Introduction
Differentiated thyroid cancer comprises the majority
of thyroid malignancies (approximately 95%). Papillary thyroid
cancer (PTC) accounts for 85% of differentiated thyroid cancers,
and follicular thyroid cancer (FTC) for 10%. Total thyroidectomy is
the initial treatment for the majority of patients with
differentiated thyroid cancer. Postoperative remnant ablation with
131I is indicated for all patients with stage 3 and 4
disease and for some patients with stage 1 and 2 disease (1). It supplements surgery by destroying
normal thyroid remnants, thus increasing the sensitivity of
subsequent 131I whole-body scanning, and of serum
thyroglobulin (Tg) measurements in detecting persistent or
recurrent disease. It also destroys microscopic neoplastic tissue,
decreasing the long-term recurrence rate (1–3).
Remnant ablation requires TSH stimulation. This may
be accomplished by withdrawing thyroid hormone treatment or by
using recombinant human thyroid-stimulating hormone (rhTSH). These
approaches have been approved for ablative therapy and diagnostic
purposes (1).
Certain studies have found that the two methods are
equally effective in preparing patients for 131I remnant
ablation, with a greater quality of life achieved when rhTSH is
used (4–6). Other authors demonstrated that the use
of rhTSH is associated with a significant decrease in whole-body
irradiation (7–12), which may be relevant with regards to
the radiation exposure of the general population. The amount of
131I retained depends on factors such as the presence of
metastatic disease, hydration level, bowel functioning,
pre-therapeutic diet and renal clearance (9). The preserved renal clearance in
patients prepared with rhTSH should explain the lower whole-body
retention in these individuals, since it is known that renal
clearance of 131I is reduced to approximately 50% in
hypothyroid patients (13).
We retrospectively evaluated 100 patients submitted
to postoperative remnant ablation with 131I. Of those,
50 patients were prepared with thyroid hormone withdrawal and 50
patients were prepared with rhTSH, with particular emphasis on the
whole-body retention of 131I, extrapolated from the
measurements of the effective dose at a 1-meter (m) distance.
Materials and methods
The study was approved by the ethics committee of
the hospital. A total of 100 randomly selected patients, submitted
to postoperative remnant ablation with 131I at the
Portuguese Oncology Institute of Lisbon (Portugal) in 2008, were
evaluated retrospectively. The patients had undergone a low-iodine
diet, and those with positive anti-Tg antibodies, <18 years of
age or with impaired renal function (estimated by the creatinine
blood level) were excluded.
Of the 100 patients, 50 were prepared with thyroid
hormone withdrawal (hypothyroidism group): levothyroxine
(LT4) was discontinued and switched to triiodothyronine
(LT3) for 6 weeks, followed by LT3 withdrawal
for 2 weeks prior to ablation. The remaining 50 patients were
prepared with rhTSH (rhTSH group): rhTSH 0.9 mg was administered
intramuscularly in the 2 days prior to ablative therapy.
Data on the maximal TSH and Tg levels were
collected. Radioiodine therapy (RIT) regimen comprised 3700 MBq
(100 mCi) of 131I and administered per os to all
patients. None of the patients were submitted to diagnostic
radioiodine scanning prior to ablative therapy.
The whole-body retention of 131I was
extrapolated from the measurements of the effective dose at a
standardized distance of 1 m between the probe and the patient, as
recommended in the ATA guidelines (14). The probe was a whole-body counter,
model TAM/S Single Area Monitor, from Tema Sinergie (Faenza,
Italy).
Statistical analysis
Data are reported as the means ± SD. The differences
between groups were tested for significance using the Student's
t-test, Mann-Whitney test and χ2 test, where
appropriate. P<0.05 was considered to indicate a statistically
significant difference.
