Introduction
The prevalence of gastric cancer is higher in East
Asia, Eastern Europe, and Central and South America compared to
other countries. Worldwide, gastric cancer ranks second among all
causes of mortality from cancer, with approximately 7,000,000
deaths confirmed annually (1). In
Japan, it is one of the most frequent causes of cancer mortality,
despite notable advances in diagnosis and treatment (2). Outcomes are extremely poor in patients
with unresectable gastric cancer, whose median survival time ranges
from 3 to 5 months even with the best supportive care (3,4).
Numerous randomized controlled trials of various treatment regimens
were previously reported, including trials of 5-fluorouracil,
doxorubicin and mitomycin (5),
epirubicin and cis-diamminedichloride platinum (CDDP) in
combination with a continuous infusion of 5-fluorouracil (6), and 5-fluorouracil and CDDP (7). However, the trials documented median
survival times of less than 1 year. Recently, two randomized
controlled trials were reported from Japan (8,9). One
was the Japanese Clinical Oncology Group 9912 trial, which
demonstrated S-1 (an oral fluoropyrimidine) to be superior to the
continuous infusion of 5-fluorouracil with respect to overall
survival (OS). The other was the SPIRITS trial, which identified
CDDP plus S-1 as the standard chemotherapy regimen for advanced or
recurrent gastric cancer in Japan (8).
The treatment of gastric cancer with docetaxel,
either as a monotherapy (10) or in
combination with other agents (11–13),
has shown promising results. In addition, phase I and II studies of
combination therapy with docetaxel plus S-1 have been performed for
patients with advanced or recurrent gastric cancer (14,15).
Recently, phase III studies were reported at the 2011
Gastrointestinal Cancers Symposium co-sponsored by the American
Society of Clinical Oncology (16).
Systemic chemotherapy is the standard choice of
treatment for patients with stage IV gastric cancer. In certain
cases, combination chemotherapy (CDDP or docetaxel plus S-1)
results in long-term survival and, in selected cases, adjuvant
surgery may result in improved long-term survival. Surgical
resection has been classified as curative (no evidence of remaining
disease following surgery; group S). Systemic chemotherapy was
continued for those patients in whom laparoscopy or imaging results
revealed non-curative surgery (group C).
This study therefore aimed to evaluate the efficacy
of surgery or continued chemotherapy in patients with advanced
gastric cancer with peritoneal dissemination, following response to
initial chemotherapy.
Materials and methods
Patients
The study included 13 patients with advanced gastric
cancer with peritoneal dissemination treated between October 2008
and March 2011 at the Hiroshima Red Cross Hospital, Japan.
Initially, all 13 patients were diagnosed with unresectable
advanced gastric cancer. Five patients underwent curative
gastrectomies following the disappearance of peritoneal
dissemination in response to chemotherapy with CDDP plus S-1 or
docetaxel plus S-1 (group S). The remaining 8 patients with
persistent peritoneal dissemination continued to receive systemic
chemotherapy (group C).
Treatment regimen
For the S-1 and CDDP regimen, S-1 (80
mg/m2) was administered orally for 3 weeks, followed by
a drug-free interval of 2 weeks. CDDP (60 mg/m2) was
diluted in 500 ml 0.9% saline and administered as a 2-h infusion on
day 8 after the hydration of each cycle (i.e., every 5 weeks). For
the docetaxel and S-1 regimen, S-1 (80 mg/m2) was
administered orally for 2 weeks, followed by a drug-free interval
of 1 week. Docetaxel (40 mg/m2) was diluted in 100 ml
0.9% saline and administered as a 1-h infusion on day 1 of each
cycle (i.e., every 3 weeks).
Responses were classified on the basis of the
guidelines of the Response Evaluation Criteria in Solid Tumors
(RECIST) (17) and the Japanese
Gastric Cancer Association (18).
To assess responses, the tumor area was measured every 4–6 weeks on
a 5-mm slice computed tomography scan for all measurable lesions.
Toxicity was graded on the basis of the Common Terminology Criteria
for Advanced Events version 3.0 (19). Informed consent was received from
all patients.
Indications for adjuvant surgery
The indications for curative resection were
anticipated on the basis of the response to chemotherapy. Such
indications included the absence of distant metastases, such as
peritoneal dissemination, extensive lymph node metastases or lung
metastases. Surgery was undertaken after laparoscopy confirmed the
disappearance of the nodules of peritoneal dissemination. CDDP plus
S-1, docetaxel plus S-1, or S-1 alone was planned for the 5
patients until recurrence or for 1 year after surgery.
