Introduction
Prostate cancer (PC) is the most common malignancy
in males and the second leading cause of cancer-related mortality
in the United States and Europe (1). The incidence of PC has significantly
increased in most developed countries, probably due to the
prevalence of the Western lifestyle in these countries and the age
distributions of their populations (1,2).
Surgical and radiation therapies are effective for treating
localized disease, but almost 30% of treated PC patients still
suffer disease relapses (3–5). Clinically high-grade PC cases with
Gleason scores of 8–10 exhibit rapid growth and are more likely to
spread beyond the prostate. Most patients with advanced disease
respond poorly to androgen deprivation therapy and often acquire a
castration-resistant phenotype. Castration-resistant PC progresses
aggressively, resulting in mortality. Thus, the development of new
therapies based on the molecular mechanisms of PC progression is
crucial.
In a previous study, we analyzed the gene expression
profiles of high-grade PC using a cDNA microarray combined with
laser microbeam microdissection to enrich populations of cancer
cells (6). In this study, among the
genes that are transactivated in high-grade PC cells, we focused on
small nuclear ribonucleoprotein polypeptide E (SNRPE) as a
novel molecular target of treatment for PC, to validate
SNRPE overexpression in clinically high-grade PC with a
Gleason score of 8–10.
Materials and methods
Cell lines
The human PC cell line 22Rv1 was obtained from the
American Type Culture Collection (ATCC; Rockville, MD, USA). Cell
lines were cultured as monolayers in Dulbecco’s modified Eagle’s
medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA) for 22Rv1 with 10%
fetal bovine serum and 1% antibiotic/antimycotic solution
(Sigma-Aldrich). The cells were maintained in incubators containing
humidified air with 5% CO2 at 37°C.
Semi-quantitative RT-PCR
Tissue samples were obtained after receiving
informed consent from high-grade PC patients undergoing prostatic
needle biopsy before androgen ablation therapy. Purification of PC
cells and normal prostatic epithelial cells from frozen PC tissues
was described previously (6). Total
RNA was extracted using the RNeasy Kit (Qiagen, Hilden, Germany)
according to the manufacturer’s instructions, treated with DNase I
(Roche, Basel, Switzerland), and reverse transcribed to
single-stranded cDNA using oligo(dT)12–18 primer and
Superscript reverse transcriptase II (Invitrogen, Carlsbad, CA,
USA). We prepared the appropriate dilutions of each single-stranded
cDNA and normalized the cDNA content to that of β-actin
(ACTB). PCR reactions were then performed using the
single-stranded cDNA as the PCR template. The primer sequences used
were: ACTB (forward: 5′-TTGGCTTG ACTCAGGATTTA-3′, reverse:
5′-ATGCTATCACCTCCCCT GTG-3′); SNRPE (forward:
5′-ACCATGGCGTACCGT GGC-3′, reverse: 5′-CTAGTTGGAGACACTTTGTAGC
AGA-3′); AR (forward: 5′-CGGAAGCTGAAGAAACT TGG-3′, reverse:
5′-CCTGGAGTTGACATTGGTGA-3′); KLK3 (PSA) (forward:
5′-CCAGACACTCACAGCAAGGA-3′, reverse: 5′-ATCCCATGCCAAAGGAAGAC-3′);
NKX3.1 (forward: 5′-TGGTTTGTGAATCCATCTTGC-3′, reverse:
5′-AACAGGCTGTCTGGGTGAAA-3′), and TMPRSS2 (forward:
5′-CGAGGAGAAAGGGAAGACCT-3′, reverse: 5′-CCAGTCCGTGAATACCATCA-3′).
The PCR conditions were as follows: initial denaturation at 95°C
for 5 min, followed by numerous cycles of denaturation at 95°C for
30 sec, annealing at 55°C for 30 sec, and elongation at 72°C for 30
sec on a TGradient Thermocycler (Biometra, Goettingen, Germany) (25
cycles for ACTB, 28 cycles for SNRPE, 28 cycles for
AR, 28 cycles for PSA, 28 cycles for NKX3.1
and 28 cycles for TMPRSS2).
