Introduction
Renal cell carcinoma (RCC), the most lethal type of
the urological cancers, accounts for 3% of all adult malignancies
(1). There are four main
histological subtypes of RCC: clear cell (CCRCC), papillary (PRCC;
divided into two groups: type I and type II) and chromophobe
(CHRCC) carcinoma. The great majority of RCC cases are clear cell
carcinoma, which is characterized by the bi-allelic inactivation of
the von Hippel-Lindau (VHL) gene (2). This genetic event leads to the
stabilization of the α-subunit of hypoxia-inducible factor 1
(HIF-1). HIF-1 is a key transcriptional regulator that promotes
cell growth and survival under hypoxic conditions. Its activation
subsequently leads to the overexpression of multiple genes
responsible for tumor angiogenesis, cell migration, glucose
transport, glycolysis and pH control. Among the genes, carbonic
anhydrase IX (CA9) is one of the most prominent markers of
tumor hypoxia mainly due to the unique localization of its hypoxia
response element at the -10/-3 position from the transcription
start site of the CA9 gene (3).
CA IX protein, as a member of the carbonic anhydrase
family, catalyzes the reversible conversion of carbon dioxide to
bicarbonate and protons and is thus involved in ion transport and
pH control (4). CA IX is a
transmembrane protein which is ectopically expressed in various
types of human cancer (e.g., cervical, lung, breast, and head and
neck). Moreover, tumors with a high CA IX expression exhibit a more
aggressive phenotype and have a poor prognosis. Paradoxically, CA
IX overexpression in CCRCC appears to be an early event and is
associated with better prognosis (5). The clinical and prognostic
significance of CA IX in RCC have been extensively evaluated. CA IX
is regarded as one of the most promising biomarkers in clear cell
RCC as it has a conclusive diagnostic, prognostic, as well as
therapeutic potential. In this study, we utilized all available
clinical material from 74 kidney cancer patients with a focus on
determining the status of CA IX. For this purpose, we investigated
the expression of CA IX in tumor tissue samples using Western
blotting (WB), ELISA and immunohistochemistry. Moreover, we
performed two different blood-based assays: RT-PCR for CA9
gene expression in circulating tumor cells (CTCs) and ELISA for CA
IX protein quantification in serum samples.
Materials and methods
Study subjects
Between March 2009 and May 2011, 74 patients with
renal tumors were treated at the Department of Urology, Derer’s
University Hospital Bratislava, Slovakia. Seventy patients
underwent partial or radical nephrectomy for malignant renal tumors
and four patients for benign renal tumors (three were oncocytomas
and one was angiomyolipoma). Of the 70 RCC patients, 57 (81.4%) had
conventional, 10 (14.3%) papillary, and 3 (4.3%) chromophobe
histological features. Patient ages ranged from 29 to 80 years,
with a mean of 59.83 years. Patients included 47 males and 27
females. Participants of this study were informed, and oral consent
was obtained from each patient.
Collection of samples
Blood samples were collected prior to surgery.
Immediately after collection, 200 μl of peripheral blood
were rapidly agitated with 800 μl of InstaPure reagent
(Eurogentec, Seraing, Belgium) and processed for RNA extraction
with 175 μl chloroform. Following centrifugation, the
aqueous phase was transferred to a fresh tube and an equal volume
of isopropanol was added and mixed. Precipitated pellets of RNA
were washed firstly with 70% ethanol and then absolute ethanol. At
the end of the procedure, RNA pellets were dried and dissolved in
10 μl of DEPC-treated water. For serum isolation, the blood
tube was centrifuged at 2500 × g for 15 min at 4°C and the serum
samples were stored at −20°C prior to use. Tissue samples were
obtained from surgical nephrectomy specimens and processed within 1
h following surgical resection. Portions of the fresh tissues from
RCCs and normal renal tissues were frozen (in liquid nitrogen) and
stored at −80°C.
RT-PCR
Reverse transcription of isolated RNA dissolved in
10 μl DEPC-treated water was performed using a High Capacity
cDNA Reverse Transcription Kit (AB Applied Biosystems, Foster City,
CA, USA). RT-PCR was carried out with 0.5 μl from a total of
20 μl of cDNA with CA9-specific primers and primers
for β-actin that served as an internal standard. The primers
were as follows (S, sense; A, antisense): CA9S
5′-TATCTGCACTCCTGCCCTCTG-3′ and CA9A
5′-CACAGGGTGTCAGAGAGGGTGT-3′ (154 bp product); β-actinS
5′-CCAACCGCGAGAAGATGACC-3′ and β-actinA
5′-GATCTTCATGAGGTAGTCAGT-3′ (236 bp product). RT-PCR was carried
out with DreamTaq™ Green PCR Master mix (Fermentas, St.
