Introduction
Cervical cancer is one of the most common forms of
cancer in females and a leading cause of mortality among
gynecological malignancies (1). In
Taiwan, cervical cancer is the sixth most common cancer in females
(2). The majority of females
diagnosed with this cancer exhibit an advanced, widely disseminated
malignancy and poor survival rate (3). Accumulating evidence has demonstrated
that oncogenic types of human papillomavirus (HPV) are important in
the development of precursors of cervical cancer (4). However, only a small fraction of
females infected with HPV develop the disease, indicating the
contribution of other factors to the progression of lesions in
invasive cervical cancer (5).
Fibroblast growth factors (FGFs) are a family of
structurally related polypeptides, comprising at least 23 members,
commonly identified as the classical FGFs. The classical
designations, acidic FGF and basic FGF, are now known as FGF1 and
FGF2, respectively (6). FGFs are
possible candidates for mitogenic factors (7). FGFs share between 35 and 50% amino
acid sequence homology, acting as mediators in a diverse range of
developmental and physiological processes, both in vitro and
in vivo (8). FGF receptors
(FGFRs) are tyrosine kinases possessing three extracellular
immunoglobulin-like domains, a transmembranous region and a
cytoplasmic split tyrosine kinase domain, which is activated upon
FGF ligand binding (9). FGFs and
their receptors are involved in the development of several human
cancers (10). Altered protein
expression levels of one or more of these receptors and ligands
have been identified in cancer of the lung (11), kidney (12), colon (13), head and neck (14), breast (15) and prostate (16). FGF2 is a ubiquitous multifunctional
regulator involved in the proliferation and differentiation of a
broad spectrum of mesodermal cells (17). FGF4, which is expressed in the
vicinity of the posterior endoderm in the gastrula and early somite
stage embryos, exhibits broad anterior-posterior patterning
activity in the gut endoderm. Specifically, FGF4 promotes posterior
and inhibits anterior endoderm cell fate (18). The study of cervical cancer provides
a good model for assessing the effect of this microenvironment on
epithelial-mesenchymal transition (EMT) (19). Little is known about the role of
FGFs in cervical cancer regarding the maintenance of normal cells
and the progression to carcinogenesis (20).
In this study, we investigated whether HPV16 E6/E7
transfection contributes to FGF2- and 4-induced tumorigenesis in
human malignant cervical cancer cells. The aim of the experiments
was to develop the scientific basis required to provide
technological support for cervical cancer therapy.
Materials and methods
Cell culture
Normal cervical epithelial cells (Epi) and cervical
cancer cells were obtained from patients with benign uterine
neoplasm, admitted to the Department of Obstetrics and Gynecology
of the National Cheng Kung University (NCKU) Medical Center,
Taiwan, to undergo surgery. The patients underwent total abdominal
hysterectomy. Specimens were removed from only typical and
clinically clear-cut (Grade II) cases.
Prior written informed consent was obtained from the
patients and all procedures had been reviewed and approved by the
ethics board at NCKU in adherence to the Declaration of
Helsinki.
Cervix tissue was dissected following surgery and
immersed in a culture medium for the preparation of normal cervical
epithelial cells. The Cx cell is a cervical cancer cell line
established by Professor Chou (21). The cells were obtained from a
48-year-old Taiwanese female with squamous cell carcinoma of the
uterine cervix, characterized as HPV-negative and
p53-mutation-negative. The CxWJ cell was established using a stable
clone from Cx cells transfected with HPV16 E6 and E7 (1). Stroma (Str) and SiHa cells were
purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). The cells were maintained on culture dishes, in
RPMI-1640 (Cx and CxWJ) or DMEM (Str and SiHa) supplemented with
10% (v/v) FBS. The cells were cultured in an incubator with an
atmosphere of 95% air and 5% CO2 at 37˚C.
Immunoblotting
Total cell lysate (30 and 50 μg) was separated using
SDS-PAGE, and transferred to nitrocellulose membranes. The
membranes were blocked with 5% BSA in PBS containing 0.1% Tween-20
(PBST) at room temperature for 1 h. The blots were incubated with
primary antibody (E-cadherin, α-SMA, vimentin, β-actin) (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) for 2 h, washed with
three exchanges of PBST for 30 min, incubated for 1 h with
secondary anti-mouse or anti-rabbit antibody conjugated with
horseradish peroxidase and then washed with three exchanges of PBST
for an additional 30 min. The proteins were visualized using a
chemiluminescence detection kit (ECL; Amersham Corp., Arlington
Heights, IL, USA).