Construction of short hairpin RNA
expression vectors and the cell viability assay
Plasmids designed to express short hairpin RNA
(shRNA) were prepared by cloning double-stranded oligonucleotides
into the pBAsi-h6 Neo vector (Takara Bio Inc., Shiga, Japan). The
target sequences for SNRPE were as follows: sense strand
sequence for siSNRPE: 5′-GCTCTATGAGCAAGTGAAT-3′, and negative
control EGFP siRNA (siEGFP): 5′-GAAGCAGCACGACTTCTTC-3′, as
previously described (6). 22Rv1
cells (2×106) that highly expressed SNRPE were seeded in
10-cm dishes, transfected with pBA-siSNRPE or pBA-siEGFP using
FuGENE6 (Roche Diagnostics) according to the manufacturer’s
instructions, and then cultured in the appropriate medium
containing 800 μg/ml Geneticin (Sigma-Aldrich) for 14 days. The
cells were fixed with 100% methanol and stained with 0.1% crystal
violet-H2O for the colony formation assay. In the cell
viability assay, cell viability was measured using the Cell
Counting Kit-8 (Dojindo, Kumamoto, Japan) 10 days after
transfection. Absorbance was measured at 450 nm as a reference with
an iMark Microplate Absorbance Reader (Bio-Rad, Hercules, CA, USA).
Preliminarily, the knockdown effects of these shRNA expression
vectors on endogenous SNRPE expression were validated 7 days
following their transfection by RT-PCR using the primers employed
for semi-quantitative RT-PCR.
Generation of SNRPE-overexpressing cells
and the cell proliferation assay
Full-length human SNRPE cDNA (accession no.
NM003094) was amplified using primers containing Myc-tag sequences
in their NH2 termini and cloned into the pIRESneo3
vector (Clontech). Human 22Rv1 cells were seeded into a 10-cm dish
(50% confluent) and transfected with 6 μg pIRESneo3 empty vector or
pIRESneo3-Myc-SNRPE expression vector using FuGENE6 reagent
(Roche), according to the manufacturer’s instructions. The cells
were selected with appropriate medium containing 400 μg/ml
Geneticin (Sigma-Aldrich) for 14 days, and then discrete colonies
were collected. Stable clones were maintained in selective medium
and assayed for exogenous SNRPE expression by RT-PCR. The
proliferation of 22Rv1 cells that stably expressed SNRPE
(22Rv1 SNRPE clone mixture) and those transfected with
pIRESneo3 empty vector (22Rv1 mock clone mixture) was examined
using the Cell Counting Kit-8 (Dojindo). The 22Rv1 SNRPE and
22Rv1 mock cells were seeded at a concentration of 5×103
cells per well in 48-well plates. The assay was performed every 48
h for 7 days, according to the manufacturer’s instructions.
cDNA microarray analysis and data
acquisition
The 22Rv1 cells were transfected with pBA-siSNRPE or
pBA-siEGFP, as described above. Total RNA was extracted using the
RNeasy Mini Kit 7 days after the transfection, according to the
manufacturer’s instructions. The GeneChip® array data
were compared using the Kurabo custom analysis service (Kurabo
Industries Ltd., Osaka, Japan; Kurabo Industries Ltd. is an
authorized service provider for Affymetrix Japan K.K., Tokyo,
Japan). Briefly, total RNA was reverse transcribed to cDNA with
T7-Oligo(dT) primer (Affymetrix, Inc.). The cDNA synthesis product
was used in an in vitro transcription (IVT) reaction
involving T7 RNA polymerase. An unlabeled ribonucleotide mix was
used in the first cycle of IVT amplification. Unlabeled cRNA was
then reverse transcribed in the first-strand cDNA synthesis step of
the second cycle using random primers. Subsequently, the
T7-Oligo(dT) promoter primer was used for the second-strand cDNA
synthesis to generate a double-stranded cDNA template containing T7
promoter sequences. The resultant double-stranded cDNA was then
amplified and labeled using a biotinylated nucleotide
analog/ribonucleotide mix in the second IVT reaction. The labeled
cRNA products were then fragmented, loaded onto the GeneChip Human
Genome U133 Plus 2.0 array (Affymetrix, Inc., CA, USA), and
hybridized according to the manufacturer’s instructions.
