Introduction
Radiotherapy is one of the most effective treatments
for metastatic spinal tumor. Pain alleviation was observed in
80–90% of patients following treatment (1–3). The
most common complication of radiotherapy in the treatment of
metastatic spinal tumors is radiation myelopathy, which is caused
by radiation damage and results in neuron apoptosis and necrosis.
The tolerance of spinal cord neuron cells to radiation is 40–50 Gy
every four or five weeks, and if an overdose occurs, this may lead
to radiation myelopathy (4). In
animal models, radiation myelopathy is reportedly closely
correlated to the form, dose and duration of radiotherapy, host
immune status and the duration of disease (5).
In brachytherapy (a form of radiotherapy), a
radiation source is permanently placed inside or next to the
treatment locus. Although 125I brachytherapy is an
improved method for killing tumor cells locally and protecting
healthy tissues, there are some negative effects, including
radiation damage to the tissue surrounding the seeds, which may
lead to complications (6,7). Appropriate animal models contribute to
experimental research for the improvement of brachytherapy on
metastastic spinal tumors. Establishment of a specific mammalian
animal model that mimics the human clinical situation is crucial.
Such a model may be a useful tool for clinicians to improve
treatment efficacy and reduce the side effects. However, only
certain rodent species have been reported to fulfill this task thus
far, and their numbers are too small to simulate the real human
physiological situation accurately (5).
A Banna mini-pig model was used in this study to
simulate spinal interstitial brachytherapy, and aimed to study the
cell-based radiation damage that was caused by 125I. The
Banna mini-pig spinal cord is similar to that of humans in terms of
anatomical structure; therefore, it is a useful model to
investigate the myelopathy pathology and the relationship between
radiation dose and duration, and tissue damage. We implanted
125I seeds to spinal dura mater at the T13 level of
mini-pigs. We then studied the dose- and time-dependent radiation
damages to the healthy cells. The cell cycle alteration, apoptosis
and necrosis ratio in spinal cord neuron cells were also
examined.
Materials and methods
Radiation source and reagents
Brachytherapy seeds iodine-125 (BT-125-1) were
purchased from Shanghai Xinke Medicine Ltd (Shanghai, China).
Apparent radioactivity was 1.00 mCi/seed and the half life of these
was 60.1 days. Prior to purchase, the 125I seeds were
randomly selected for activity testing in order to confirm the seed
container integrity and apparent activity of the seeds.
X-ray computed tomography (CT) was purchased from
Siemens, Germany; the digital subtraction angiography (DSA) was
purchased from Philips, The Netherlands; and treatment planning
systems (TPSs) were purchased from Hejie Medical Instruments
(Tianjin, China). The CRC-15R calibrator was purchased from
Capintec Inc. (Ramsey, NJ, USA).
Propidium iodide (PI), RNAase Triton X-100 and
Trypsin were purchased from Sigma (St. Louis, MO, USA). The
terminal deoxynucleotidyl transferase dUTP nick end-labeling
(TUNEL) kit was from Millipore (Temecula, CA, USA).
Animal
Twenty healthy, adult, female Banna mini-pigs were
selected for the experiment. The animals were provided and raised
by the Animal Center at Kunming Medical College (China). The
weights of the animals ranged from 20 to 25 kg (average 22.7 kg).
The mini-pigs adapted to the laboratory environment for 1 week
prior to modeling. This housing facility is a barrier housing
facility, and is in keeping with national standards (Laboratory
Animal-Requirements of Environment and Housing Facilities). The
care of laboratory animals and the animal experimental surgery
conformed to the Chinese Administration Rule of Laboratory
Animal.
Grouping
The pigs were randomly divided into four groups: A,
B, C and D, with 6 pigs in groups A, B and C, and 2 in group D. In
group A, four brachytherapy seeds were implanted into the spinal
dura mater at the T13 level. The pigs were raised for eight months
(equal to 4 half-lives of 125I). In group B, eight
brachytherapy seeds were inserted into the same location. The pigs
were monitored for two months (equal to 1 half-life of
125I). In group C, eight seeds were treated similarly
and pigs were monitored for eight months (4 half-lives of
125I). Group D acted as an age-matched normal control,
without 125I implantation.