Invasive assay
The invasive assay of cells through type IV collagen
was performed as previously reported (22). Briefly, modified Boyden chambers
containing polycarbonate filters with 8-μm pores (Becton-Dickinson,
Boston, MA, USA) were coated with 0.25 mg/ml type IV collagen
(Sigma, St. Louis, MO, USA). Following incubation for 48 h, invaded
cells were stained with 0.1% crystal violet solution and
photographed using a QImaging Retiga EXi digital camera (Burnaby,
BC, Canada) under a Leica DMIRE2 microscope. The number of invaded
cells was then counted and subjected to statistical analysis.
Cell proliferation assay
Cells were seeded in culture plates at 5000
cells/well. Different cell wells were treated with 0, 25 and 100 μM
FGFs (FGF2 and 4) for 24 h. The number of cells was determined
microscopically using a hemocytometer. MTS dye (1 mg/ml) was added
to each well for an additional 4 h following treatment. The
reaction was stopped by the addition of sodium dodecyl sulfate and
optical density was measured at 492 nm using a multi-well plate
reader (Powerwave XS, BioTek, Winooski, VT, USA). Background
absorbance of the medium was subtracted in the absence of cells.
The samples were assayed in at least triplicate, and the mean was
calculated for each experiment. Each assay was carried out in
triplicate and the results are expressed as the mean (±SEM).
Statistical analysis
Data are shown as the mean (±SEM) of at least three
separate experiments. Statistical analysis was performed using the
t-test, with significance set at P<0.05.
Results
EMT is expressed between the Cx and CxWJ
cells
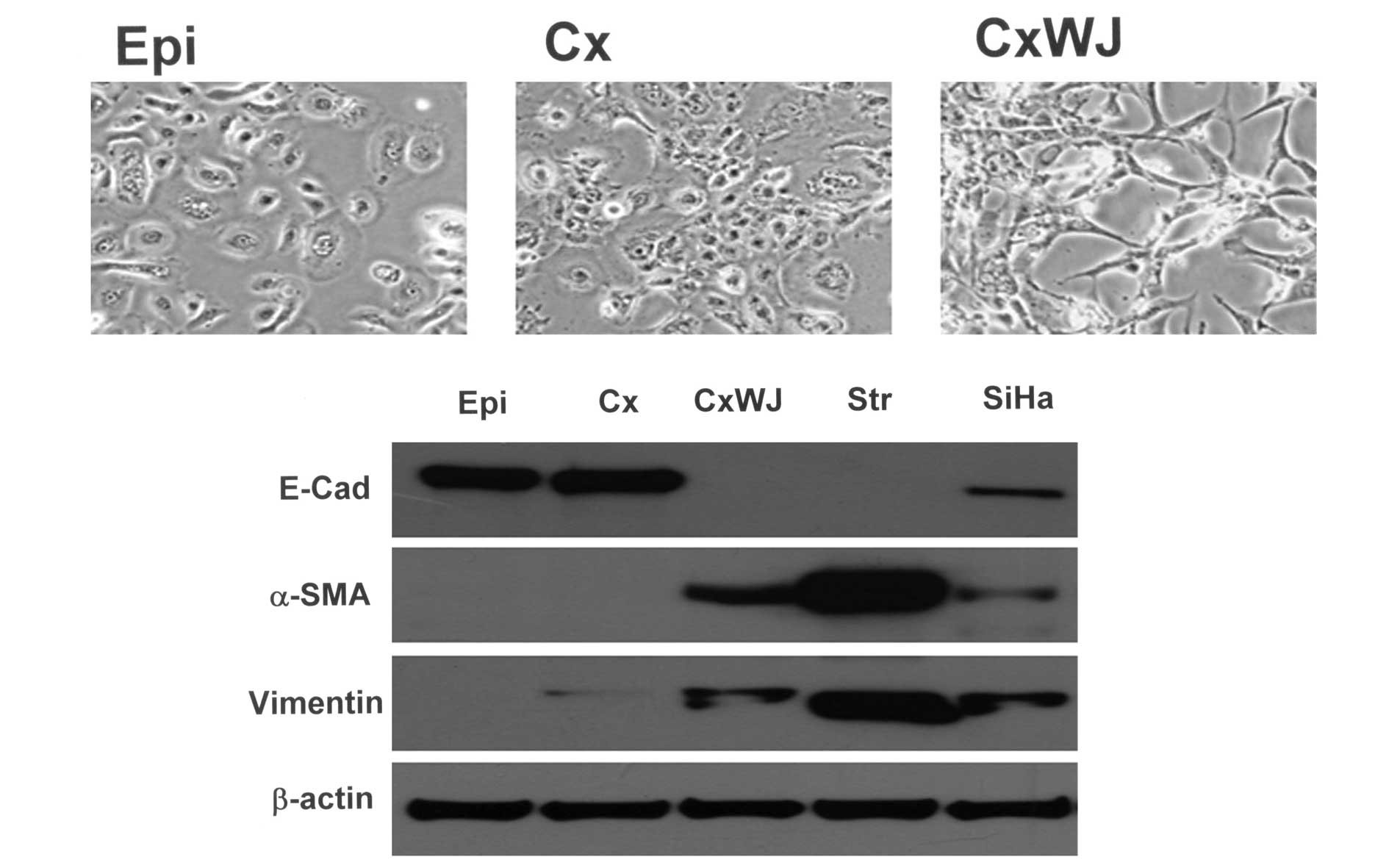
Using an Olympus CKX41 microscope, the cell lines
(Epi, Cx and CxWJ) were found to exhibit changes in cell
morphology. In Fig. 1A, a
microscopic examination revealed Cx cells with polygonal epithelial