Streptavidin-phycoerythrin (Molecular Probes) was used as a
fluorescent conjugate to detect the hybridized target sequences.
Raw intensity data from the GeneChip array were analyzed using
GeneChip Operating Software (Affymetrix, Inc.).
Results
SNRPE overexpression in high-grade PC
cells
In a previous study, we analyzed the expression
profiles of high-grade PC cells purified from clinically high-grade
PC tissues with high PSA levels and high Gleason scores (6). Among the genes that were
transactivated in the high-grade PC cells compared with normal
prostatic epithelial cells, we focused on SNRPE in this
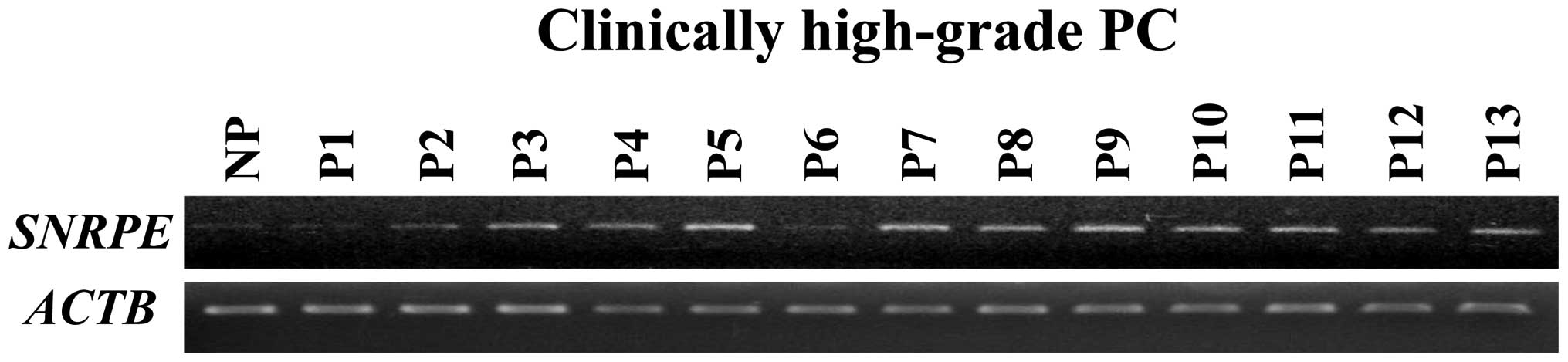
study. Semi-quantitative RT-PCR (Fig.
1) confirmed that SNRPE expression was elevated in 11
out of 13 clinically high-grade PC cells.
