nucl Med.
11:7–12. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rehling M, Møller ML, Thamdrup B, Lund JO
and Trap-Jensen J: Simultaneous measurement of renal clearance and
plasma clearance of 99mTc-labelled diethylenetriaminepenta-acetate,
51Cr-labelled ethylenediaminetetra-acetate and inulin in man. Clin
Sci (Lond). 66:613–619. 1984.
|