Leon-Rot, Germany). Following the initial denaturation at 94°C for
3 min, the amplification program was set as follows: denaturation
at 94°C for 30 sec, annealing at 60/65°C
(β-actin/CA9) for 30 sec, and extension at 72°C
during 30 sec for a total of 30/35 cycles
(β-actin/CA9), and final extension at 72°C for 7 min.
Positive RT-PCR control was provided by cDNA from HeLa cells. The
PCR reactions were repeated three times.
Western blotting
For Western blotting analysis, frozen tissue
specimens were homogenized in ice-cold lysis buffer (1% Triton
X-100; 150 mM NaCl; 50 mM Tris, pH 7.5; 0.5% Nonidet P-40; 50mM
NaF) containing inhibitors of proteases (Roche Applied Science,
Mannheim, Germany). The protein concentration was determined using
the BCA protein assay reagent (Pierce, Rockford, IL, USA). Total
protein extracts (100 μg/lane) were separated in 10%
SDS-PAGE and transferred onto PVDF membranes
(Immobilon™-P, Millipore, Billerica, MA, USA). For CA IX
detection, the membrane was incubated with M75 primary antibody
(6) and diluted 1:2 in blocking
buffer for 1 h. Secondary anti-mouse peroxidase-conjugated antibody
(Dako, Glostrup, Denmark) was diluted 1:5000 in blocking buffer. As
a loading control, the membrane was probed with anti-actin antibody
(Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and the
polyclonal rabbit anti-goat IgG-HRP (Dako). Following treatment,
the membrane was washed and developed by enhanced chemiluminescence
using an ECL kit (Amersham Pharmacia Biotech, Buckinghamshire,
UK).
ELISA
Serum samples were initially analyzed with
commercially available ELISA DuoSet® Human Carbonic
Anhydrase IX (R&D Systems, Inc., Minneapolis, MN, USA)
according to the manufacturer’s instructions. Quantification was
performed with V/10 antibody (7)
which replaced capture antibody in the ELISA kit supplied by
R&D. Samples were assayed in duplicate and repeated three
times. For CA IX tissue quantification, sandwich ELISA with V/10 as
a capture antibody was performed. The same protein lysates as in
Western blotting were used and the final CA IX concentration was
calculated from 100 μg of total proteins.
Immunohistochemistry
Dissected tissues were embedded in paraffin
according to the standard histological procedure.
Immunohistochemistry was performed on an automated immunostainer
(Dako Autostainer) using DakoCytomation EnVision®+
System-HRP (DAB) according to the manufacturer’s instructions.
Sections (4 μm) were incubated with M75 antibody (hybridoma
medium diluted 1:100) for 1 h at room temperature. Staining was
visualized with DAB solution for 1 min with 3,3′-diaminobenzidine
as a chromogenic substrate. Finally, the sections were
counterstained with Mayer’s hematoxylin and mounted in Aquamount
(Merck, Darmstadt, Germany). The stained sections were examined
using a Leica DM4500B microscope and images were captured by a
Leica DFC480 camera. CA IX is a membrane-associated antigen and
thus, cells exhibiting clear and sharp membrane staining were
interpreted as CA IX-positive. The percentage of CA IX-stained
pixels was evaluated on the entire tissue section with ImageJ 1.38x
software (Rasband, W.S., ImageJ, U.S. National Institutes of
Health, Bethesda, MD, USA, http://rsb.info.nih.gov/ij/, 1997–2007). ImageJ is
able to create density histograms and calculate area and pixel
value statistics of user defined selections. The proportion as well
as intensity of CA IX-stained pixels was calculated as an average
from 10 images captured from each tissue sample. Specimens that had
no evidence of specific immunostaining were marked as negative.
Statistical analysis
Results were analyzed by the two-tailed unpaired t
test (Student’s t-test). P<0.05 was considered statistically
significant. The correlation between different variables was
examined using the Pearson’s correlation coefficient.