Results
Patient characteristics
The characteristics of patients in groups S and C
are shown in Tables I and II, respectively. The median age of
patients in group S was 61.6 years, while that of patients in group
C was 50.3 years.
 | Table IThe characteristics of patients with
advanced gastric cancer with peritoneal dissemination (group
S). |
Table I
The characteristics of patients with
advanced gastric cancer with peritoneal dissemination (group
S).
| Case no. | Age | Gender | Clinical stage | Regimen before
surgery | No. of cycles | Response | Surgical
curability |
|---|
|
|---|
| T | N | M | P(CY) |
|---|
| 1 | 59 | F | 3 | 2 | 0 | 1 | TXT+S-1 | 2 | SD | R0 |
| 2 | 68 | M | 3 | 2 | 0 | 1 | TXT+S-1 | 5 | SD | R0 |
| 3 | 59 | F | 3 | 1 | 0 | 1 | TXT+S-1 | 10 | PR | R0 |
| 4 | 57 | M | 3 | 1 | 0 | 1 | CDDP+S-1 | 4 | PR | R0 |
| 5 | 65 | M | 4 | 1 | 0 | 1 | CDDP+S-1 | 4 | PR | R0 |
 | Table IIThe characteristics of patients with
advanced gastric cancer with peritoneal dissemination (group
C). |
Table II
The characteristics of patients with
advanced gastric cancer with peritoneal dissemination (group
C).
| Case no. | Age | Gender | Clinical stage | Regimen | No. of cycles on 1st
line | Response on 1st
line | Surgery |
|---|
|
|---|
| T | N | M | P(CY) |
|---|
| 1 | 38 | M | 3 | 1 | 0 | 1 | TXT+S-1 | 3 | SD | No |
| 2 | 57 | M | 3 | 2 | 0 | 1 | TXT+S-1 | 12 | PR | No |
| 3 | 42 | F | 4 | 0 | 0 | 1 | TXT+S-1 | 3 | SD | No |
| 4 | 53 | F | 3 | 2 | 0 | 1 | TXT+S-1 | 7 | PR | No |
| 5 | 41 | M | 3 | 2 | 0 | 1 | CDDP+S-1 | 3 | PD | No |
| 6 | 61 | M | 3 | 2 | 1 | 1 | CDDP+S-1 | 4 | PD | No |
| 7 | 71 | F | 4 | 2 | 0 | 1 | CDDP+S-1 | 1 | SD | No |
| 8 | 39 | M | 3 | 2 | 0 | 1 | CDDP+S-1 | 6 | PR | No |
The 5 cases shown in Table I (group S) underwent curative
resection following the disappearance of peritoneal dissemination
in response to systemic chemotherapy. The median duration of
preoperative chemotherapy was 4.6 months per patient (range, 6–30
weeks). Patients were assessed for their response following
surgery. No cases demonstrated a complete response (CR) or
progressive disease (PD), while 3 (60%) demonstrated a partial
response (PR) and 2 (40%) demonstrated stable disease (SD). One of
the 5 (20%) patients developed pancreatic fistula following
surgery. At a median follow-up of 664 days, the median duration of
OS was 794 days in these patients.
The 8 cases shown in Table II (group C) received systemic
chemotherapy without surgery. The median duration of first-line
chemotherapy was 4.5 months per patient (range, 5–30 weeks),
following which patients were assessed for their response. No case
of CR was observed, while PR was observed in 3 cases (37.5%), SD in
3 (37.5%) and PD in 2 (25.0%). At a median follow-up of 376 days,
the median duration of OS was 505 days in these patients.
Comparison of OS in patients with various
responses
The survival time of the patients is shown in
Fig. 1. The median was 660 days, at
a median follow-up of 524 days. The survival time of patients with
PR did not differ from that of patients with SD or PD (Fig. 2).
