Introduction
The concept of extraskeletal myxoid chondrosarcoma
(EMC) was described by Enzinger and Shiraki in 1972 (1). As cartilaginous areas are not common
despite the name ‘EMC’, making a diagnosis based solely on
histopathological findings is often difficult. At present, the
number of reported cytogenetic studies of EMC remains small due to
the rarity of the tumor (2–21). We report an additional case of EMC
and review the literature on cytogenetic studies. This study was
conducted following a clinical research review by our ethics
committee. The patient was informed that data from the case would
be submitted for publication and gave his consent.
Case report
A 41-year-old man presented with more than a 4-month
history of a tumor in the left thigh. No history of disease or
trauma was noted. The size of the tumor was 5×10 cm. The surface
was smooth with no signs of inflammation, but the borders were
unclear. Radiological studies revealed an expansion of the soft
tissue, but no bony changes or calcifications.
Magnetic resonance imaging (MRI) revealed a
low-intensity lesion with T1-weighted imaging and a high-intensity
lesion with T2-weighted imaging (Fig.
1A and B). The tumor exhibited enhancement following
intravenous administration of gadolinium. The inside was
heterogeneous, and the border was ill-defined (Fig. 1C). 67Ga scintigraphy did
not show abnormal uptake. Chest radiography and computed tomography
showed no evidence of lung metastasis. Laboratory findings were
normal.
Open biopsy was performed. Uniform round- to
spindle-like tumor cells proliferated with myxomatous stroma.
Well-formed hyaline cartilage and rhabdoid-like cells were not
observed. The specimen was submitted for cytogenetic analysis.
Karyotype analysis was performed with the G-banded method.
Karyotypes were described using the International System for Human