Knockdown of SNRPE expression by shRNA
decreased the PC cell number
To examine the biological roles of SNRPE
overexpression in PC cells, we constructed vectors designed to
express shRNA specific to SNRPE and EGFP, and
transfected them into 22Rv1 cells, which express endogenous
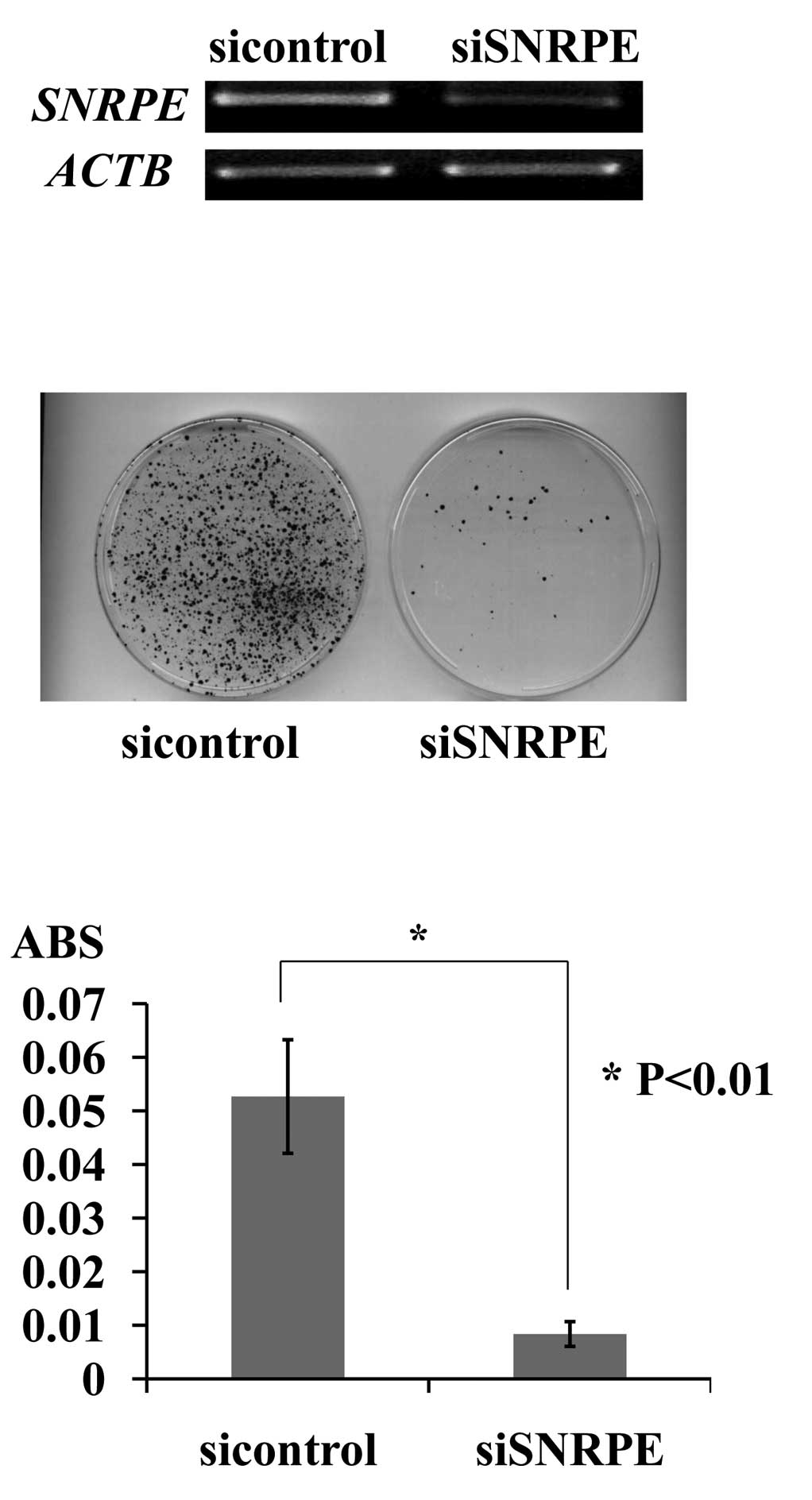
SNRPE (Fig. 2A). Of the two
shRNA expression vectors, siSNRPE had a significant knockdown
effect on the endogenous SNRPE transcript, and its
transfection resulted in a decreased number of PC colonies and a
decreased number of viable PC cells as measured by the cell
viability assay. By contrast, the transfection of the negative
control (siEGFP) had little or no effect on SNRPE expression
and did not affect the viability of 22Rv1 cells (Fig. 2B and C).