cell characteristics and CxWJ cells in the shape of a spindle. The
expression of epithelial-mesenchymal cell markers was determined
using western blotting. Compared with the Cx cells, the expression
of E-cadherin protein was downregulated in CxWJ cells and Str cells
(Fig. 1B). The expression of α-SMA
and vimentin protein was upregulated in CxWJ cells. A similar
pattern was observed in Str and SiHa cells. Taken together, these
results suggest the occurrence of EMT between the Cx and CxWJ
cells. HPV16 E6/E7 transfection therefore induces EMT in cervical
epithelial cells.
HPV E6/E7 transfection repressed the
number of CxWJ cells compared with Cx cells
To investigate the proliferation of FGF (FGFs 2 and
4) ligands in cervical cancer cell lines (Cx and CxWJ), the number
of cells (Fig. 2A) and MTS analysis
were used to monitor the reduction percentage of R-tetrazolium into
R-formazan, which was quantified using a spectrophotometer at 492
nm (Fig. 2B). Fig. 2A shows that the number of Cx and
CxWJ cells increased following treatment with the FGF2 and 4
ligands (P<0.05 vs. 0 ng/ml group). Transfection with HPV16
E6/E7 reduced cell growth in CxWJ cervical cancer cells (P<0.01
Cx vs. CxWJ cells FGFs 0 ng/ml). Similar results were observed with
the 25 and 100 ng/ml treatment of FGFs (2 and 4). HPV E6/E7
transfection repressed the number of CxWJ cells compared with Cx
cells (P<0.05 vs. Cx cells).
Treatment with FGF ligand (50 ng/ml) also enhanced
cell growth in Cx and CxWJ cells (P<0.05 vs. SF group; Fig. 2B). As shown in Fig. 2B, the proliferation of CxWJ cells
decreased following transfection with HPV16 E6/E7 (P<0.05 vs. Cx
cells). Taken together, these results indicate that HPV16 E6/E7
transfection is important in the duplication of cervical epithelial
cells.