Results
Study subjects
A total of 74 patients were included in this study
(Table I). CA IX expression was
evaluated in blood samples using RT-PCR and ELISA as well as in
tissue specimens using WB and immunohistochemistry.
 | Table IPatient and tumor
characteristics. |
Table I
Patient and tumor
characteristics.
|
Characteristics | No. |
|---|
| Patient (No.) | 74 |
| Male | 47 |
| Female | 27 |
| Mean age
(years) | 59.83 |
| Male | 58.92 |
| Female | 60.74 |
| Histological
subtype (No.) | |
| Clear cell
RCC | 57 |
| Papillary
RCC | 10 |
| Chromophobe
RCC | 3 |
| Benign tumor | 4 |
| T classification
(in CCRCC) | |
| T1 | 28 |
| T2 | 5 |
| T3 | 24 |
| T4 | 0 |
CA IX as a biomarker in blood-based
assays
RT-PCR for CA9 detection was performed on
blood samples obtained from 74 patients. Cells expressing
CA9 were detected in 24 of 74 (32.43%) kidney cancer
patients. The CA9-positive samples were obtained from
patients with malignant renal tumors: 18 (75%) were diagnosed with
CCRCC, 4 (16.67%) with PRCC and 2 (8.33%) with CHRCC. Four patients
with benign tumors (BTs) were CA9-negative. No blood samples
from the healthy controls were found to be positive for CA9
(data not shown) and thus, the specificity was 100% in our
study.
From 57 CCRCC patients, 18 (32%) were found to be
positive for CA9 expression in the peripheral blood.
Decreased positivity was observed with the higher tumor stage
(Table II). Patients with stage T3
exhibited CA9-positive CTCs in 17% of the cases compared
with 50% for the patients with T1 disease. The small number of
specimens in stage T2 limited any statistical evaluation.
 | Table IIRT-PCR results of CA9
expression in circulating tumor cells according to the histological
subtype of RCC and tumor stage of CCRCC patients. |
Table II
RT-PCR results of CA9
expression in circulating tumor cells according to the histological
subtype of RCC and tumor stage of CCRCC patients.
| Subtype |
CA9-positive |
CA9-negative |
|---|
| CCRCC | 18 | 39 |
| T1 | 14 | 14 |
| T2 | 0 | 5 |
| T3 | 4 | 20 |
| T4 | 0 | 0 |
| PRCC | 4 | 6 |
| CHRCC | 2 | 1 |
| BT | 0 | 4 |
Soluble forms of CA IX (s-CA IX) shed from renal
tumors were initially determined by commercially available ELISA
(DuoSet® Human Carbonic Anhydrase IX, R&D Systems).
Although a significant association between serum CA IX and the
histological subtype of RCC was observed, the association with
tumor stage did not reach statistical significance (Table III). Therefore, the following
evaluation was performed with V/10 as a capture antibody. The s-CA
IX levels in serum samples from CCRCC patients were found to be
significantly higher compared to samples in the non-CCRCC (p=0.002)
and benign tumors (p=0.002) (Table
III). The mean level of s-CA IX was 209.22 pg/ml in 57 CCRCC
patients, 28.78 pg/ml in 13 non-CCRCC (PRCC and CHRCC) patients and
21.84 pg/ml in 4 BTs. Stratification of the CCRCC patients
according to the tumor stage revealed a significant association
between s-CA IX and tumor stage (p=0.046) with the mean value for
stage T1 and T3 87.51 pg/ml and 341.98 pg/ml, respectively.
 | Table IIIRelationship between CA IX levels
(measured by ELISA in serum and tissue) and clinicopathological
variables. |
Table III
Relationship between CA IX levels
(measured by ELISA in serum and tissue) and clinicopathological
variables.
| Serum CA IX (pg/ml)
V/10 | Serum CA IX (pg/ml)
R&D | Tissue CA IX
(pg/100 μg of total proteins) |
|---|
| CCRCC (all
stages) | 209.22 | 308.53 | 6057.83 |
| CCRCC stage T1 | 87.51 | 269.67 | 8278.05 |
| CCRCC stage T3 | 341.98 | 312.39 | 4478.82 |
| Non-CCRCC | 28.78 | 73.49 | 172.82 |
| BT | 21.84 | 18.94 | 20.79 |
| p-value | CCRCC vs.
non-CCRCC, 0.002
CCRCC vs. BT, 0.002
T1 vs. T3 CCRCC, 0.046 | CCRCC vs.
non-CCRCC, 0.01
CCRCC vs. BT, 0.002
T1 vs. T3 CCRCC, 0.830 | CCRCC vs.