Cytogenetic Nomenclature (22). The
composite karyotype was
46,XY,t(9;17)(q22;q11),t(9;21)(q21;p13)[16]/46,XY[4] (Fig. 2). The diagnosis of EMC was made
based on the histopathological and cytogenetic findings.
Wide resection of the tumor was performed in July,
2006. The tumor was completely resected along with a portion of the
rectus femoris, tensor fascia latae and the fascia of vastus
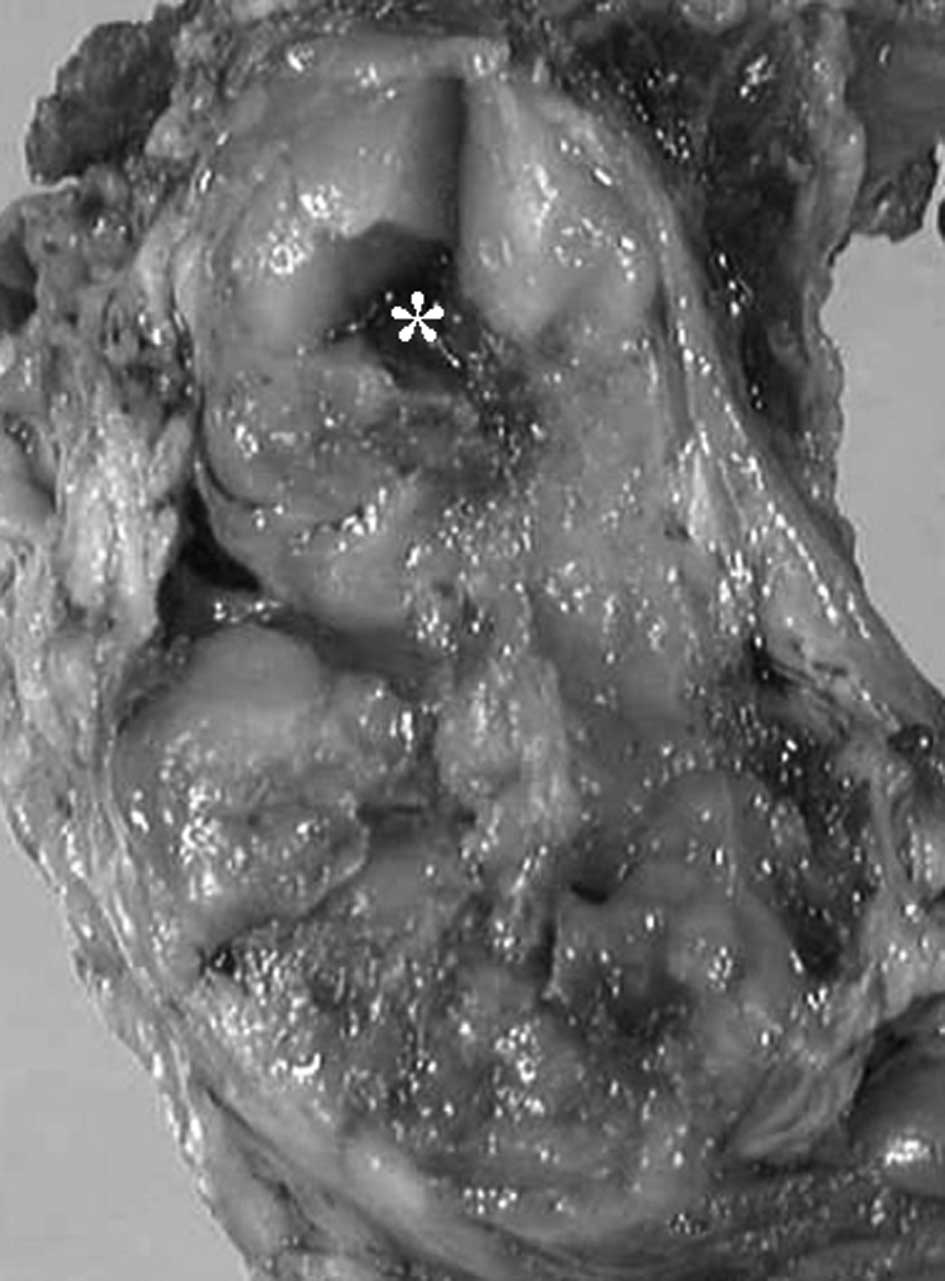
lateralis. The tumor was contained within a pseudocapsule and was
multinodular and yellowish in color (Fig. 3). Histopathologically, the tumor had
a multinodular architecture consisting of myxomatous areas
demarcated by fibrous septa. Each lobule was composed of
proliferating tumor cells and showed a lace-like appearance with an
abundant myxoid matrix (Fig. 4A).
The proliferation of uniform, round tumor cells with oval nuclei
was observed (Fig. 4B). Mitoses
were rare. Immunohistochemically, tumor cells were positive for
vimentin and S-100, and negative for epithelial membrane antigen.
Chemotherapy and radiotherapy were not performed. No masses
recurred during the follow-up period of 4 years and 7 months.
Discussion
EMC is a rare tumor, accounting for less than 3% of
primary soft tissue sarcomas (23)
and most commonly affecting individuals older than 35 years of age
(1). Males are affected
approximately twice as often as females. EMC arises in the deep
soft tissues of proximal extremities. Patients with EMC tumors have
long survival and prolonged follow-up, but EMC leads to a high
disease-associated mortality rate (24,25).
Local recurrence and metastasis each occur in approximately half of
cases. Meis-Kindblom et al (24) reported 5-, 10-, and 15-year survival
rates of 90, 70, and 60%, respectively. Therefore, EMC should be
considered an intermediate-grade rather than a low-grade malignant
tumor, and long follow-up is necessary.