Comparison of OS between patients in
groups S and C
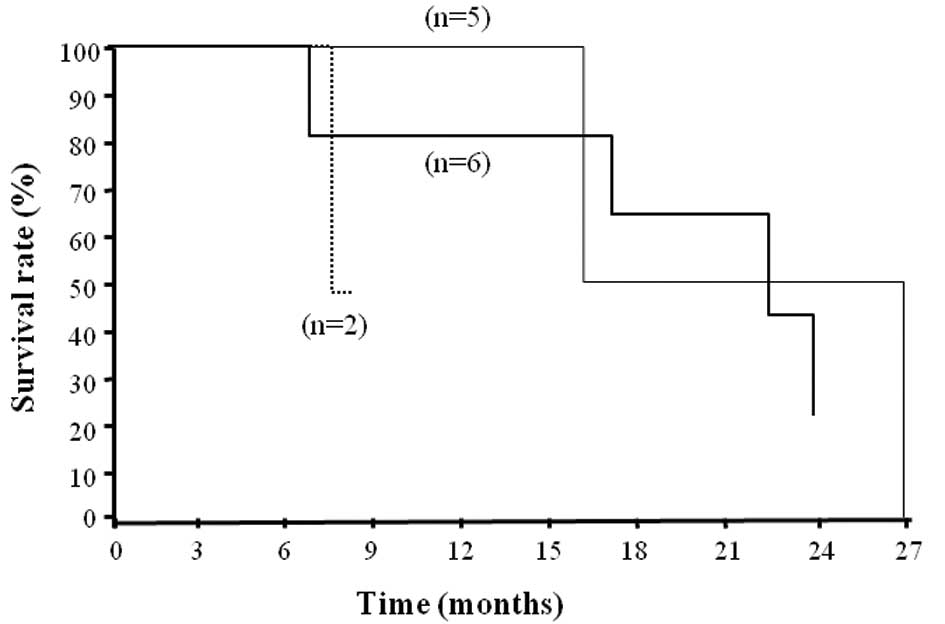
The median survival time of patients in group S was
794 days, which was significantly longer than that in group C (505
days, Fig. 3, p<0.05). One- and
2-year survival was observed in 100 and 60% of patients,
respectively, in group S, and 66.7 and 0% of patients,
respectively, in group C. In group S, relapse-free survival (RFS)
was 294 days (Fig. 4).
Discussion
Various treatment regimens for advanced or recurrent
gastric cancer have been developed (5,6,9) and
have improved patient survival. In Japan, the combination of
docetaxel plus S-1 or CDDP plus S-1 is effective as a first-line
chemotherapy for advanced or recurrent gastric cancer. In the
SPIRITS and STAT studies, the median survival times were reported
to be 13.0 and 12.8 months, respectively, and the median time to
tumor progression was 5.0 and 5.3 months, respectively (9,16).
Although advances in chemotherapy have resulted in improved
long-term survival, a number of patients have required alternative
or adjuvant treatments or a reduction in dose levels due to drug
resistance or adverse effects. The patients were usually
administered another regimen in such cases (second- or
third-line).
Although chemotherapy is the standard treatment for
unresectable advanced gastric cancer, it may prove inadequate in
certain cases. In particular, advanced gastric cancer with
peritoneal dissemination has a poor prognosis. The actual rate of
CR with S-1-based combination chemotherapy has been found to be
relatively low (8,15,20,21).
Peritoneal dissemination is the greatest barrier to an improved
prognosis for advanced gastric cancer. In this study, peritoneal
dissemination disappeared macroscopically in 5 cases following
systemic chemotherapy. However, this disappearance may have been
temporary and macroscopic. Therefore, while systemic chemotherapy
may have been effective at that time, it may have been insufficient
to prevent the recurrence of peritoneal dissemination at a later
date.
Curative surgery should be undertaken after a
reduction in tumor size, and before the appearance of drug
resistance. In this study, the average duration of preoperative
chemotherapy was 4.5 months. It is difficult to evaluate the period
of preoperative chemotherapy due to the small sample size. However,
the duration of preoperative systemic chemotherapy using docetaxel
plus S-1 or CDDP plus S-1 may indicate time to progression for
advanced gastric cancer (5–5.3 months).
In this study, the survival time of patients with
peritoneal dissemination who underwent surgery following
preoperative chemotherapy was significantly longer than that of
patients treated with chemotherapy alone. However, the effects of
surgery with combined chemotherapy such as CDDP plus S-1 or
docetaxel plus S-1 were weaker than expected, with an RFS of only
9.4 months. Recently, Suzuki et al reported that the effect
of adjuvant surgery on stage IV gastric cancer was weaker in cases
with peritoneal dissemination than in cases with lymph node or
liver metastases (22).