Radiation dose calculations
This study adopted Monte Carlo-aided dosimetry to
calculate the accurate radiation dose that the mini-pig spinal
surface received during the whole of the brachytherapy process.
Briefly, we calculated the initial dose (termed D(0))
immediately following implantation of the 125I particles
into the spinal cord at the T13 level. The formula used was
D(0)=A0×1.27xΛxg(r)xF(r
θ)/r2, where A0 is the particle initial
radiation dose, which was tested by the CRC-15R calculator on the
day prior to implantation. Λ is the constant parameter for
125I, and in our study the value is 1.06; r is the
distance between the spinal surface to the 125I
particles, which was obtained from magnetic resonance imaging
(MRI)-detecting data; g(r) is the radial dose functions;
and F(r θ) is the anisotropy constant, the detailed data
of the calculations were utilized according to previously published
methods (8,9). After D0 was confirmed, the
radiation dose received by the spine was calculated using the
formula
D(T)=D(0)*T1/2*1.443*[1-e−T*0.693/T1/2].
D(T) refers to the total received dose within the time
interval T, and e is a natural constant.
Surgerical procedures
Our preliminary data revealed that the T13 level was
the best locus for surgery (data not shown). The mini-pigs were
anaesthetized with sodium pentobarbital through the ear vein, and
were then inserted in prone positions followed by skin preparation
and sterilization. Digital subtraction angiography was used to
precisely localize the surface projection of the T13 vertebra body
and the vertebral pedicle. Following the template, a syringe
needle, mounted with a 20 to 30 degree angle to the coronal plate,
was inserted into the pedicle of the vertebral arch, where it
connects vertebra, and was inserted into the T13 anterior spinal
canal without damaging the dura mater. Meglumine diatrizoate was
used to confirm the location of the needle. 125I seeds
were then implanted into the spinal canal (i.e., between the dura
mater and anterior canal). Following the surgery, DSA was used to
confirm the location of the 125I seeds at the T13
level.
Cell phase analysis
Neuron cells in the T13 level of the spinal cord
were collected and stored in Eppendorf tubes and diluted at a
density of 1.0×1.06 per 100 μl. The cell type was
identified as neuronal cells by histological staining (data not
shown). The cells were treated with 70% alcohol for DNA
precipitation and stored at −20°C (for less than a week). Cells
were re-suspended in cell cycle buffer [0.4 ml phosphate-buffered
saline (PBS), 0.5 mg RNase, and 0.5 μl Triton-X100] following
removal of the alcohol. The cells passed through nylon mesh
filtration prior to being applied to Falcon 2052 tubes. PI (10 μl
of 5 mg/ml) was incubated with the cells for 10 min. The cells were
then applied to flow cytometry (Beckman-Coulter, Brea, CA, USA) for
analysis. For each sample, data were collected from
1×104 cells and analyzed using Coulten-cycle software.
Experiments were repeated at least three times.
TUNEL assay
Digoxogenin-11-dUTP forms hetero-oligomers with dUTP
at the 3-OH terminus of double-strand or single-strand DNA in
apoptotic cells catalyzed by the TdT enzyme. FITC and PI
fluorescent dyes labeled with dUTP were used to distinguish
apoptotic, necrotic and normal cells by flow cytometry. TUNEL
assays were performed to examine the rates of apoptosis and
necrosis due to varying dose and durations of brachytherapy.
Data analysis
Standard statistical software (SPSS version 11.0;
SPSS, Inc., Chicago, IL, USA) was used for data analysis. The
Student’s t-test and χ2 test were used for variable and
attribute data respectively. P<0.05 was considered statistically
significant. The data were expressed as the means ± standard
deviation (SD).