Results
Demographic characteristics did not vary
significantly between the two groups (Table I).
 | Table IBaseline characteristics of the
patients. |
Table I
Baseline characteristics of the
patients.
| Hypothyroidism
group | rhTSH group | p-value |
|---|
| Number of
patients | 50 | 50 | |
| Mean age | 50.4±13.2 | 48.3±14.6 | 0.456 |
| Gender (M:F) | 12:38 | 6:44 | 0.118 |
| Histology |
| Papillary | 32 | 33 | |
| Follicular | 6 | 2 | |
| Papillary,
follicular variant | 10 | 13 | 0.442 |
| Papillary, tall cell
variant | 0 | 1 | |
| Papillary, oncocytic
variant | 2 | 1 | |
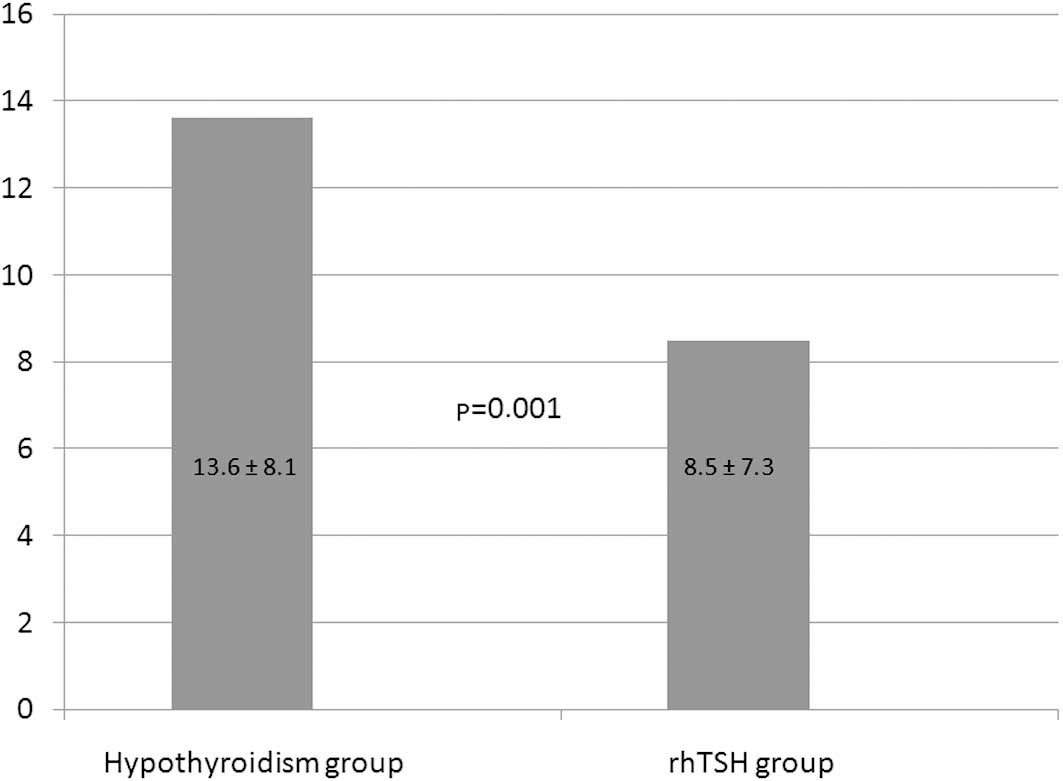
The whole-body retention of 131I,
extrapolated from the measurements of the effective dose at 1 m,
was significantly lower in the rhTSH group at 8.5±7.3 μS/h versus
13.6±8.1 μS/h in the hypothyroid group (p=0.001; Fig. 1).
