Introduction
Malignant pleural mesothelioma (MPM) is an
aggressive malignant tumor of mesothelial origin associated with
asbestos exposure (1–3). The lifetime risk of MPM is associated
with a history of occupational and/or environmental asbestos
exposure (4). Due to the long
latency period (typically over 30 years) between the first asbestos
exposure and the onset of the disease, MPM remains a universally
fatal disease of increasing incidence worldwide (1,2,5),
although asbestos usage has recently decreased in Western countries
and Japan.
Malignant tumor progression requires the destruction
of the basement membrane (BM), which is constructed from
extracellular matrix (ECM) materials. Various human tumor cells are
reported to produce ECM-degrading proteases that are important in
tumor progression (6). Among this
group of proteolytic enzymes, matrix metalloproteinases (MMPs) are
thought to be important due to their wide degrading function. MMPs
are zinc-dependent endopeptidases, whose activities are targeted to
all components of the ECM (7).
MMP-3 is known to be involved in tumor cell invasion
and metastasis (8). The increased
expression of MMP-3 has been reported in several malignant tumors,
including esophageal cancer (9),
breast cancer (10) and glioma
(11). Moreover, a correlation
between a higher MMP-3 expression and disease progression has been
reported in patients with gastric cancer (12), hepatocellular carcinoma (13) and bladder cancer (14). However, the clinical importance of
MMP-3 in MPM patients has not been fully investigated, although
MMP-3 expression has been reported in certain MPM cells (15,16).
In this study, we evaluated the clinical role of the pleural
effusion MMP-3 concentration as a biomarker in MPM.
Materials and methods
Patients and pleural effusion
samples
The MMP-3 levels in pleural effusion samples
collected from 119 individuals presenting at the Department of
Respiratory Medicine of Hyogo College of Medicine between 2005 and
2009 were examined. The pleural effusions were obtained by
thoracocentesis. All cases were diagnosed by pathologists, and it
was confirmed that their clinical course matched their diagnosis.
Fifty-two individuals had MPM involving a documented asbestos
exposure history. These cases were diagnosed by pathologists
skilled in the diagnosis of MPM using histopathological samples.
The patients were classified using the staging system of the
International Mesothelioma Interest Group (IMIG) (17). Patients with MPM were treated
according to our therapeutic guidelines: combination chemotherapy
including the multi-target anti-folate pemetrexed was performed for
patients with performance status (PS) 0–1 who were aged <70, and
the best supportive care was selected for the remaining patients.
Surgical treatment was not performed in any patient in the present
study. Thirty-three individuals, including 8 cases with benign
asbestos pleurisy, had non-malignant pleural effusion. Thirty-four
individuals had lung cancer involving malignant pleural effusion
without asbestos exposure. We verified the asbestos exposure by
interview. The study was approved by our ethics committee in
accordance with the 1975 Declaration of Helsinki. Informed consent
was obtained from all patients. Fresh pleural effusion samples were
collected prior to treatment and centrifuged for 10 min at 2000 × g
at 4°C, and the resultant supernatants were immediately frozen in
liquid nitrogen and stored at −80°C until use.
Measurement of MMP-3
The MMP-3 concentrations of the pleural effusions
were measured using an enzyme-linked immunosorbent assay (ELISA)
kit (R&D Systems, Oxford, UK) according to the manufacturer's
instructions.
Statistical analysis
The non-parametric Mann-Whitney U test was used to
compare three groups of samples. In all tests, p<0.05 was
considered to indicate a statistically significant result. To
estimate the significance of the pleural effusion MMP-3 values,
receiver operating characteristic (ROC) curves, the area under the
ROC curve (AUC) and their 95% confidence intervals (95% CI) were
calculated using standard techniques. To examine the cut-off values
for pleural effusion MMP-3 levels, we calculated the total
sensitivity and specificity for each cut-off value and then
selected the cut-off values that maximized each factor. Estimates
of the probability of survival were calculated by the Kaplan-Meier
method and compared using the log-rank test to evaluate the
prognostic significance of MMP-3 with regard to the survival of
patients with MPM.