SNRPE overexpression promoted PC cell
proliferation
To investigate the potential oncogenic function of
SNRPE, we established stable transformants from 22Rv1 cells,
which constitutively expressed exogenous SNRPE. We also
prepared control 22Rv1 cells, which were transfected with the empty
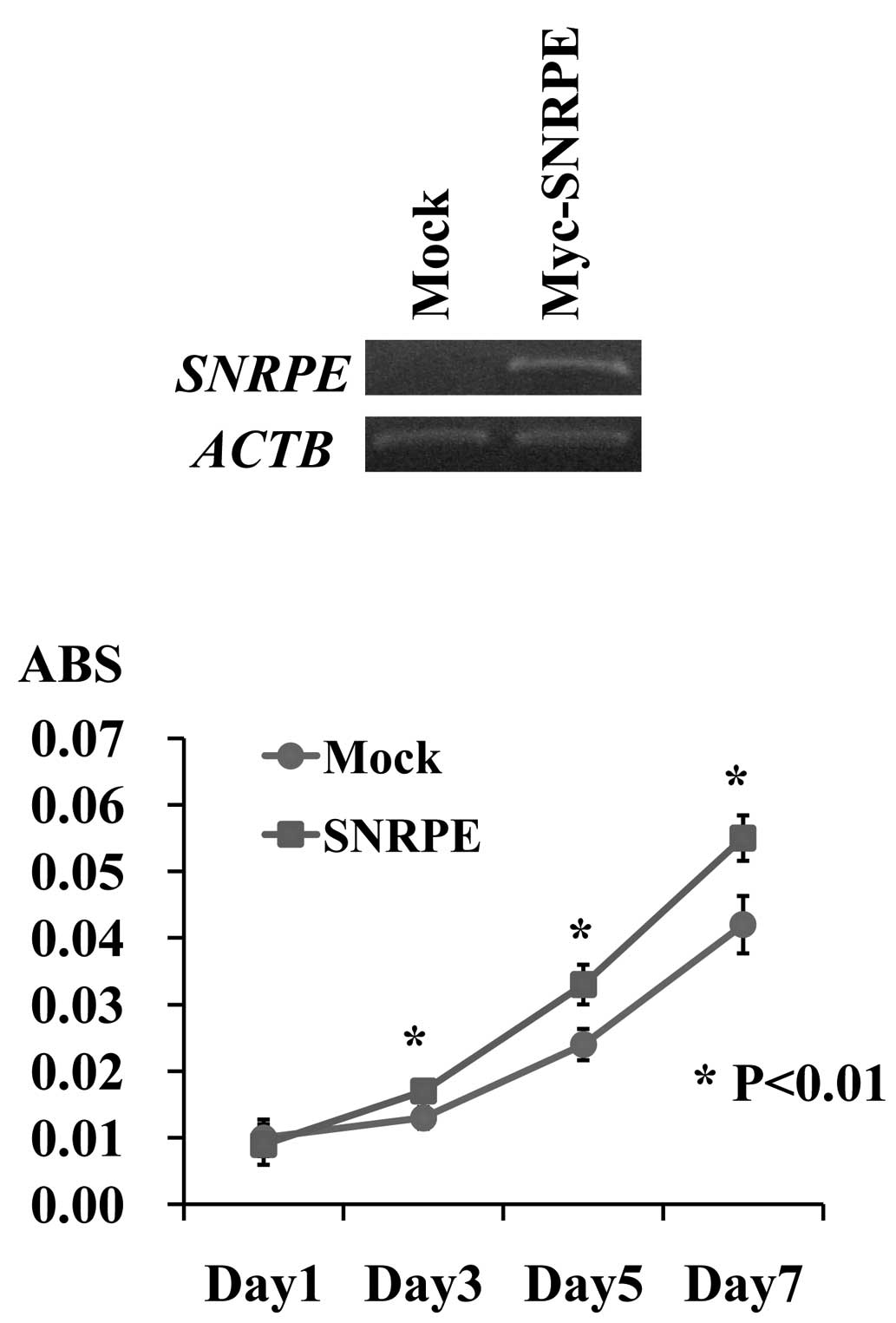
vector (mock), and compared their proliferation. RT-PCR detected a
high level of exogenous SNRPE expression in the stable
clone. A cell proliferation assay revealed that the 22Rv1
SNRPE-overexpressing clones grew more rapidly than the 22Rv1
mock clones (*P<0.01, Student’s t-test), indicating
that SNRPE overexpression promoted PC cell proliferation
(Fig. 3).