Results
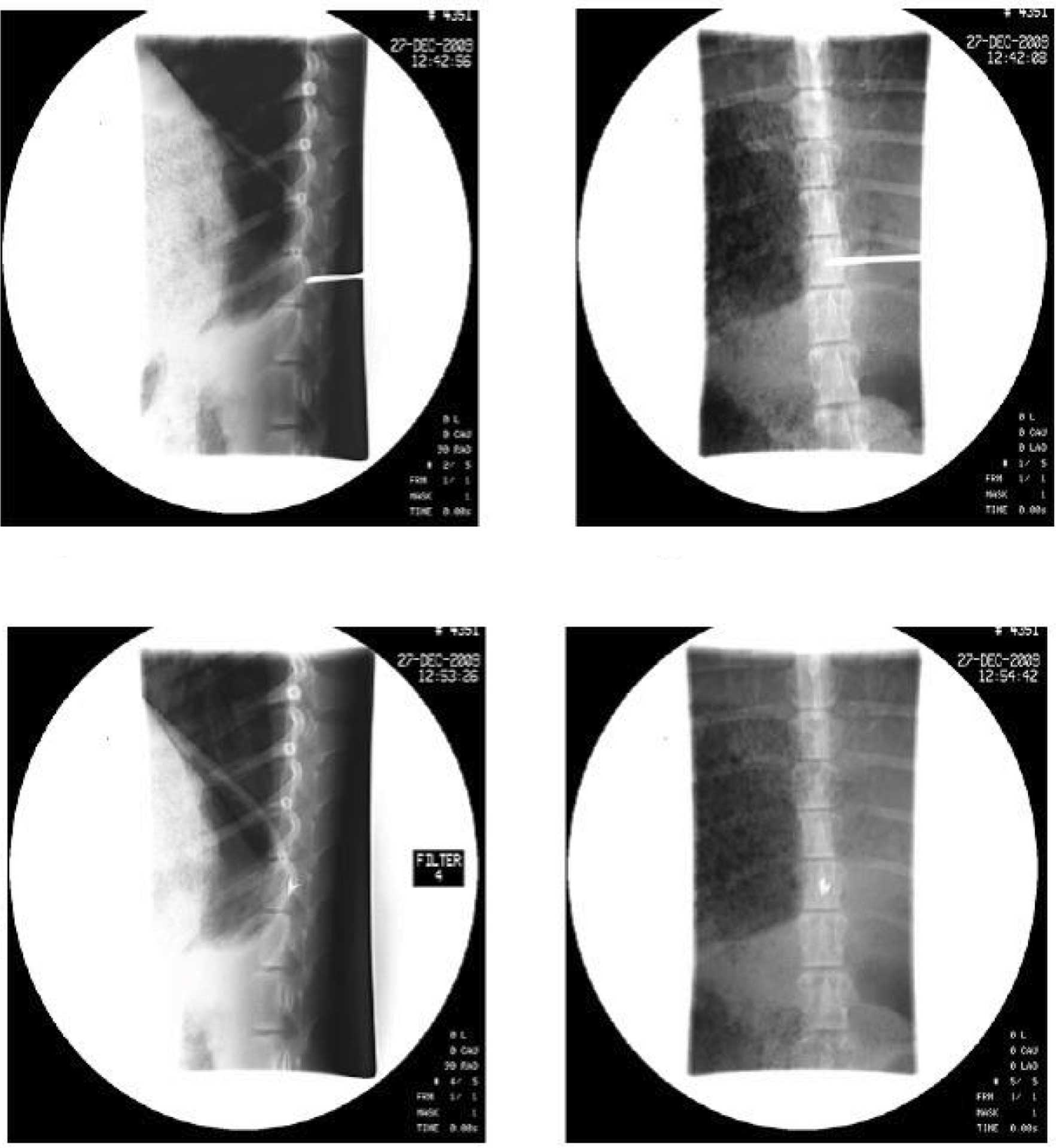
Confirmation of 125I seeds at
the spinal T13 level by DSA and CT scanning
Following surgery, the Banna mini-pigs were
consecutively treated with antibiotics for 3 days to avoid
infection. CT-scanned data proved that 125I seeds had
been precisely inserted at the T13 target level, and that the
procedure complied with TPS requirements. DSA images of one pig in
group A were randomly selected to reveal the location of the
I125 seeds (Fig. 1).