Results
MMP-3 pleural effusion levels in MPM and
non-MPM patients
We recruited a total of 119 subjects presenting with
pleural effusion. Of the 119 patients, 52 had confirmed MPM, 33 had
non-malignant pleural effusion, and 34 had lung cancer involving
malignant pleural effusion. Their characteristics are shown in
Table I. Of the 52 patients with
MPM, 40 were of epithelioid histology, 10 sarcomatoid and 2
biphasic.
 | Table ICharacteristics of the MPM and non-MPM
patients. |
Table I
Characteristics of the MPM and non-MPM
patients.
| Patient group | Cases (%) | Total |
|---|
| MPM | | 52 |
| Age, years (mean ±
SD) | 69.1±10.3 | |
| Gender |
| Male | 39 (75.0) | |
| Female | 13 (25.0) | |
| Histology |
| Epithelioid | 40 (76.9) | |
| Sarcomatoid | 10 (19.2) | |
| Biphasic | 2 (3.9) | |
| Stage |
| I | 8 (15.4) | |
| II | 5 (9.6) | |
| III | 8 (15.4) | |
| IV | 31 (59.6) | |
| Non-malignant | | 33 |
| Age, years (mean ±
SD) | 70.6±11.1 | |
| Gender |
| Male | 28 (84.8) | |
| Female | 5 (15.2) | |
| Histology |
| Benign asbestos
pleurisy | 8 (24.1) | |
| Tuberculous (Tb)
pleurisy | 9 (27.3) | |
| Infectious (non-Tb)
pleurisy | 9 (27.3) | |
| Empysema | 2 (6.1) | |
| Heart failure | 2 (6.1) | |
| Hepatic
failure | 1 (3.0) | |
| Renal
failure | 2 (6.1) | |
| Lung cancer | | 34 |
| Age, years (mean ±
SD) | 67.6±11.1 | |
| Gender |
| Male | 23 (67.6) | |
| Female | 11 (32.4) | |
| Histology |
|
Adenocarcinoma | 31 (91.2) | |
| Squamous cell
carcinoma | 2 (5.9) | |
| Small cell
carcinoma | 1 (2.9) | |
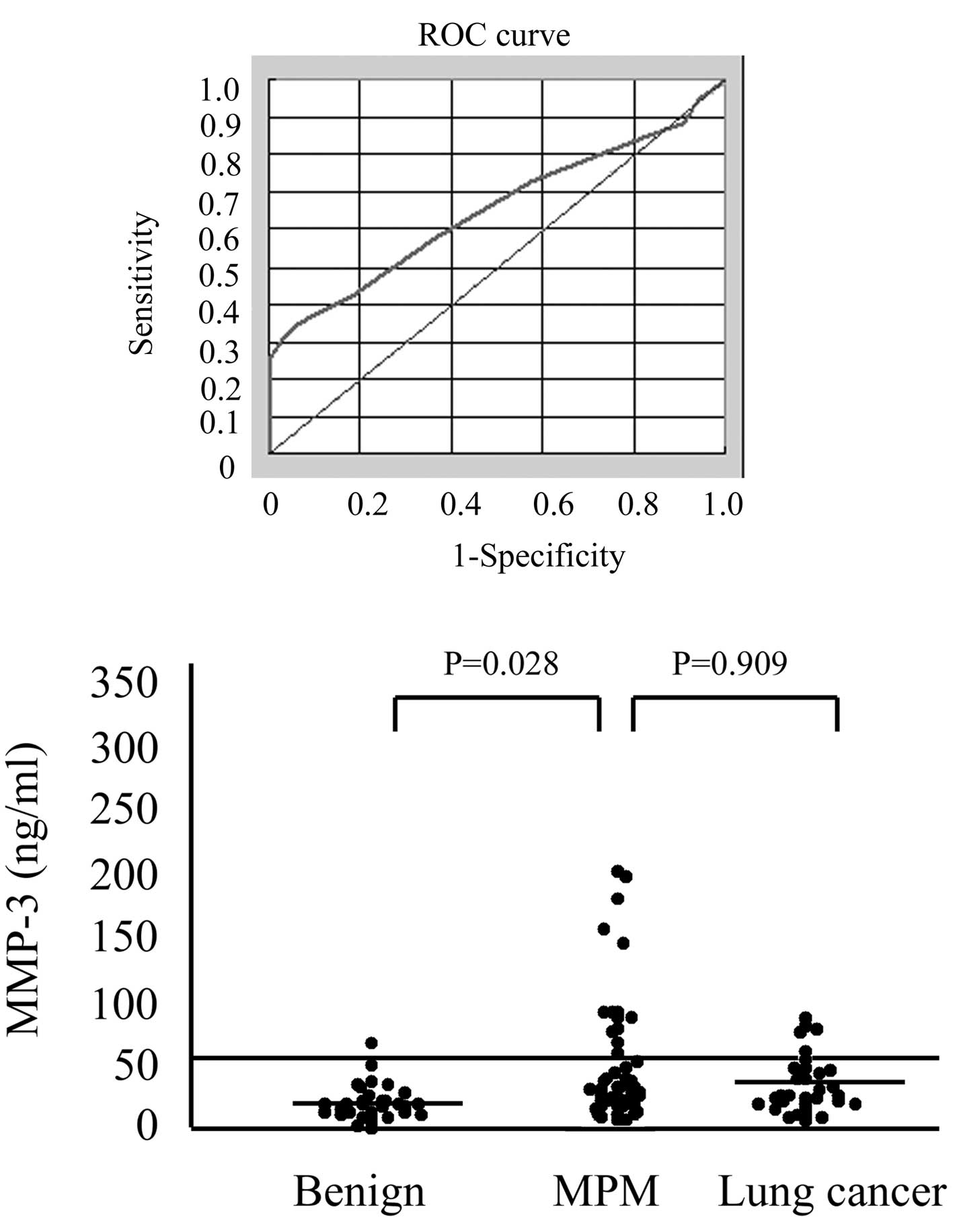
The ROC curves for pleural effusion MMP-3 levels
(Fig. 1A) reveal that the patients
with MPM had an AUC of 0.651 in comparison with those with