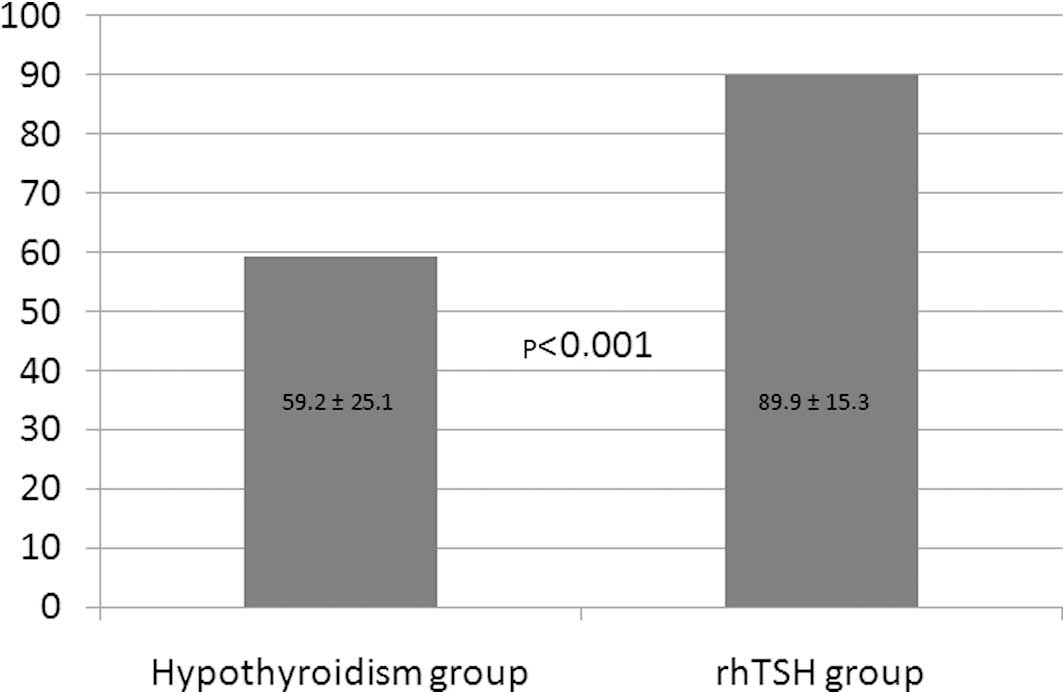
All rhTSH-stimulated patients reached TSH >30
mU/l, while 7 patients (14%) in the hypothyroid group had maximal
TSH <30 mU/l (Fig. 2). Mean
maximal TSH values were significantly higher in the rhTSH group at
89.9±15.3 mU/l versus 59.2±25.1 mU/l in the hypothyroid group
(p<0.001).
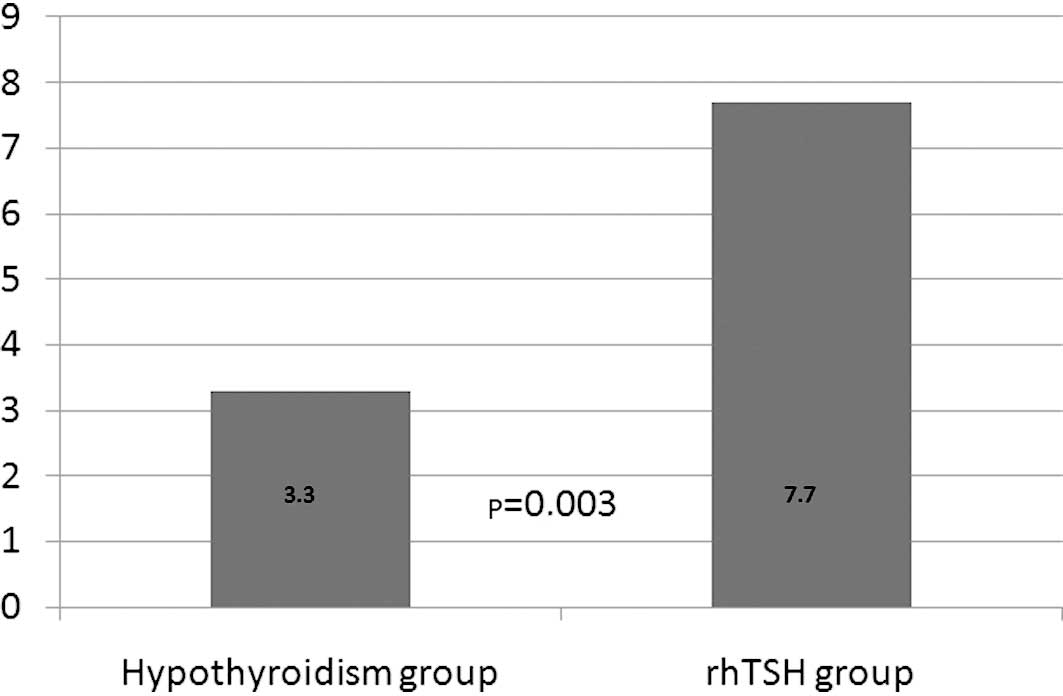
Maximal Tg values were also significantly different
between the two groups (p=0.003) with a median of 7.7 ng/ml in the
rhTSH group versus 3.3 ng/ml in the hypothyroid group (Fig. 3). Undetectable Tg levels were
present in 20 cases (40%) in the hypothyroid group and in 11 cases
(22%) in the rhTSH group (p=0.05).