Radiation dose measurement
Using CT scanning, radiation dose distribution was
determined through the axial, sagittal and coronal planes of the
spinal cord. Based on the formula mentioned in Materials and
methods, the average radiation doses of the T13 level of the spinal
cord were obtained for each group. The average radiation dose for
group A was 10.14±0.087 Gy, group B was 14.05±0.61 Gy and group C
was 18.53±1.4 Gy.
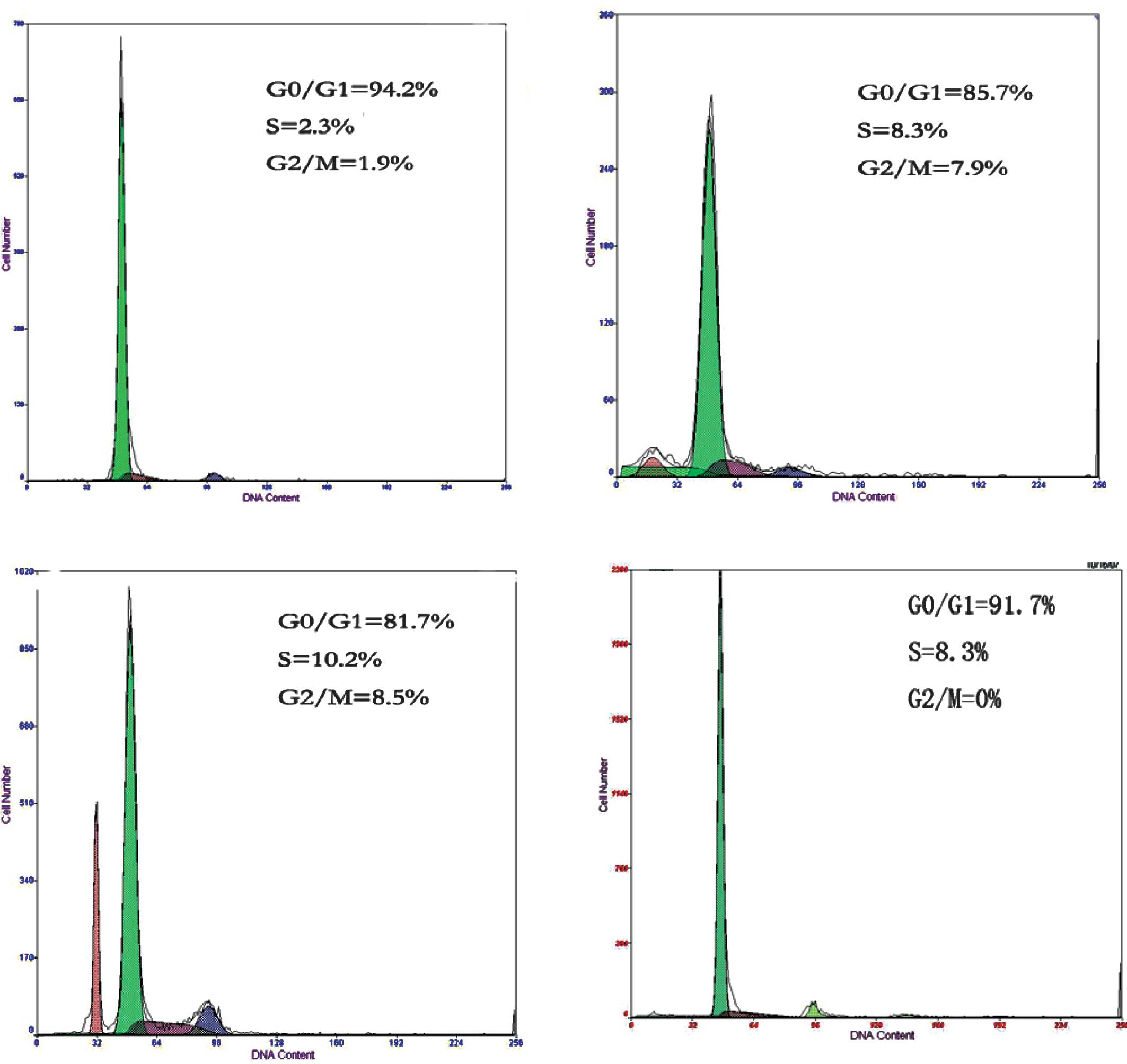
Cell cycle analysis
The focus of the present study was neuronal cells in
the gray matter of the spinal cord, which mainly included the
ventral horn and dorsal horn cells. These cells are more sensitive
to radiation and induce myelopathy. Cells in the gray matter were
carefully collected for cell cycle analysis. The effect of dose and
duration of radiation on cell cycle distribution were investigated.