non-malignant pleural effusion (95% CI 0.555–0.747). At the optimal
cut-off value of 50 ng/ml, the diagnostic sensitivity was 30.8% and
the specificity was 97.0%. The mean pleural effusion MMP-3
concentration of the patients with MPM was significantly higher
(49.1±59.1 ng/ml) than that of the patients with non-malignant
pleural effusion (19.8±13.8 ng/ml; p=0.028; Fig. 1B). Although the mean pleural
effusion MMP-3 concentrations of the patients with MPM was higher
than that of the patients with lung cancer involving malignant
pleural effusion (33.7±22.4 ng/ml), no statistically significant
difference was found between them (p=0.909; Fig. 1B). No statistically significant
differences were observed between the pleural effusion MMP-3 levels
of the MPM histological groups (epithelioid, 54.2±62.9 ng/ml;
non-epithelioid, 32.1±45.5 ng/ml) or the different disease stages
(stage I, 33.1±30.2 ng/ml; stage II, 34.0±32.2 ng/ml; stage III,
84.6±120.3 ng/ml; and stage IV, 49.9±58.8 ng/ml) and there were no
significant differences between the pleural effusion MMP-3 levels
of the subjects with benign asbestos pleurisy and those with benign
pleurisy without a history of asbestos exposure (14.4±9.0 ng/ml and
20.3±14.2 ng/ml, respectively).
Correlation between pleural effusion
MMP-3 levels and overall survival
Among the 52 MPM patients, we were able to follow 47
patients closely for up to 1,700 days. Twenty patients had died by
the end of the follow-up. Five patients were lost due to lack of
information following transfer to other hospitals. These 5 subjects
were homogenously distributed in the two groups compared (3
subjects were >50 ng/ml and 2 subjects were ≤50 ng/ml).