Based on histopathological and immunohistochemical
findings, EMC is difficult to distinguish from myxoid tumors
including myxomas, myxofibrosarcomas (also known as myxoid
malignant fibrous histiocytomas), and myxoid liposarcomas. Myxomas
exhibit a similar paucity of vascular structures, but are less
cellular. Myxofibrosarcomas have a similar fibrous septum and
myxoid stroma but show a broad spectrum of cellularity and
pleomorphism. Most myxofibrosarcomas are negative for S-100. Myxoid
liposarcomas exhibit a marked plexiform vascular pattern and
contain lipoblasts. S-100 positivity is found in approximately 40%
of myxoid liposarcomas (26) and
50% of EMCs (27). Thus, S-100 does
not help distinguish myxoid liposarcomas from EMC. Therefore, we
performed a cytogenetic examination. The karyotype was
t(9;17)(q22;q11), and the diagnosis of EMC was made in this
case.
At present, only 50 cytogenetic cases of EMC,
including our case, have been reported in the English language
literature (2–21). Cytogenetic findings of EMC are
occasionally complex (12%). In addition, a diploid or near-diploid
chromosomal complement has been observed. Recurrent chromosomal
aberrations in EMC include t(9;22)(q22;q12), t(9;17)(q22;q11), and
trisomies 7, 8, 12, 18, 19 and 22. t(9;22)(q22;q12) is the most
specific chromosomal aberration in EMC and was observed in 29 cases
(58%). t(9;22)(q22;q12) results in the fusion of NR4A3,
located at 9q22, to EWSR1, located at 22q12, to form the
abnormal fusion gene EWSR1/NR4A3, which is thought to play a
primary role in the causation and development of EMC (9).
t(9;17)(q22;q11) is less common than
t(9;22)(q22;q12), but is a specific chromosomal aberration in EMC.
Of the 50 cases, t(9;17)(q22;q11) was observed in nine cases (18%).
t(9;17)(q22;q11) results in the fusion of TAF15, located at
17q11, to NR4A3, located at 9q22, to form the abnormal
fusion gene TAF15/NR4A3 (15,19,27).
t(9;22)(q22;q12) and t(9;17)(q22;q11) have not been found in any
other tumors. t(9;15)(q22;q21), which results in the
TCF12/NR4A3 fusion gene (16,27),
and t(3;9)(q12;q22), which results in the TFG/NR4A3 fusion
gene (28), were reported in only
one case each. It is unclear whether the fusion genes identified in
EMC are associated with particular morphological features and
clinical significance.
We reviewed the site, size and prognosis of tumors
with t(9;22)(q22;q12) and t(9;17)(q22;q11). EMC tumors with
t(9;17)(q22;q11) tended to be larger than those with
t(9;22)(q22;q12). However, we did not observe a significant
difference due to the small number of cases. In our case,
t(9;17)(q22;q11) and t(9;21)(q21;p13) were observed.
t(9;21)(q21;p13) has not previously been reported in EMC or any
other tumor. A number of genes located at 9q21, including
ALDH1A1, NTRK2 and GAS1 were identified, but
tumor-related genes located at 21p13 have not yet been
identified.
EMC usually lacks overtly cartilaginous areas.
Therefore, EMC was provisionally classified as a tumor of uncertain
differentiation in the revised version of the World Health
Organization classification of tumors of soft tissue and bone
(29). Although a possible
association between t(9;17)(q22;q11) and neural differentiation has
been suggested (30), the number of
cases examined is insufficient to draw any conclusions.
Comprehensive studies examining the nature of the tumor and
biological properties are required in EMC.
References
|
1
|
Enzinger FM and Shiraki M: Extraskeletal
myxoid chondrosarcoma. An analysis of 34 cases. Hum Pathol.
3:421–435. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hinrichs SH, Jaramillo MA, Gumerlock PH,
Gardner MB, Lewis JP and Freeman AE: Myxoid chondrosarcoma with a
translocation involving chromosomes 9 and 22. Cancer Genet
Cytogenet. 14:219–226. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Turc-Carel C, Dal Cin P, Rao U, Karakousis
C and Sandberg AA: Recurrent breakpoints at 9q31 and 22q12.2 in
extraskeletal myxoid chondrosarcoma. Cancer Genet Cytogenet.