Discussion
Preparation for RIT following hormone withdrawal is
usually well tolerated but may be harmful in a subset of patients
(6,7,15),
such as the elderly, patients with cardiac dysfunction or patients
with psychiatric conditions. Other individuals, including those
with hypopituitarism, may be unable to endogenously raise TSH. In
these circumstances the use of rhTSH is advisable.
There is general agreement that rhTSH administration
provides a better quality of life. One of the major drawbacks for
its more generalized use is its cost (16). Although it is believed that it can
be counterbalanced by improved productivity and reduced work
absenteeism when hypothyroidism is avoided (17,18),
its exact economic impact is difficult to ascertain (19).
Our primary goal was to evaluate the whole-body
retention time of 131I, extrapolated from the
measurements of the effective dose at a 1-m distance, associated
with each strategy of preparing patients for postoperative remnant
ablation with 131I. We found lower whole-body retention
of 131I in the rhTSH group. Our results, regarding
whole-body retention of 131I and maximal TSH values
reached, support previously reported data (5,10–13,20).
Although it was not our primary objective, the
comparison of peak Tg levels between groups demonstrated that the
levels were significantly higher in the rhTSH group. There are
several possible explanations for this finding. One is that TSH
levels in the rhTSH group are higher than in the hypothyroid group,
which clearly stimulates the release of Tg by the remaining thyroid
tissue. Another reason is that the acute stimulation obtained with
rhTSH releases Tg stored in thyroid cells, whereas a more chronic
TSH stimulation, as obtained with thyroid hormone withdrawal,
gradually depletes the pool of stored Tg. A previous study has also
documented higher Tg levels with rhTSH preparation compared to
those of hypothyroidism (12),
although it was interpreted as a consequence of the more aggressive
tumor subtypes in the group of patients prepared with rhTSH. This
is not the case in our study, since no differences were observed in
TNM staging between the two groups (data not shown).
The safety of rhTSH use for diagnostic and ablative
purposes has been well documented (16,21).
The question of whether the sudden increase in TSH resulting from
rhTSH administration may lead to tumor expansion (10) in patients with occult metastatic
disease, or whether the more prolonged TSH stimulation related to
hypothyroidism is more life-threatening, remains to be resolved.
Although studies have been carried out to address the efficacy and
safety of rhTSH in metastatic disease (22,23),
its use has not yet been approved for this purpose.
In conclusion, in our series, the use of rhTSH in
the preparation of patients for postoperative remnant ablation with
131I was associated with lower radioiodine toxicity and
with greater efficacy in achieving the desirable TSH level (>30
mU/l). However, the use of rhTSH was also associated with greater
Tg levels and an undetectable Tg level was more frequently found in
the hypothyroid group (40%) as compared to the rhTSH group (22%;
p=0.05). Since an undetectable Tg level at the time of
postoperative 131I remnant ablation generally reflects
complete remission (24–26), patients treated with rhTSH who do
not have undetectable Tg levels when treated with 131I
may not be regarded as disease-free. Therefore, we believe that
clinicians should not disregard the possibility of complete tumor
eradication in patients presenting with low, but not undetectable,
Tg levels at the time of ablation with rhTSH.
References
|
1
|
Cooper DS, Doherty GM, Haugen BR, Kloos
RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F,
Schlumberger M, et al: Revised management guidelines for patients
with thyroid nodules and differentiated thyroid cancer. Thyroid.
19:1167–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eustatia-Rutten CF, Smit JW, Romijn JA,
Van der Kleij- Corssmit EP, Pereira AM, Stokkel MP and Kievit J:
Diagnostic value of serum thyroglobulin measurements in the
follow-up of differentiated thyroid carcinoma, a structured
meta-analysis. Clin Endocrinol (Oxf). 61:61–74. 2004. View Article : Google Scholar
|
|
3
|
Toubeau M, Touzery C, Arveux P, Chaplain
G, Vaillant G, Berriolo A, Riedinger J, Boichot C, Cochet A and
Brunotte F: Predictive value for disease progression of serum
thyroglobulin levels measured in the postoperative period and after
131I ablation therapy in patients with differentiated
thyroid cancer. J Nucl Med. 45:988–994. 2004.PubMed/NCBI
|
|
4
|
Edmonds CJ, Hayes S, Kermode JC and
Thompson BD: Measurement of serum TSH and thyroid hormones in the
management of treatment of thyroid carcinoma with radioiodine. Br J
Radiol. 50:799–807. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pacini F, Ladenson PW, Schlumberger M,
Driedger A, Luster M, Kloos RT, Sherman S, Haugen B, Corone C,
Molinaro E, et al: Radioiodine ablation of thyroid remnants after
preparation with recombinant human thyrotropin in differentiated
thyroid carcinoma: results of an international, randomized,
controlled study. J Clin Endocrinol Metab. 91:926–932. 2006.