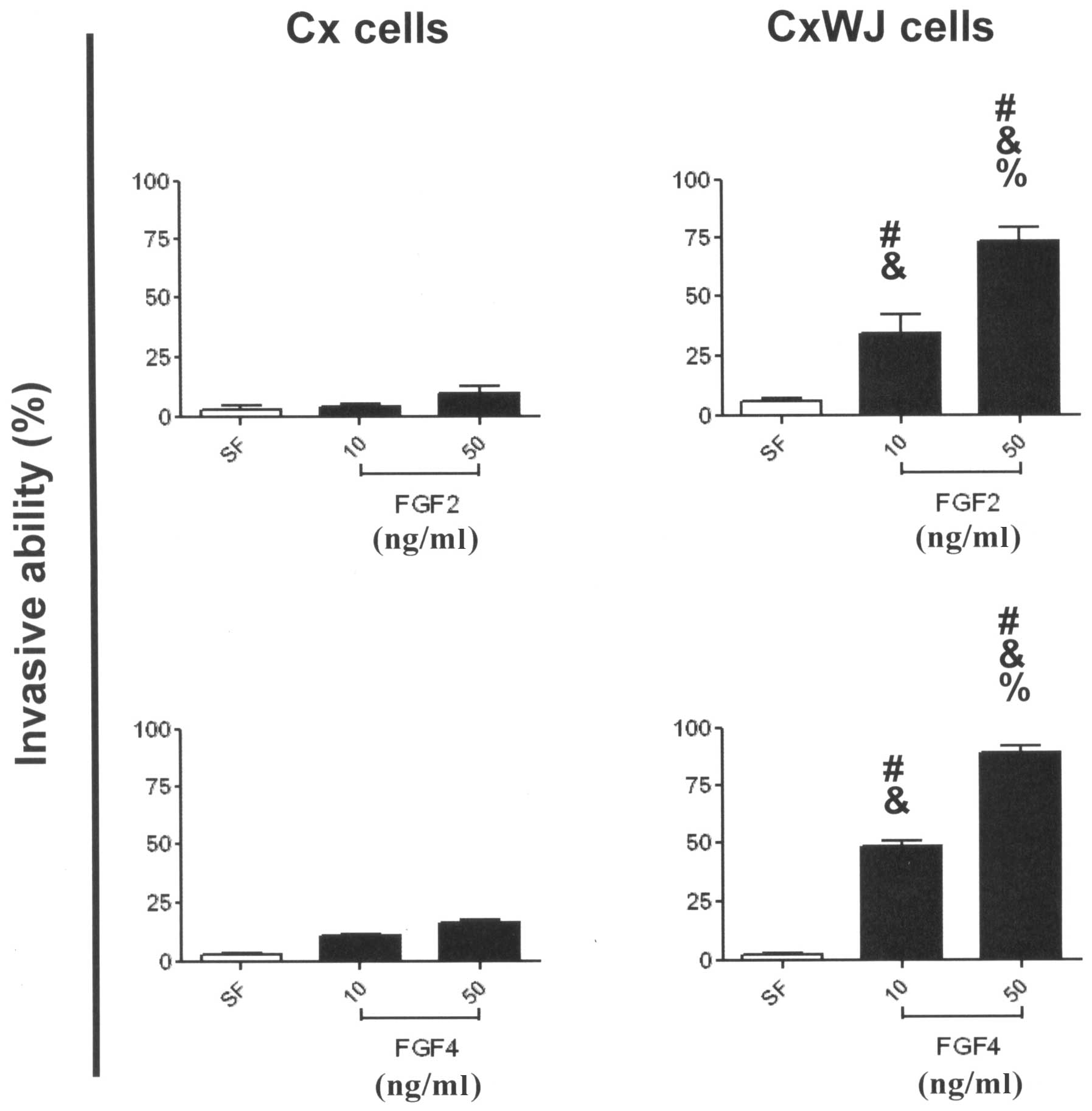
FGF2 and 4 induce invasive activity via
HPV16 E6/E7 transfection
To examine the potential invasive activity of FGFs
in HPV16 E6/E7-transfected cervical cancer cells, we employed
modified Boyden chambers and a digital camera attached to a
microscope to identify the activity of Cx and CxWJ cells. Invasive
ability increased significantly in CxWJ cells following ligand
stimulation with FGF2 (Fig. 3B;
P<0.05 vs. SF) and FGF4 (Fig.
3D; P<0.05 vs. SF), but not in Cx cells (Fig. 3A and C). Treatment with FGFs
enhanced the invasive ability of CxWJ cells compared with Cx cells
(P<0.05 CxWJ vs. Cx cells). FGF ligand stimulated invasive
ability in CxWJ cells (P<0.05 50 vs. 10 ng/ml) in a
dose-dependent manner. The results in Fig. 3 show that FGF2 and 4 induce invasive
activity via HPV16 E6/E7 transfection.