30:145–150. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bridge JA, Sanger WG and Neff JR:
Translocations involving chromosomes 2 and 13 in benign and
malignant cartilaginous neoplasms. Cancer Genet Cytogenet.
38:83–88. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Orndal C, Carlén B, Akerman M, et al:
Chromosomal abnormality t(9;22)(q22;q12) in an extraskeletal myxoid
chondrosarcoma characterized by fine needle aspiration cytology,
electron microscopy, immunohistochemistry and DNA flow cytometry.
Cytopathology. 2:261–270. 1991. View Article : Google Scholar
|
|
6
|
Bridge JA, Bhatia PS, Anderson JR and Neff
JR: Biologic and clinical significance of cytogenetic and molecular
cytogenetic abnormalities in benign and malignant cartilaginous
lesions. Cancer Genet Cytogenet. 69:79–90. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stenman G, Andersson H, Mandahl N,
Meis-Kindblom JM and Kindblom LG: Translocation t(9;22)(q22;q12) is
a primary cytogenetic abnormality in extraskeletal myxoid
chondrosarcoma. Int J Cancer. 62:398–340. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirabayashi Y, Ishida T, Yoshida MA, et
al: Translocation (9;22)(q22;q12). A recurrent chromosome
abnormality in extraskeletal myxoid chondrosarcoma. Cancer Genet
Cytogenet. 81:33–37. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sciot R, Dal Cin P, Fletcher C, et al:
t(9;22)(q22-31;q11-12) is a consistent marker of extraskeletal
myxoid chondrosarcoma: evaluation of three cases. Mod Pathol.
8:765–768. 1995.PubMed/NCBI
|
|
10
|
Rao UN, Surti U, Hoffner L, et al:
Extraskeletal and skeletal myxoid chondrosarcoma: A multiparameter
analysis of three cases including cytogenetic analysis and
fluorescence in situ hybridization. Mol Diagn. 1:99–107. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kilpatrick SE, Inwards CY, Fletcher CD,
Smith MA and Gitelis S: Myxoid chondrosarcoma (chordoid sarcoma) of
bone: a report of two cases and review of the literature. Cancer.
79:1903–1910. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Day SJ, Nelson M, Rosenthal H, Vergara GG
and Bridge JA: Der(16)t(1;16)(q21;q13) as a secondary structural
aberration in yet a third sarcoma, extraskeletal myxoid
chondrosarcoma. Genes Chromosomes Cancer. 20:425–427. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sjögren H, Meis-Kindblom JM, Kindblom LG,
Åman P and Stenman G: Fusion of the EWS-related gene TAF2N to TEC
in extraskeletal myxoid chondrosarcoma. Cancer Res. 59:5064–5067.
1999.PubMed/NCBI
|
|
14
|
Attwooll C, Tariq M, Harris M, Coyne JD,
Telford N and Varley JM: Identification of a novel fusion gene
involving hTAFII68 and CHN from a t(9;17)(q22;q11.2) translocation
in an extraskeletal myxoid chondrosarcoma. Oncogene. 18:7599–7601.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bjerkehagen B, Dietrich C, Reed W, et al:
Extraskeletal myxoid chondrosarcoma: multimodal diagnosis and
identification of a new cytogenetic subgroup characterized by
t(9;17)(q22;q11). Virchows Arch. 435:524–530. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sjögren H, Wedell B, Meis-Kindblom J,
Kindblom LG and Stenman G: Fusion of the NH2-terminal domain of the
basic helix-loop-helix protein TCF12 to TEC in extraskeletal myxoid
chondrosarcoma with translocation t(9;15)(q22;q21). Cancer Res.