As the amounts of brachytherapy seeds and the duration of radiation
increased, compared to group D, a marked changed was observed in
the cell cycle distribution of the spinal cord cells in groups A, B
and C. The average ratios of cells in the G0/G1 phase were
95.33±2.16% in group A, 84.42±2.25% in group B and 81.00±1.41% in
group C. The average ratios of spinal cord cells in the S phase
were 2.10±0.26% in group A, 8.35±0.15% in group B and 10.40±1.25%
in group C. The average ratios of spinal cord cells in the G2/M
phase were 2.03±0.19% in group A, 7.78±0.38% in group B and
8.43±0.27% in group C. The differences between any two groups were
statistically significant (P<0.05, Table I, Fig.
2). Our data suggest that 125I brachytherapy
substantially affected the cell cycle distribution of spinal cord
cells. The ratio of cells in the G0/G1 phase decreased, while that
in the G2/M phase increased significantly as the radiation dose and
time increased.
 | Table ICell cycle distribution of spinal cord
cells following treatment with various doses and durations of
radiation (%, mean ± SD). |
Table I
Cell cycle distribution of spinal cord
cells following treatment with various doses and durations of
radiation (%, mean ± SD).
| Group | n | G0/G1phase | S phase | G2/M phase |
|---|
| A | 6 | 95.33±2.16 | 2.10±0.26 | 2.03±0.19 |
| B | 6 | 84.42±2.25 | 8.35±0.15 | 7.78±0.38 |
| C | 6 | 81.00±1.41 | 10.40±1.25 | 8.43±0.27 |
| D | 3 | 99.21±0.56 | 0.45±0.34 | 0.34±0.17 |
Apoptosis and necrosis in spinal cord
cells
The apoptotic and necrotic ratio of the spinal cord
cells exhibited a dose- and duration-dependent trend. When the
number of brachytherapy seeds and radiation time was extended, the
apoptosis and necrosis rates in the spinal cord cells increased
significantly. The average apoptosis rate was 1.18±0.11% in group
A, 6.78±0.38% in group B and 17.88±1.02% in group C. The average
necrosis rate was 0.48±0.21% in group A, 0.80±0.05% in group B and
2.43±0.29% in group C. In group D, no obvious apoptosis or necrosis
was observed. Differences between any two groups were statistically
significant (P<0.05, Table II
and Fig. 3). The results indicate
that, as the dose and time of brachytherapy increased, the survival
of cells was reduced, whereas the apoptotic and necrotic cells
significantly increased. We also monitored the behavioral changes
of the mini-pigs as radiation accumulated. No obvious abnormality
was noted in the mini-pigs of group A. One pig in group B had hair
loss on the left hind leg and clumsy tail movement. Two pigs in
group C exhibited slow movement in the hind legs, and one pig
exhibited incontinence.
 | Table IIThe ratio of apoptosis and necrosis
following varying doses of radiation (%, mean ± SD). |
Table II
The ratio of apoptosis and necrosis
following varying doses of radiation (%, mean ± SD).
| Group | n | Apoptosis | Necrosis |
|---|
| A | 6 | 1.18±0.11 | 0.48±0.21 |
| B | 6 | 6.78±0.38 | 0.80±0.05 |
| C | 6 | 17.38±1.02 | 2.43±0.29 |
| D | 3 | 0.12±0.11 | 0.05±0.04 |
Discussion
125I brachytherapy was introduced into
radiation therapy in the 1970s, earlier documents regarding this
method can be traced back to 1979 when clinical practitioners
treated prostate cancer patients with 125I implantation
(10). This method has been widely
applied in prostate, brain (11)
and lung cancer treatment (12),
and has been proven to be effective for the inhibition of cancer
progression.