Discussion
Cervical cancer remains a fatal disease despite the
development of various advanced forms of treatment (23). Following human papillomavirus (HPV)
infection, cervical epithelial cells develop from premalignant
cervical lesions to malignant invasive cancer via a multi-step
process.
E-cadherin, the major cadherin molecule expressed by
epithelial cells, maps to chromosome 16q22 (4) and serves as a mediator in the adhesion
of epithelial cells. The role of E-cadherin as a tumor invasion
suppressor in epithelial cells is well supported (24). Loss of the gene expression of
E-cadherin has been shown to disrupt E-cadherin-mediated
intercellular adhesion and is frequently associated with high-grade
tumors, infiltrative growth and lymph node metastasis in a variety
of human malignancies. The expression of E-cadherin in transgenic
mice has also been associated with the development of invasive
carcinoma from well-differentiated adenoma. In this study, we found
that HPV16 E6/E7 transfection repressed the expression of
E-cadherin protein in CxWJ cells. HPV infection may thus play a
significant role in E-cadherin-mediated cervical cancer
malignancy.
During the development of tumors, the stroma
surrounding the fibroblasts and myofibroblasts express α-smooth
muscle actin (α-SMA) constituting the ‘desmoplastic reaction’ in
the process of invasion and metastasis. Vimentin is a
developmentally regulated intermediate filament protein found in
cells of mesenchymal origin. It is frequently co-expressed with
other members of the intermediate filament family including
cytokeratins and neoplasms such as melanoma and breast carcinoma
(25,26). Vimentin has been used as a sarcoma
tumor marker to identify mesenchyme (27). The α-SMA and vimentin play a key
role in the progression of tumors. Our results reveal that HPV16
E6/E7 transfection enhances the expression of α-SMA and vimentin
protein in CxWJ cells. HPV infection may be associated with α-SMA
and the vimentin-induced metastasis of cervical cancer cells.
In conclusion, our results indicate that HPV16 E6/E7
transfection of CxWJ cells causes them to respond differently to
FGF ligand stimulation and increase invasive ability. Further study
regarding changes in the epithelium and mesenchymal characteristics
should be conducted to elucidate the role of HPV16 E6/E7 and FGF
alteration in cervical carcinogensis.
Acknowledgements
The authors would like to express their appreciation
for the funding support provided by the National Cheng Kung
University Hospital.
References
|
1
|
Hsu KF, Wu CL, Huang SC, et al:
Conditionally replicating E1B-deleted adenovirus driven by the
squamous cell carcinoma antigen 2 promoter for uterine cervical
cancer therapy. Cancer Gene Ther. 15:526–534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lang HC and Wu SL: Lifetime costs of the
top five cancers in Taiwan. Eur J Health Econ. Mar 27–2011.(Epub
ahead of print).
|
|
3
|
Chen CC, Lin JC, Jan JS, Ho SC and Wang L:
Definitive intensity-modulated radiation therapy with concurrent
chemotherapy for patients with locally advanced cervical cancer.
Gynecol Oncol. Apr 22–2011.(Epub ahead of print).
|
|
4
|
Schiffman M, Wentzensen N, Wacholder S,
Kinney W, Gage JC and Castle PE: Human papillomavirus testing in
the prevention of cervical cancer. J Natl Cancer Inst. 103:368–383.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mühlen S, Behren A, Iftner T and Simon C:
Influence of HPV16 E2 and its localisation on the expression of
matrix metalloproteinase-9. Int J Oncol. 37:337–345.
2010.PubMed/NCBI
|
|
6
|
Chen GJ and Forough R: Fibroblast growth
factors, fibroblast growth factor receptors, diseases, and drugs.
Recent Pat Cardiovasc Drug Discov. 1:211–224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ucuzian AA, Brewster LP, East AT, Pang Y,
Gassman AA and Greisler HP: Characterization of the chemotactic and
mitogenic response of SMCs to PDGF-BB and FGF-2 in fibrin
hydrogels. J Biomed Mater Res A. 94:988–996. 2010.PubMed/NCBI
|
|
8
|
Dorey K and Amaya E: FGF signalling:
diverse roles during early vertebrate embryogenesis. Development.