non-CCRCC, <0.001
CCRCC vs. BT, <0.001
T1 vs. T3 CCRCC, 0.043 |
CA IX as a tissue marker
Seventy-four frozen tissue specimens were used to
determine the expression of CA IX by Western blotting. The same
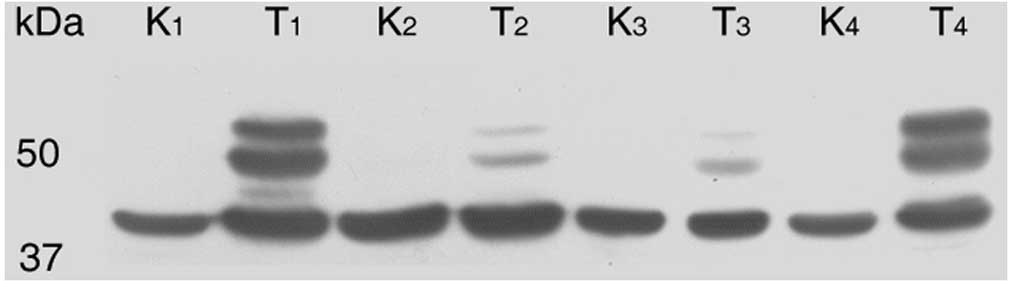
twin band of CA IX (54 and 58 kDa) as in protein lysates from tumor
cell cultures was observed in the protein lysates from tissues. CA
IX expression was assessed in 74.32% of tumor tissue samples. The
majority of the CA IX-positive samples (50 of 55) were CCRCC
tumors. The rest of the positive tissues were four papillary RCCs
and one chromophobe RCC. Specimens from BTs were negative. Within
the CCRCC group, 88% positivity for CA IX expression was obtained.
By contrast, parallel tissue samples from healthy kidneys
demonstrated a complete absence of CA IX protein bands (Fig. 1).
For CA IX quantification, the same protein lysates
from 74 tumor tissues were analyzed by ELISA. According to the
protein standard, the final concentration of CA IX in 100 μg
of total proteins was calculated. As expected, CA IX tissue levels
were significantly higher in CCRCC than in non-CCRCC and BTs
(6057.83 pg vs. 172.82 and 6057.83 pg vs. 20.79 pg/ml, p<0.001
and p<0.001). The CA IX expression was significantly higher in
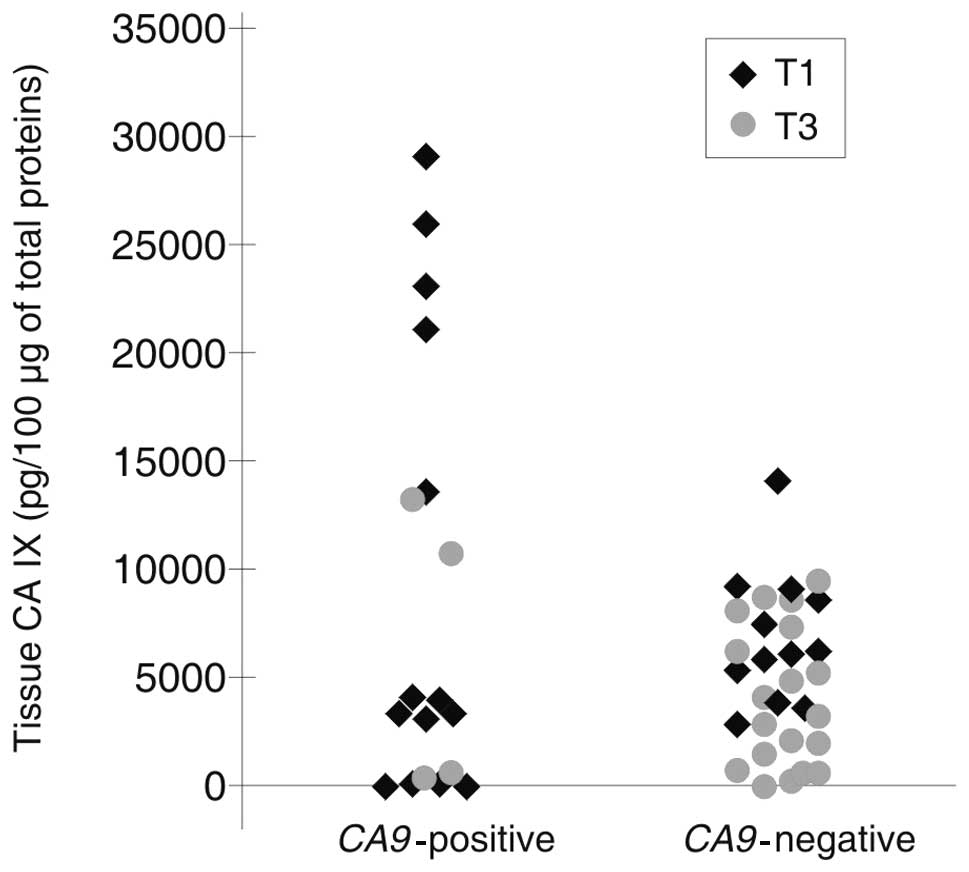
stage T1 when compared with stage T3 (p=0.043) (Table III). Additionally, there was a trend
toward a higher detection of CA9 mRNA in CTCs using RT-PCR
in a group of T1 tumors (the mean value is 8278.05 pg) exhibiting a
significantly higher mean CA IX expression when compared with T3
tumors (4478.82 pg) (Fig. 2).