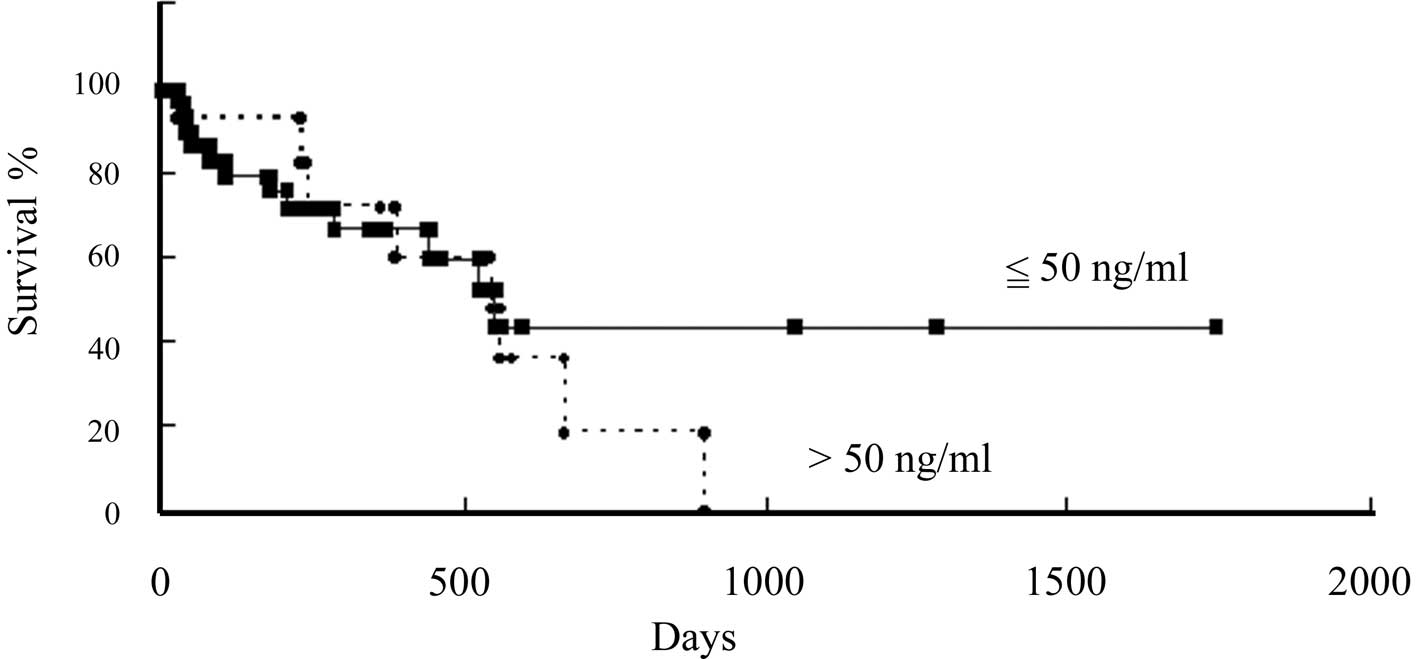
To study the correlation between pleural effusion
MMP-3 levels and patient clinical courses, we separated the
patients according to their pleural effusion MMP-3 levels at the
time of the first measurement. The first group included patients
with pleural effusion MMP-3 levels lower than 50 ng/ml, the cut-off
value that we selected. In this group of 34 patients, the mean
MMP-3 value was 18.2 ng/ml (interquartile range, 10.5–26.2). The
other group included the remaining 13 patients, who had pleural
effusion MMP-3 levels higher than 50 ng/ml and whose mean MMP-3
pleural effusion concentration was 109.5 ng/ml (interquartile
range, 71.8–139.1). The difference in overall survival between the
groups with lower and higher pleural effusion MMP-3 concentrations
than the cut-off point of 50 ng/ml was not statistically
significant (p=0.51; Fig. 2).
However, there was a tendency for the survival of the patients with
lower pleural effusion MMP-3 levels to be longer than that of the
patients with higher pleural effusion MMP-3 levels.
Discussion
MPM shows a limited response to conventional
chemotherapy and radiotherapy. Although the multi-target
anti-folate pemetrexed has recently been approved as a first-line
agent for use in combination with cisplatin for the treatment of
MPM, survival of patients remains extremely poor (18), with a median survival duration of
8–18 months (19). Although in
advanced cases, resection of the tumor only prolongs survival by
approximately 3 months, it has been reported that patients with
stage IA disease survive for five or more years following total
resection of the tumor (20).