In the present study, we successfully established a
Banna mini-pigs model to investigate the side effects of
brachytherapy. The Banna mini-pig has a similar spinal structure to
the human. Thus, our study may provide a valuable tool for use in
brachytherapy for the treatment of metastastic spinal cancer in an
animal model.
In conventionally fractionated radiotherapy (1.8–2.0
Gy per fraction), the tolerance of the spinal cord is only two
thirds compared to that of regular tissues with regard to
irreversible damages (13). Chronic
progressive radiation myelopathy developed in patients after 0.5 to
2 years of treatment (13–17). Van den Aardweg et al
previously evaluated the effects of local irradiation on various
lengths of the spinal cord in mature pigs (37–43 weeks) (18). In that study, the effective dose 50
(ED50) values for chronic progressive radiation myelopathy were
found to be 27.02±0.36, 27.68±0.57 and 28.28±0.78 Gy on a field
length of 10, 5 and 2.5 cm, respectively, with a single high dose
of radiation (25–32 Gy). In another study on the canine brain
(19), the geographically
circumscribed radiation from 125I seeds was accompanied
by increased permeability in blood-brain barrier (BBB), which may
persist for more than 1 year following insertion of the
125I seed. This altered BBB function was probably
responsible for the cerebral edema associated with 125I
brachytherapy (19). It was
reported that the high dose radiation more efficiently treated
brain tumors; at the same time, however, more damage was induced to
the normal nerve tissues, resulting in a debilitating cognitive
decline (20). We examined the
changes of cell cycle distribution in spinal cord cells following
radiation seed implantation with flow cytometry. Our results
revealed that the ratio of spinal cord cells in the G2 and S phases
increased as the radiation accumulated in mini-pigs. These data
suggest that the cell cycle was blocked in the G2 and S phases
after radiation. The cells in the G2 and S phases are more
sensitive to radiation (21), and
therefore more tumor cells were eliminated, while the apoptosis of
normal cells also increased, which may lead to radiation
myelopathy.
We analyzed the ratios of apoptosis and necrosis in
spinal cord cells with TUNEL assay by flow cytometry. Our results
demonstrated that the ratios of apoptosis and necrosis in spinal
cord cells increased significantly as the dose and duration of
radiation increased. Additionally, the mini-pigs exhibited
behavioral signs of radiation damages. As the dose enhanced
gradually, mini-pigs in group A exhibited signs of pain and
sickness. Hair loss in the left hind leg and clumsy tail movement
were observed in one pig from group B. Two pigs had paralysis of
the hind legs in group C. After one half-life of 125I
(i.e., 2 months), all the animals were normal. After four
half-lives, three pigs had slow movements of the hind legs. Our
data indicate that higher doses caused greater damage to spinal
cord cells and increased the chance of inducing radiation
myelopathy, which develops chronically and irreversibly.
In conclusion, radiation myelopathy is closely
correlated to the dose and duration of brachytherapy. A low dose
and short-term radiation effectively reduces the apoptosis and
necrosis of spinal cord cells, thus eliminating the occurrence of
radiation myelopathy. Our results demonstrate that brachytherapy
may cause damage to normal tissues, and that the dose and duration
of brachytherapy requires careful calculation to treat metastatic
spinal tumors.
Acknowledgements
This study was supported by the Joint Specialized
Research Fund from Yunnan Provincial Science and Technology
Department and Kunming Medical College (grant no. grant no.
2011FB201) and Kunming Major Program of Science and Technology
Development (no. 11S030003).
References
|
1
|
Jhaveri P, Teh BS, Bloch C, et al:
Stereotactic body radiotherapy in the management of painful bone
metastases. Oncology (Williston Park). 22:782–788; discussion 8–9.
2008.PubMed/NCBI
|
|
2
|
Moser L, Schubert T and Hinkelbein W:
Hormone-refractory and metastatic prostate cancer - palliative
radiotherapy. Front Radiat Ther Oncol. 41:117–125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rock JP, Ryu S and Yin FF: Novalis
radiosurgery for metastatic spine tumors. Neurosurg Clin N Am.