60:6832–6835. 2000.PubMed/NCBI
|
|
17
|
Panagopoulos I, Mertens F, Isaksson M, et
al: Molecular genetic characterization of the EWS/CHN and RBP56/CHN
fusion genes in extraskeletal myxoid chondrosarcoma. Genes
Chromosomes Cancer. 35:340–52. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reid R, de Silva MV and Paterson L: Poorly
differentiated extraskeletal myxoid chondrosarcoma with
t(9;22)(q22;q11) translocation presenting initially as a solid
variant devoid of myxoid areas. Int J Surg Pathol. 11:137–141.
2003. View Article : Google Scholar
|
|
19
|
Sjögren H, Meis-Kindblom JM, Örndal C, et
al: Studies on the molecular pathogenesis of extraskeletal myxoid
chondrosarcoma: cytogenetic, molecular genetic, and cDNA microarray
analyses. Am J Pathol. 162:781–792. 2003.PubMed/NCBI
|
|
20
|
Domanski HA, Carlén B, Mertens F and
Akerman M: Extraskeletal myxoid chondrosarcoma with neuroendocrine
differentiation: a case report with fine-needle aspiration biopsy,
histopathology, electron microscopy, and cytogenetics. Ultrastruct
Pathol. 27:363–368. 2003. View Article : Google Scholar
|
|
21
|
Nilsson M, Meza-Zepeda LA, Mertens F,
Forus A, Myklebost O and Mandahl N: Amplification of chromosome 1
sequences in lipomatous tumors and other sarcomas. Int J Cancer.
109:363–369. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shaffer LG and Tommerup N: An
International System for Human Cytogenetic Nomenclature. S Karger;
Basel: 2005
|
|
23
|
Tsuneyoshi M, Enjoji M, Iwasaki H and
Shinohara N: Extraskeletal myxoid chondrosarcoma. A
clinicopathologic and electron microscopic study. Acta Pathol Jpn.
31:439–447. 1981.PubMed/NCBI
|
|
24
|
Meis-Kindblom JM, Bergh P, Gunterberg B
and Kindblom LG: Extraskeletal myxoid chondrosarcoma. A reappraisal
of its morphologic spectrum and prognostic factors based on 117
cases. Am J Surg Pathol. 23:636–650. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saleh G, Evans HL, Ro JY and Ayala AG:
Extraskeletal myxoid chondrosarcoma. A clinicopathologic study of
ten patients with long-term follow-up. Cancer. 702:827–830.
1992.PubMed/NCBI
|
|
26
|
Hashimoto H, Daimaru Y and Enjoji M: S-100
protein distribution in liposarcoma. An immunoperoxidase study with
special reference to the distinction of liposarcoma from myxoid
malignant fibrous histiocytoma. Virchows Arch A Pathol Anat
Histopathol. 405:1–10. 1984. View Article : Google Scholar
|
|
27
|
Okamoto S, Hisaoka M, Ishida T, et al:
Extraskeletal myxoid chondrosarcoma: a clinicopathologic,
immunohistochemical, and molecular analysis of 18 cases. Hum
Pathol. 32:1116–1124. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hisaoka M, Ishida T, Imamura T and
Hashimoto H: TFG is a novel fusion partner of NOR1 in extraskeletal
myxoid chondrosarcoma. Genes Chromosomes Cancer. 40:325–328. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lucas DR and Heim S: Extraskeletal myxoid
chondrosarcoma. WHO Classification of Tumours, Pathology and
Genetics of Tumours of Soft Tissue and Bone. Fletcher CDM, Unni K
and Martens F: IARC Press; Lyon: pp. 213–215. 2002
|
|
30
|
Harris M, Coyne J, Tariq M, et al:
Extraskeletal myxoid chondrosarcoma with neuroendocrine
differentiation: a pathologic, cytogenetic, and molecular study of
a case with a novel translocation t(9;17)(q22;q11.2). Am J Surg
Pathol. 24:1020–1026. 2000. View Article : Google Scholar : PubMed/NCBI
|