Moreover, in several centers, potentially curative surgery combined
with some form of adjuvant therapy has been performed. Early
diagnosis may provide an opportunity for early treatment using new
treatment regimens, although whether early intervention results in
good prognosis has yet be confirmed.
Due to the difficulty of diagnosing MPM by
radiological and/or histological examinations, efficient and
practical pleural effusion biomarkers are required to aid the
diagnosis of MPM. Several cytokines, including interleukin (IL)-6
(21), transforming growth factor
(TGF)-β1 (22–24), platelet-derived growth factor (PDGF)
(25), TGF-α (26), and IL-8 (27), are significant in the development of
MPM. Pleural effusion biomarkers for MPM, including hyaluronic acid
and CYFRA 21–1, have also been reported and used to assist the
diagnosis of MPM (20,28–32).
The level of mesothelin-related protein (SMRP), the soluble form of
mesothelin, has been reported to be a useful pleural effusion
marker in MPM (33). However,
little is known about their biological functions or effects on MPM
cells.
A number of studies have focused on the expression
of MMPs, including MMP-2 and MMP-9 in MPM cells, or in patients
with MPM or lung cancer (34,35).
In the present study, we examined the serum and pleural effusion
MMP-2 levels of MPM patients and found no significant differences
between samples from patients with MPM and those from non-MPM
patients (data not shown). MMP-2 and MMP-9, which are also known as
gelatinase A and B, respectively, cleave type IV collagen. However,
MMP-3 degrades several components of the ECM, including
fibronectin, laminin and collagen type IV (36). MMP-3 is also involved in tumor cell
invasion and metastasis (8).
In this study, we evaluated the clinical role of the
pleural effusion MMP-3 concentration as a biomarker in MPM, and
demonstrated that the pleural effusion MMP-3 concentrations of
patients with MPM were significantly higher than those of patients
with non-malignant pleural effusion. At the optimal cut-off value
of 50 ng/ml, the diagnostic sensitivity of the MMP-3 pleural
effusion concentration was low (30.8%), and its negative predictive
value (non-MPM patients/all patients with pleural effusion MMP-3
levels of <50 ng/ml) was not high (47.1%), suggesting that the
pleural effusion MMP-3 concentration cannot be used to select MPM
patients from individuals with lower pleural effusion MMP-3 levels.
However, its specificity was high (97.0%) and its positive
predictive value of 94.1% (MPM patients/all patients with pleural
effusion MMP-3 levels of >50 ng/ml), suggests that the MMP-3
concentration could be used to differentiate MPM patients from
patients with higher MMP-3 pleural effusion levels. Although the
difference in overall survival between the groups with lower and
higher pleural effusion MMP-3 values than the cut-off point (50
ng/ml) was not statistically significant, the survival of patients
with higher pleural effusion MMP-3 levels showed a tendency to be
shorter than that of the patients with lower pleural effusion MMP-3
levels. This observation is compatible with the previous studies
demonstrating a correlation between higher MMP-3 expression and
disease progression in patients with certain malignancies (12–14).
It is well known that MPM patients with higher stage and/or
non-epithelioid tumor have a poor prognosis. In the present study,
however, there were no significant differences in pleural effusion
MMP-3 levels among disease stages as well as histological types
(epithelioid versus non-epithelioid). We consider that the
prognostic impact of pleural effusion MMP-3 levels needs further
evaluation. From these findings, patients with high pleural
effusion MMP-3 levels may be suspected of having MPM and a poor
prognosis.
We evaluated the clinical role of the pleural
effusion MMP-3 concentration as a biomarker in MPM, and
demonstrated that patients with MPM had significantly higher
pleural effusion MMP-3 levels than a population with non-malignant
pleuritis involving benign asbestos pleurisy, suggesting MMP-3 to
be a useful diagnostic marker of MPM. The Kaplan-Meier method
revealed that the survival of the patients with higher pleural
effusion MMP-3 levels showed a tendency to be shorter than that of
the patients with lower pleural effusion MMP-3 levels, indicating
the usefulness of MMP-3 as a prognostic marker.