View Article : Google Scholar
|
|
6
|
Ladenson PW, Braverman LE, Ebner SA,
Brucker-Davis F, Cooper D, Garber J, Wondisford F, Davies T,
DeGroot L, Daniels G, et al: Comparison of administration of
recombinant human thyrotropin with withdrawal of thyroid hormone
for radioactive iodine scanning in patients with thyroid carcinoma.
New Eng J Med. 337:888–896. 1997. View Article : Google Scholar
|
|
7
|
Haugen B, Pacini F, Reiners C,
Schlumberger M, Ladenson P, Sherman S, Cooper D, Graham K,
Braverman L, Skarulis M, et al: A comparison of recombinant human
thyrotropin and thyroid hormone withdrawal for the detection of
thyroid remnant cancer. J Clin Endocrinol Metab. 84:3877–3885.
1999.PubMed/NCBI
|
|
8
|
Pilli T, Brianzoni E, Capoccetti F,
Castagna MG, Fattori S, Poggiu A, Rossi G, Ferretti F, Guarino E,
Burroni L, et al: A comparison of 1850 (50 mCi) and 3700MBq (100
mCi) 131-iodine administered doses for recombinant
thyrotropin-stimulated postoperative thyroid remnant ablation in
differentiated thyroid cancer. J Clin Endocrinol Metab.
92:3542–3546. 2007. View Article : Google Scholar
|
|
9
|
Sisson JC, Shulkin BL and Lawson S:
Increasing efficacy and safety of treatments of patients with
well-differentiated thyroid carcinoma by measuring body retentions
of 131I. J Nucl Med. 44:898–903. 2003.PubMed/NCBI
|
|
10
|
Papadimitriou D, Kottou S, Oros L, Ilias
I, Molfetas M, Tsapaki V, Perris A and Christakopoulou I:
Differentiated thyroid cancer: comparison of therapeutic iodine 131
biological elimination after discontinuation of levothyroxine
versus administration of recombinant human thyrotropin. Annals Nucl
Med. 20:63–67. 2006. View Article : Google Scholar
|
|
11
|
Remy H, Borget I, Leboulleux S, Guilabert
N, Lavielle F, Garsi J, Bournaud C, Gupta S, Schlumberger M and
Ricard M: 131I effective half-life and dosimetry in
thyroid cancer patients. J Nucl Med. 49:1445–1450. 2008. View Article : Google Scholar
|
|
12
|
Menzel C, Kranert W, Döbert N, Diehl M,
Fietz T, Nadja H, Berner U and Grünwald F: rhTSH stimulation before
radiodine therapy in thyroid cancer reduces the effective half-life
of 131I. J Nucl Med. 44:1065–1068. 2003.PubMed/NCBI
|
|
13
|
Hänscheid H, Lassmann M, Luster M, Thomas
S, Pacini F, Ceccarelli C, Ladenson P, Wahl R, Schlumberger M,
Ricard M, et al: Iodine biokinetics and dosimetry in radioiodine
therapy of thyroid cancer: procedures and results of a prospective
international controlled study of ablation after rhTSH or hormone
withdrawal. J Nucl Med. 47:648–654. 2006.
|
|
14
|
Sisson JC, Freitas J, McDougall IR, Dauer
LT, Hurley JR, Brierley JD, Edinboro CH, Rosenthal D, Thomas MJ,
Wexler JA, et al: Radiation safety in the treatment of patients
with thyroid diseases by radioiodine 131I: practice
recommendations of the American Thyroid Association. Thyroid.