The ACTS-GC trial advocated adjuvant chemotherapy
following curative resection of advanced gastric cancer. Therefore,
patients were administered S-1 for 1 year following curative
resection of stage II and III gastric cancer according to the
Japanese Classification. The 5 cases in this study were
administered CDDP plus S-1, docetaxel plus S-1 or S-1 alone
following surgery. However, 2 of these patients were not
administered the combined chemotherapy for more than 3 months. In
the ACTS-GC trial, 66.4% of patients were administered S-1 alone
for 1 year (23). Kodera et
al demonstrated that CDDP plus S-1 was extremely toxic as a
postoperative treatment (24).
Considering these results, it is difficult to prescribe
post-gastrectomy combination chemotherapy for Japanese gastric
cancer patients. To improve the prognosis of gastric cancer with
peritoneal dissemination, strong combined preoperative chemotherapy
(for example, docetaxel and CDDP plus S-1) (25) should be administered rather than
postoperative combination chemotherapy with docetaxel plus S-1 or
CDDP plus S-1.
This study aimed to evaluate the efficacy of
adjuvant surgery following an initial response to systemic
chemotherapy for advanced gastric cancer with peritoneal
dissemination. The OS of patients was prolonged following curative
surgery. To prove the efficacy of adjuvant surgery, a randomized
controlled study with a larger sample of patients is necessary. A
number of obstacles remain to be addressed, including the selection
of combination drugs, the timing of adjuvant surgery, and the
selection of postoperative chemotherapy.
References
|
1
|
Kamanger F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparties in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
2
|
Inoue M and Tsugane S: Epidemiology of
gastric cancer in Japan. Postgrad Med J. 81:419–424. 2005.
View Article : Google Scholar
|
|
3
|
Murad AM, Santiago FF, Petroianu A, Rocha
PR, Rodrigues MA and Rausch M: Modified therapy with
5-fluorouracil, doxorubicin, and methotrexate in advanced gastric
cancer. Cancer. 72:37–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Glinelinus B, Ekstrom K, Hoffman K, Graf
W, Sjoden PO, Haglund U, Svensson C, Enander LK, Linne T, Sellstrom
H and Heuman R: Randomized comparison between chemotherapy plus
best supportive care with best supportive care in advanced gastric
cancer. Ann Oncol. 8:163–168. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
MacDonald JS, Schein PS, Woolley PV,
Smythe T, Ueno W, Hoth D, Smith F, Boiron M, Gisselbrecht C, Brunet
R and Lagarde C: 5-Fluorouracil, doxorubicin, and mitomycin (fam)
combination chemotherapy for advanced gastric cancer. Ann Intern
Med. 93:533–536. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Findlay M, Cuningham D, Norman A, Mansi J,
Nicolson M, Hickish T, Nicolson V, Nash A, Sacks N, Ford H, Carter
R and Hill A: A phase II study in advanced gastro-esophageal cancer
using epirubicin and cisplatin in combination with continuous
infusion 5-fluorouracil (ECF). Ann Oncol. 5:609–616.
1994.PubMed/NCBI
|
|
7
|
Ohtsu A, Shimoda Y, Shirao K, Boku N,
Hyodo I, Saito H, Yamamichi N, Miyata Y, Ikeda N, Yamamoto S,
Fukuda H and Yoshida S: Randomized phase III trial of fluorouracil
alone vs. fluorouracil plus cisplatin vs uracil and tergafur plus
mitomycin in patients with unresectable, advanced gastric cancer:
the Japan Clinical Oncology Study (JCOG9205). J Clin Oncol.
21:54–59. 2003. View Article : Google Scholar
|
|
8
|
Koizumi W, Narahara H, Hara T, Takegane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin vs. S-1 alone for first-line treatment
of advanced gastric cancer (SPIRITS): A phase III trial. Lancet
Oncol. 9:215–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boku N, Yamamoto S, Fukuda H, Shirao K,
Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H, Nasu
J and Ohtsu A: Gastrointestinal Oncology Study Group of the Japan
Clinical Oncology Group: Fluorouracil vs. combination of irinotecan
plus cisplatin vs S-1 in metastatic gastric cancer A randomized
phase 3 study. Lancet Oncol. 10:1063–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Einzig AI, Neuberg D, Remick SC, Karp DD,
O’Dwyer PJ, Stewart JA and Benson AB 3rd: Phase II trial of
docetaxel (taxotere) in patients with adenocarcinoma of the upper
gastrointestinal tract previously untreated with cytotoxic
chemotherapy: the Eastern Cooperative Oncology Group (ECOG) results
of protocol el293. Med Oncol. 13:87–93. 2006. View Article : Google Scholar
|
|
11
|
Thuss-Patience PC, Kretzchmar A and
Reichardt P: Docetaxel in the treatment of gastric cancer. Future
Oncol. 10:603–620. 2009.
|
|
12
|
Fushida S, Fujimura T, Oyama K, Yagi Y,
Kinoshita J and Ohta T: Feasibility and efficacy of preoperative
chemotherapy with docetaxel, cisplatin and S-1 in gastric cancer
with para-aortic lymph node metastases. Anticancer Drug.