Acknowledgements
We thank Ms. Hidemi Kitai for providing technical
assistance. This study was supported by grants from KAKENHI, a
Grant-in-Aid for Scientific Research (C) (20590936), Funds for
Cancer Research from the Hyogo Prefecture Health Promotion
Association and Special Coordination Funds for Promoting Science
and Technology (H18-1-3-3-1).
Abbreviations:
|
AUC
|
area under the ROC curve
|
|
BM
|
basement membrane
|
|
CI
|
confidence interval
|
|
ECM
|
extracellular matrix
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
IL
|
interleukin
|
|
MMP
|
matrix metalloproteinase
|
|
MPM
|
malignant pleural mesothelioma
|
|
PDGF
|
platelet-derived growth factor
|
|
ROC
|
receiver operating characteristic
|
|
TGF
|
transforming growth factor
|
References
|
1
|
Robinson BW, Musk AW and Lake RA:
Malignant mesothelioma. Lancet. 366:397–408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robinson BW and Lake RA: Advances in
malignant mesothelioma. N Engl J Med. 353:1591–1603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wagner JC, Sleggs CA and Marchand P:
Diffuse pleural mesothelioma and asbestos exposure in the North;
Western Cape Province. Br J Ind Med. 17:260–271. 1960.PubMed/NCBI
|
|
4
|
Rake C, Gilham C, Hatch J, et al:
Occupational, domestic and environmental mesothelioma risks in the
British population: a case-control study. Br J Cancer.
100:1175–1183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Selikoff IJ, Hammond EC and Seidman H:
Latency of asbestos disease among insulation workers in the United
States and Canada. Cancer. 15:2736–2740. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liotta LA and Stetler-Stevenson WG:
Metalloproteinases and cancer invasion. Semin Cancer Biol.
1:99–106. 1990.PubMed/NCBI
|
|
7
|
Johnson LL, Dyer R and Hupe DJ: Matrix
metalloproteinase. Curr Opin Chem Biol. 2:466–471. 1998. View Article : Google Scholar
|
|
8
|
Kleiner DE and Stetler-Stevenson WG:
Structural biochemistry and activation of matrix metalloproteases.
Curr Opin Cell Biol. 5:891–897. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mukherjee S, Roth MJ, Dawsey SM, et al:
Increased matrix metalloproteinase activation in esophageal
squamous cell carcinoma. J Transl Med. 8:912010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Phromnoi K, Yodkeeree S, Anuchapreeda S,
et al: Inhibition of MMP-3 activity and invasion of the MDA-MB-231
human invasive breast carcinoma cell line by bioflavonoids. Acta
Pharmacol Sin. 30:1169–1176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee EJ, Kim SY, Hyun JW, et al: Glycitein
inhibits glioma cell invasion through down-regulation of MMP-3 and
MMP-9 gene expression. Chem Biol Interact. 185:18–24. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yeh YC, Sheu BS, Cheng HC, et al: Elevated
serum matrix metalloproteinase-3 and -7 in H. pylori-related
gastric cancer can be biomarkers correlating with a poor survival.
Dig Dis Sci. 55:1649–1657. 2010.PubMed/NCBI
|
|
13
|
Okamoto K, Ishida C, Ikebuchi Y, et al:
The genotypes of IL-1 beta and MMP-3 are associated with the
prognosis of HCV-related hepatocellular carcinoma. Intern Med.