15:503–509. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baumann M, Budach V and Appold S:
Radiation tolerance of the human spinal cord. Strahlenther Onkol.
170:131–139. 1994.PubMed/NCBI
|
|
5
|
Chiang CS, Mason KA, Withers HR, et al:
Alteration in myelin-associated proteins following spinal cord
irradiation in guinea pigs. Int J Radiat Oncol Biol Phys.
24:929–937. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Merrick GS, Wallner KE and Butler WM:
Permanent interstitial brachytherapy for the management of
carcinoma of the prostate gland. J Urol. 169:1643–1652. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roeloffzen EM, Monninkhof EM, Battermann
JJ, et al: Acute urinary retention after I-125 prostate
brachytherapy in relation to dose in different regions of the
prostate. Int J Radiat Oncol Biol Phys. 80:76–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rivard MJ, Coursey BM, DeWerd LA, et al:
Update of AAPM Task Group No. 43 Report: a revised AAPM protocol
for brachytherapy dose calculations. Med Phys. 31:633–674. 2004.
View Article : Google Scholar
|
|
9
|
Jianping SLLJC: Monte Carlo calculations
of the dosimetry parameters for the 125I brachytherapy source.
Tsinghua Sci Technol. 49:1593–1596. 2006.
|
|
10
|
Charyulu K, Block N and Sudarsanam A:
Preoperative extended field radiation with I-125 seed implant in
prostatic cancer: a preliminary report of a randomized study. Int J
Radiat Oncol Biol Phys. 5:1957–1961. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bogart JA, Ungureanu C, Shihadeh E, et al:
Resection and permanent I-125 brachytherapy without whole brain
irradiation for solitary brain metastasis from non-small cell lung
carcinoma. J Neurooncol. 44:53–57. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee W, Daly BD, DiPetrillo TA, et al:
Limited resection for non-small cell lung cancer: observed local
control with implantation of I-125 brachytherapy seeds. Ann Thorac
Surg. 75:237–242; discussion 42–43. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lo SS, Sahgal A, Wang JZ, et al:
Stereotactic body radiation therapy for spinal metastases. Discov
Med. 9:289–296. 2010.PubMed/NCBI
|
|
14
|
Kavanagh BD, McGarry RC and Timmerman RD:
Extracranial radiosurgery (stereotactic body radiation therapy) for
oligometastases. Semin Radiat Oncol. 16:77–84. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khrizman P, Small JW, Dawson L, et al: The
use of stereotactic body radiation therapy in gastrointestinal
malignancies in locally advanced and metastatic settings. Clin
Colorectal Cancer. 9:136–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carey Sampson M, Katz A and Constine LS:
Stereotactic body radiation therapy for extracranial
oligometastases: does the sword have a double edge? Semin Radiat
Oncol. 16:67–76. 2006.PubMed/NCBI
|
|
17
|
Maranzano E, Trippa F, Pacchiarini D, et
al: Re-irradiation of brain metastases and metastatic spinal cord
compression: clinical practice suggestions. Tumori. 91:325–330.
2005.PubMed/NCBI
|
|
18
|
van den Aardweg GJ, Hopewell JW and
Whitehouse EM: The radiation response of the cervical spinal cord
of the pig: effects of changing the irradiated volume. Int J Radiat
Oncol Biol Phys. 31:51–55. 1995.PubMed/NCBI
|
|
19
|
Groothuis DR, Wright DC and Ostertag CB:
The effect of 125I interstitial radiotherapy on
blood-brain barrier function in normal canine brain. J Neurosurg.
67:895–902. 1987.
|
|
20
|
Monje ML and Palmer T: Radiation injury
and neurogenesis. Curr Opin Neurol. 16:129–134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Knox SJ, Sutherland W and Goris ML:
Correlation of tumor sensitivity to low-dose-rate irradiation with
G2/M-phase block and other radiobiological parameters. Radiat Res.
135:24–31. 1993. View
Article : Google Scholar : PubMed/NCBI
|