SNRPE regulates AR signaling pathways in
PC cells
To identify the RNA targets regulated by
SNRPE to explain its effect on cell proliferation, we
performed a gene expression analysis using a cDNA microarray. The
gene expression patterns of the 22Rv1 cells transfected with the
two shRNA expression vectors (siSNRPE and siEGFP) were compared.
The gene expression patterns of the 22Rv1
SNRPE-overexpressing clone and 22Rv1 mock clone were also
compared. As a result, we found that the expression of the
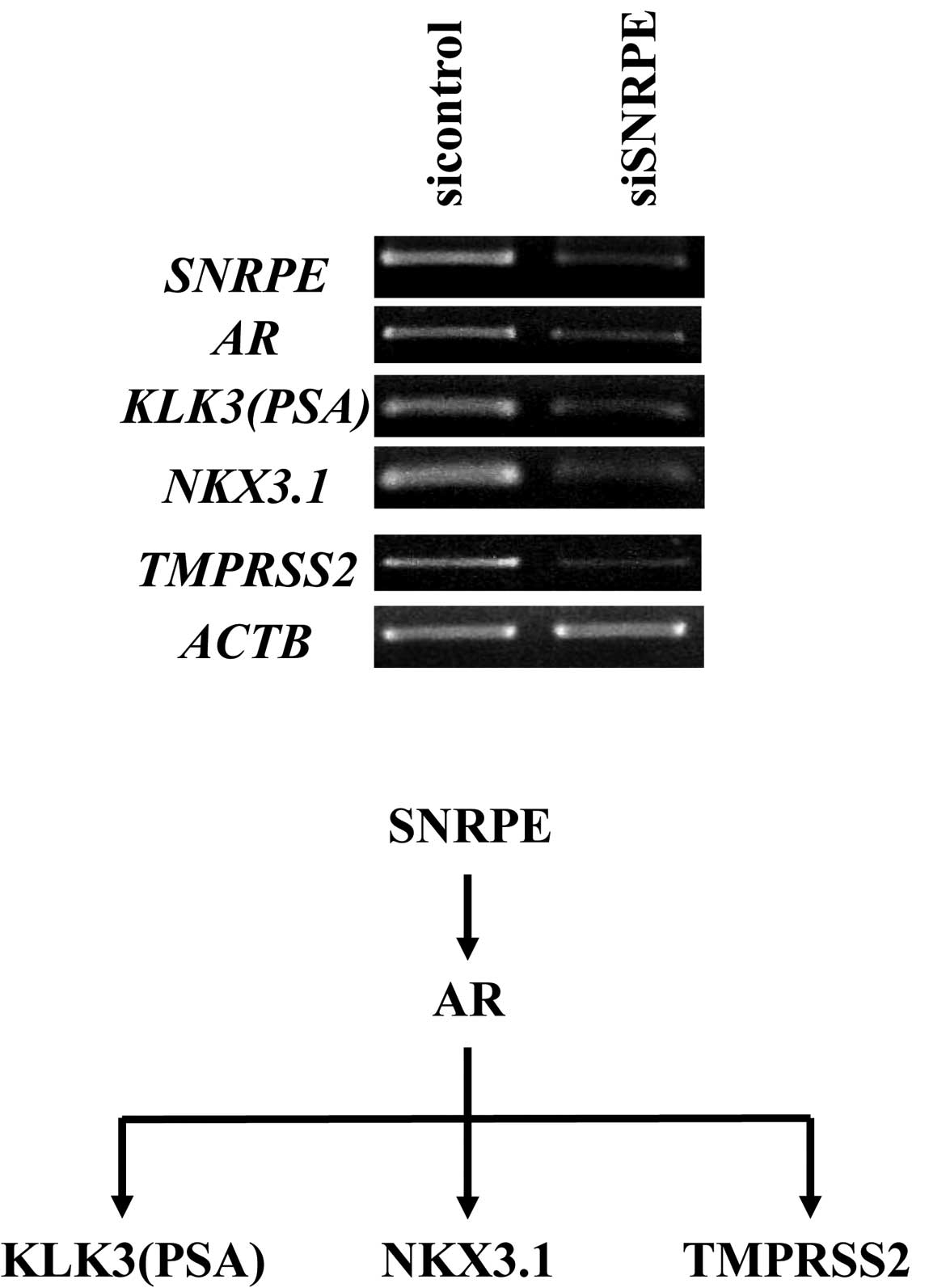
AR gene was downregulated by ≥80% in the SNRPE
knockdown cells and upregulated by ≥20% in the
SNRPE-overexpressing clone. Semi-quantitative RT-PCR
confirmed the significant downregulation of AR and its
downstream target genes (PSA, NKX3.1 and TMPRSS2) in
the SNRPE knockdown clone (Fig.