137:3731–3742. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Caverzasio J and Thouverey C: Activation
of FGF receptors is a new mechanism by which strontium ranelate
induces osteoblastic cell growth. Cell Physiol Biochem. 27:243–250.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Knights V and Cook SJ: De-regulated FGF
receptors as therapeutic targets in cancer. Pharmacol Ther.
125:105–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pardo OE, Latigo J, Jeffery RE, et al: The
fibroblast growth factor receptor inhibitor PD173074 blocks small
cell lung cancer growth in vitro and in vivo. Cancer Res.
69:8645–8651. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsimafeyeu I, Demidov L, Stepanova E, Wynn
N and Ta H: Overexpression of fibroblast growth factor receptors
FGFR1 and FGFR2 in renal cell carcinoma. Scand J Urol Nephrol.
45:190–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Katoh M and Katoh M: FGF signaling network
in the gastrointestinal tract. Int J Oncol. 29:163–168.
2006.PubMed/NCBI
|
|
14
|
Yura Y, Yoshioka Y, Yamamoto S, et al:
Enhancing effects of fibroblast growth factor on the proliferation
of salivary gland carcinoma cells and salivary gland
carcinogenesis. J Oral Pathol Med. 30:159–167. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Manuvakhova M, Thottassery JV, Hays S, et
al: Expression of the SNT-1/FRS2 phosphotyrosine binding domain
inhibits activation of MAP kinase and PI3-kinase pathways and
antiestrogen resistant growth induced by FGF-1 in human breast
carcinoma cells. Oncogene. 25:6003–6014. 2006. View Article : Google Scholar
|
|
16
|
Schwertfeger KL: Fibroblast growth factors
in development and cancer: insights from the mammary and prostate
glands. Curr Drug Targets. 10:632–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vodyanik MA, Yu J, Zhang X, et al: A
mesoderm-derived precursor for mesenchymal stem and endothelial
cells. Cell Stem Cell. 7:718–729. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morrison GM, Oikonomopoulou I, Migueles
RP, et al: Anterior definitive endoderm from ESCs reveals a role
for FGF signaling. Cell Stem Cell. 3:402–415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hardy KM, Yatskievych TA, Konieczka J,
Bobbs AS and Antin PB: FGF signalling through RAS/MAPK and PI3K
pathways regulates cell movement and gene expression in the chicken
primitive streak without affecting E-cadherin expression. BMC Dev
Biol. 11:202011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Katoh M: Network of WNT and other
regulatory signaling cascades in pluripotent stem cells and cancer
stem cells. Curr Pharm Biotechnol. 12:160–170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chou CY, Chen YH, Tzeng CC, Cheng YC,
Chang CF and Chen TM: Establishment and characterization of a
human-papillomavirus negative, p53-mutation negative human cervical
cancer cell line. Cancer Lett. 102:173–181. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Benton G, Kleinman HK, George J and
Arnaoutova I: Multiple uses of basement membrane-like matrix
(BME/Matrigel) in vitro and in vivo with cancer cells. Int J
Cancer. 128:1751–1757. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hasimu A, Ge L, Li QZ, Zhang RP and Guo X:
Expressions of Toll-like receptors 3, 4, 7, and 9 in cervical
lesions and their correlation with HPV16 infection in Uighur women.
Chin J Cancer. 30:344–350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simionescu C, Mărgăritescu C, Stepan A,
Georgescu CV, Niculescu M and Muntean M: The utility of p16,
E-cadherin and Ki67 in cervical squamous intraepithelial lesions
diagnosis. Rom J Morphol Embryol. 51:621–626. 2010.PubMed/NCBI
|
|
25
|
De Chiara A, Losito S, Terracciano L, Di
Giacomo R, Iaccarino G and Rubolotta MR: Primary plasmacytoma of
the breast. Arch Pathol Lab Med. 125:1078–1080. 2001.
|
|
26
|
Sun H, Qin M, Xiao Y, Yang F, Ni W and Liu
S: Haemangiomas, leiomyosarcoma and myeloma caused by subgroup J
avian leukosis virus in a commercial layer flock. Acta Vet Hung.
58:441–451. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu QS, Rosenblatt K, Huang KL, et al:
Vimentin is a novel AKT1 target mediating motility and invasion.
Oncogene. 30:457–470. 2011. View Article : Google Scholar : PubMed/NCBI
|