49:887–895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Srivastava P, Mandhani A, Kapoor R, et al:
Role of MMP-3 and MMP-9 and their haplotypes in risk of bladder
cancer in North Indian cohort. Ann Surg Oncol. 17:3068–3075. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harvey P, Clark IM, Jaurand MC, et al:
Hepatocyte growth factor/scatter factor enhances the invasion of
mesothelioma cell lines and the expression of matrix
metalloproteinases. Br J Cancer. 83:1147–1153. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Z, Ivanoff A and Klominek J:
Expression and activity of matrix metalloproteases in human
malignant mesothelioma cell lines. Int J Cancer. 91:638–643. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rusch VW: A proposed new international TNM
staging system for malignant pleural mesothelioma. From the
International Mesothelioma Interest Group. Chest. 108:1122–1128.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
et al: Phase III study of pemetrexed in combination with cisplatin
versus cisplatin alone in patients with malignant pleural
mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nowak AK, Lake RA, Kindler HL, et al: New
approaches for mesothelioma: biologics, vaccines, gene therapy, and
other novel agents. Semin Oncol. 29:82–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pass HI, Lott D, Lonardo F, et al:
Asbestos exposure, pleural mesothelioma, and serum osteopontin
levels. N Engl J Med. 353:1564–1573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adachi Y, Aoki C, Yoshio-Hoshino N, et al:
Interleukin-6 induces both cell growth and VEGF production in
malignant mesotheliomas. Int J Cancer. 119:1303–1311. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tabata C, Tabata R, Hirayama N, et al:
All-trans-retinoic acid inhibits tumor growth of malignant pleural
mesothelioma in mice. Eur Respir J. 34:1159–1167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fitzpatrick DR, Bielefeldt-Ohmann H,
Himbeck RP, et al: Transforming growth factor-beta: antisense
RNA-mediated inhibition affects anchorage-independent growth,
tumorigenicity and tumor-infiltrating T-cells in malignant
mesothelioma. Growth Factors. 11:29–44. 1994. View Article : Google Scholar
|
|
24
|
Marzo AL, Fitzpatrick DR, Robinson BW, et
al: Antisense oligonucleotides specific for transforming growth
factor beta2 inhibit the growth of malignant mesothelioma both in
vitro and in vivo. Cancer Res. 57:3200–3207. 1997.PubMed/NCBI
|
|
25
|
Versnel MA, Claesson-Welsh L, Hammacher A,
et al: Human malignant mesothelioma cell lines express PDGF
beta-receptors whereas cultured normal mesothelial cells express
predominantly PDGF alpha-receptors. Oncogene. 6:2005–2011.
1991.
|
|
26
|
Mórocz IA, Schmitter D, Lauber B, et al:
Autocrine stimulation of a human lung mesothelioma cell line is
mediated through the transforming growth factor alpha/epidermal
growth factor receptor mitogenic pathway. Br J Cancer. 70:850–856.
1994.
|
|
27
|
Galffy G, Mohammed KA, Dowling PA, et al:
Interleukin 8: an autocrine growth factor for malignant
mesothelioma. Cancer Res. 59:367–371. 1999.PubMed/NCBI
|
|
28
|
Fuhrman C, Duche JC, Chouaid C, et al: Use
of tumor markers for differential diagnosis of mesothelioma and
secondary pleural malignancies. Clin Biochem. 33:405–410. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Paganuzzi M, Onetto M, Marroni P, et al:
Diagnostic value of CYFRA 21-1 tumor marker and CEA in pleural
effusion due to mesothelioma. Chest. 119:1138–1142. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Robinson BW, Creaney J, Lake R, et al:
Mesothelin-family proteins and diagnosis of mesothelioma. Lancet.
362:1612–1616. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Frebourg T, Lerebours G, Delpech B, et al:
Serum hyaluronate in malignant pleural mesothelioma. Cancer.
59:2104–2107. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schouwink H, Korse CM, Bonfrer JM, et al:
Prognostic value of the serum tumour markers Cyfra 21-1 and tissue
polypeptide antigen in malignant mesothelioma. Lung Cancer.
25:25–32. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Creaney J, Yeoman D, Naumoff LK, et al:
Soluble mesothelin in effusions: a useful tool for the diagnosis of
malignant mesothelioma. Thorax. 62:569–576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Edwards JG, McLaren J, Jones JL, et al:
Matrix metalloproteinases 2 and 9 (gelatinases A and B) expression
in malignant mesothelioma and benign pleura. Br J Cancer.
88:1553–1559. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roomi MW, Monterrey JC, Kalinovsky T, et
al: Modulation of MMP-2 and MMP-9 by cytokines, mitogens and
inhibitors in lung cancer and malignant mesothelioma cell lines.
Oncol Rep. 22:1283–1291. 2009.PubMed/NCBI
|
|
36
|
Murphy GJ, Murphy G and Reynolds JJ: The
origin matrix metalloproteinases and their familial relationships.
FEBS Lett. 289:4–7. 1991. View Article : Google Scholar : PubMed/NCBI
|