4).
Discussion
We identified SNRPE as a novel molecular
target of cancer drug therapy for high-grade PC. SNRPE, which is
also known as spliceosomal protein E (SmE), was originally
identified as an essential component of the spliceosomal complex,
which is involved in pre-mRNA processing. SNRPE (SmE) belongs to a
large family of polypeptides containing Sm and Sm-like (Lsm)
proteins, which are conserved in eukaryotes and archaebacteria
(7,8). Seven Sm proteins (SmB/B’, SmD1, SmD2,
SmD3, SmE, SmF and SmG) form a core ring structure that encompasses
RNA. All Sm proteins contain the Sm domain, which consists of two
blocks of conserved amino acids (Sm motif 1 and Sm motif 2) that
are responsible for the assembly of snRNAs (U1, U2, U4, U5 and U6)
in an ordered manner to form the Sm core of the spliceosomal snRNPs
(8,9) and are thereby involved in pre-mRNA
processing (10). Generally, mRNA
processing factors are considered to function only during the
control of global gene expression, during which they are involved
in essential pre-mRNA splicing. However, several studies have
revealed that Sm and Lsm proteins perform other physiological
activities that are distinct from pre-mRNA splicing (11–13).
In this study, we confirmed that high-grade PC cells
overexpress SNRPE. The knockdown of SNRPE expression
by siRNA resulted in the marked suppression of PC cell
proliferation. By contrast, exogenous SNRPE expression in
transfected cells promoted PC cell proliferation. These findings
demonstrate the involvement of SNRPE in the proliferation
and viability of PC cells.
Notably, using cDNA microarray analysis we have
shown that SNRPE regulates AR mRNA expression in PC cells.
The knockdown of SNRPE expression by siRNA resulted in the
marked suppression of AR and its downstream target genes at
the mRNA level. AR plays a key role in the modulation of
prostate cell proliferation and is involved in the development and
progression of PC. Several studies using in vitro prostate
cancer cell lines and mouse models have suggested that the
progression to castration-resistant PC is associated with increased
levels of AR expression, indicating that AR
downregulation using siRNA or other methods may inhibit tumor
growth even in castration-resistant PC (14–17). A
study by Tamura et al (18)
provided new insights into the molecular characteristics that
distinguish castration-resistant PC from castration-naïve PC. These
authors revealed a significant association between high-levels of
AR, SNRPE expression and castration-resistant PC.
High-grade PC has a similar molecular profile to
castration-resistant PC, which may reflect dedifferentiation and
explain the clinical association between grade and prognosis
(19). Our findings regarding
SNRPE overexpression in high-grade PC have shown that SNRPE
is crucial in PC progression through the regulation of AR
expression.
In conclusion, we suggest that the regulation of
AR expression by SNRPE is essential for the cell
proliferation and progression of high-grade PC, although the
detailed mechanisms responsible for these effects remain unknown
and should be elucidated by further investigations. We also
demonstrated its positive involvement in cell proliferation and the
regulation of AR expression. Our findings could provide new
insights into the molecular mechanisms of PC progression and
indications that will aid the development of new therapeutic drugs
for PC.