21:335–346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dow KH, Ferrell BR and Anello C:
Quality-of-life changes in patients with thyroid cancer after
withdrawal of thyroid hormone therapy. Thyroid. 7:613–619. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haugen B, Cooper D, Emerson C, Luster M,
Maciel R, Biscolla R, Mazzaferri E, Medeiros-Neto G, Reiners C,
Robbins R, et al: Expanding indications for recombinant human TSH
in thyroid cancer. Thyroid. 18:687–694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mernagh P, Campbell S, Dietlein M, Luster
M, Mazzaferri E and Weston AR: Cost-effectiveness of using
recombinant human TSH prior to radioiodine ablation for thyroid
cancer, compared with treating patients in a hypothyroid state: the
German perspective. Eur J Endocrinol. 155:405–414. 2006. View Article : Google Scholar
|
|
18
|
Borget I, Corone C, Nocaudie M, Allyn M,
Iacobelli S, Schlumberger M and De Pouvourville G: Sick leave for
follow-up control in thyroid cancer patients: comparison between
stimulation with Thyrogen and thyroid hormone withdrawal. Eur J
Endocrinol. 156:531–538. 2007. View Article : Google Scholar
|
|
19
|
Wang T, Cheung K, Mehta P, Roman S, Walker
H and Sosa J: To stimulate or withdraw? A cost-utility analysis of
recombinant human thyrotropin versus thyroxine withdrawal for
radioiodine ablation in patients with low-risk differentiated
thyroid cancer in the United States. J Clin Endocrin Metab.
96:1672–1680. 2010. View Article : Google Scholar
|
|
20
|
Rosário P, Borges M and Purisch S:
Preparation with recombinant human thyroid-stimulating hormone for
thyroid remnant ablation with 131I is associated with
lowered radiotoxicity. J Nucl Med. 49:1776–1782. 2008.PubMed/NCBI
|
|
21
|
David A, Blotta A, Bondanelli M, Rossi R,
Roti E, Braverman L, Busutti L and Uberti E: Serum thyroglobulin
concentrations and 131I whole-body scan results in
patients with differentiated thyroid carcinoma after administration
of recombinant human thyroid-stimulating hormone. J Nucl Med.
42:1470–1475. 2001.
|
|
22
|
Lippi F, Capezzone M, Angelini F, Taddei
D, Molinaro E, Pinchera A and Pacini F: Radioiodine treatment of
metastatic differentiated thyroid cancer in patients on
L-thyroxine, using recombinant human TSH. Eur J Endoc. 144:5–11.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jarzab B, Handkiewicz-Junak D, Roskosz J,
Puch Z, Wygoda Z, Kukulska A, Jurecka-Lubieniecka B, Hasse-Lazar K,
Turska M and Zajusz A: Recombinant human TSH-aided radioiodine
treatment of advanced differentiated thyroid carcinoma: a
single-centre study of 54 patients. Eur J Nucl Med Molec Imaging.
30:1077–1086. 2003. View Article : Google Scholar
|
|
24
|
Kim TY, Kim WB, Kim ES, Ryu JS, Yeo JS,
Kim SC, Hong SJ and Shong YK: Serum thyroglobulin levels at the
time of 131I remnant ablation just after thyroidectomy
are useful for early prediction of clinical recurrence in low-risk
patients with differentiated thyroid carcinoma. J Clin Endocrin
Metab. 90:1440–1445. 2005.PubMed/NCBI
|
|
25
|
Piccardo A, Arecco F, Morbelli S, Bianchi
P, Barbera F, Finessi M, Corvisieri S, Pestarino E, Foppiani L,
Villavecchia G, Cabria M and Orlandi F: Low thyroglobulin
concentrations after thyroidectomy increase the prognostic value of
undetectable thyroglobulin levels on levo-thyroxine suppressive
treatment in low-risk differentiated thyroid cancer. J Enddocrinol
Invest. 33:83–87. 2010. View Article : Google Scholar
|
|
26
|
Karam M, Feustel PJ, Postal ES, Cheema A
and Goldfarb CR: Successful thyroid tissue ablation as defined by a
negative whole-body scan or an undetectable thyroglobulin: a
comparative study. Nucl Med Commun. 26:331–336. 2005. View Article : Google Scholar : PubMed/NCBI
|