20:752–756. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zang DY, Yang DH, Kim MJ, Jang KM, Hwang
SW, Yoo KS, Han T, Kim HY, Kim HJ, Kwon JH, et al: Dose-finding
study of docetaxel, oxaliplatin, and S-1 for patients with advanced
gastric cancer. Cancer Chemother Pharmacol. 64:877–883. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshida K, Hirabayashi N, Takiyama W,
Ninomiya M, Takakura N, Sakamoto J, Nishiyama M and Toge T: Phase I
study of combination therapy with S-1 and docetaxel (TXT) for
advanced or recurrent gastric cancer. Anticancer Res. 24:1843–1851.
2004.PubMed/NCBI
|
|
15
|
Yoshida K, Ninomiya M, Takakura N,
Hirabayashi N, Takiyama W, Sato Y, Toda S, Terashima M, Gotoh M,
Sakamoto J and Nishiyama M: Phase II study of docetaxel and S-1
combination therapy for advanced gastric cancer. Clin Cancer Res.
12:3402–3407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim YH, Koizumi W, Lee KH, Kishimoto T,
Chung HC, Hara T, Cho JY, Nakajima T, Kim H and Fujii M: American
Society of Clininal Oncology – Gastrointestinal Symposium 2011,
Board #A8. J Clin Oncol. 29(Suppl 4): abs. 72011.
|
|
17
|
Eisenhauer EA, Terasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumors:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar
|
|
18
|
Japanese Gastric Cancer Association.
Japanese classification of gastric carcinoma − 3rd English edition.
Gastric Cancer. 14:101–112. 2011.
|
|
19
|
Trotti A, Colevas AD, Setser A, Rusch H,
Jaques D, Budach V, Langer C, Murphy B, Cumberline R, Coleman CN
and Rubin P: CTCAC v3.0: development of a comprehensive grading
system for the adverse effects of cancer treatment. Semin Radiat
Oncol. 13:176–181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lenz HJ, Lee FC, Haller DG, Singh D,
Benson AB 3rd, Strumberg D, Yanagihara R, Yao JC, Phan AT and
Ajiani JA: Extended safety and efficacy data on S-1 plus cisplatin
in patients with untreated, advanced gastric carcinoma in a
multicenter phase II study. Cancer. 109:33–40. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park SR, Kim HK, Kim CG, Choi IJ, Lee JS,
Lee JH, Ryu KW, Kim YW, Bae JM and Kim NK: Phase I/II study of S-1
combined with weekly docetaxel in patients with metastatic gastric
carcinoma. Br J Cancer. 98:1305–1311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki T, Tanabe K, Taomoto J, Yamamoto H,
Tokumoto N, Yoshida K and Ohdan H: Preliminary trial of adjuvant
surgery for advanced gastric cancer. Oncol Lett. 1:743–747.
2010.PubMed/NCBI
|
|
23
|
Sakuramoto S, Sasako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Fukukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: Adjuvant chemotherapy for gastric cancer with
S-1, an oral fluoropyrimidine. N Engl J M. 357:1810–1820. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kodera Y, Ishikawa A, Yoshikawa T,
Kinoshita T, Ito S, Yokoyama H, Michizuki Y, Ito H, Tsuburaya A,
Sakamoto J and Nakao A: A feasibility study of postoperative
chemotherapy with S-1 and cisplatin (CDDP) for gastric carcinoma.
Gastric Cancer. 13:197–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fushida S, Fujimura T, Oyama K, Kinoshita
J, Fujita H, Ninomiya I and Ohta T: Neoadjuvant chemotherapy
combining docetaxel, cisplatin, and S-1 in gastric cancer with
para-aortic lymph node metastases: report of five cases.
Hepatogastroenterol. 57:1650–1654. 2010.PubMed/NCBI
|