Acknowledgements
This study was supported in part by a grant for
Research for Promoting Technological Seeds A (discovery type) no.
14-063 (to K. Tamura) from the Japan Science and Technology
Agency.
References
|
1
|
Grönberg H: Prostate cancer epidemiology.
Lancet. 361:859–64. 2003.PubMed/NCBI
|
|
2
|
Hsing AW and Devesa SS: Trends and
patterns of prostate cancer: what do they suggest? Epidemiol Rev.
23:3–13. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feldman BJ and Feldman D: The development
of androgen-independent prostate cancer. Nat Rev Cancer. 1:34–45.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scher HI and Sawyers CL: Biology of
progressive, castration-resistant prostate cancer: directed
therapies targeting the androgen-receptor signaling axis. J Clin
Oncol. 23:8253–8261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han M, Partin AW, Piantadosi S, et al: Era
specific biochemical recurrence-free survival following radical
prostatectomy for clinically localized prostate cancer. J Urol.
166:416–419. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Satake H, Tamura K, Furihata M, et al: The
ubiquitin-like molecule interferon-stimulated gene 15 is
overexpressed in human prostate cancer. Oncol Rep. 23:11–16.
2010.PubMed/NCBI
|
|
7
|
Mayes AE, Verdone L, Legrain P, et al:
Characterization of Sm-like proteins in yeast and their association
with U6 snRNA. EMBO J. 18:4321–4331. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salgado-Garrido J, Bragado-Nilsson E,
Kandels-Lewis S, et al: Sm and Sm-like proteins assemble in two
related complexes of deep evolutionary origin. EMBO J.
18:3451–3462. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hermann H, Fabrizio P, Raker VA, et al:
snRNP Sm proteins share two evolutionarily conserved sequence
motifs which are involved in Sm protein-protein interactions. EMBO
J. 14:2076–2088. 1995.PubMed/NCBI
|
|
10
|
Séraphin B: Sm and Sm-like proteins belong
to a large family: identification of proteins of the U6 as well as
the U1, U2, U4 and U5 snRNPs. EMBO J. 14:2089–2098. 1995.PubMed/NCBI
|
|
11
|
Albrecht M and Lengauer T: Novel Sm-like
proteins with long C-terminal tails and associated
methyltransferases. FEBS Lett. 569:18–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barbee SA and Evans TC: The Sm proteins
regulate germ cell specification during early C. elegans
embryogenesis. Dev Biol. 291:132–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bilinski SM, Jaglarz MK, Szymanska B, et
al: Sm proteins, the constituents of the spliceosome, are
components of nuage and mitochondrial cement in Xenopus oocytes.
Exp Cell Res. 299:171–178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scher HI and Sawyers CL: Biology of
progressive, castration-resistant prostate cancer: directed
therapies targeting the androgen-receptor signaling axis. J Clin
Oncol. 23:8253–8261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gregory CW, Hamil KG, Kim D, et al:
Androgen receptor expression in androgen-independent prostate
cancer is associated with increased expression of androgen
regulated genes. Cancer Res. 58:5718–5724. 1998.PubMed/NCBI
|
|
16
|
Chen CD, Welsbie DS, Tran C, et al:
Molecular determinants of resistance to anti-androgen therapy. Nat
Med. 10:33–39. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zegarra-Moro OL, Schmidt LJ, Huang H, et
al: Disruption of androgen receptor function inhibits proliferation
of androgen-refractory prostate cancer cells. Cancer Res.
62:1008–1013. 2002.PubMed/NCBI
|
|
18
|
Tamura K, Furihata M, Tsunoda T, et al:
Molecular features of hormone-refractory prostate cancer cells by
genome-wide gene expression profiles. Cancer Res. 67:5117–5125.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomlins SA, Mehra R, Rhodes DR, et al:
Integrative molecular concept modeling of prostate cancer. Nat
Genet. 39:41–51. 2007. View
Article : Google Scholar : PubMed/NCBI
|