Despite a relative increased positivity in T1 tumors >4 cm and
decreased positivity in T3 tumors >7 cm, the p-value did not
reach a statistical cut-off (data not shown).
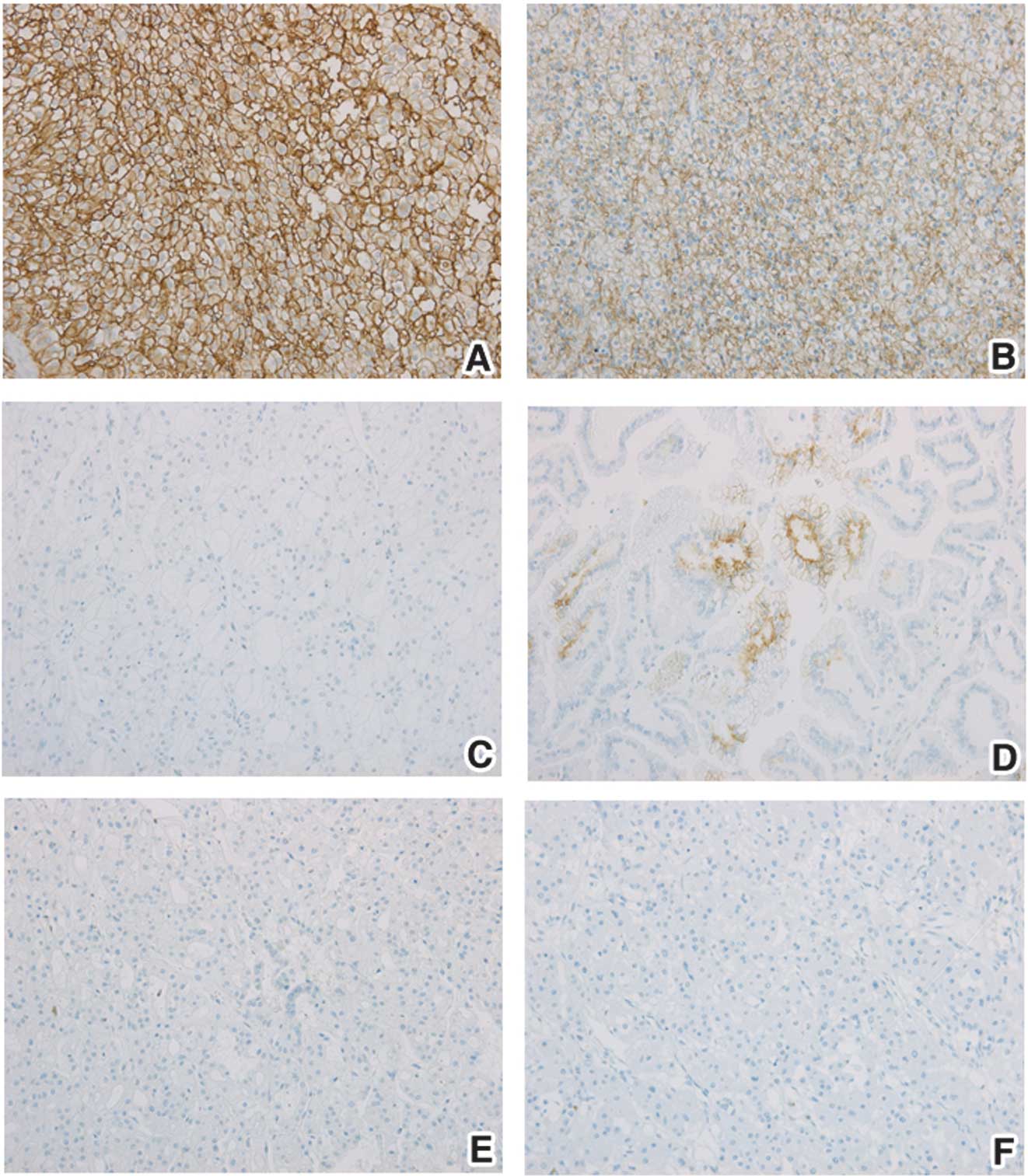
CA IX expression in tumor tissues was also evaluated
by immunohistochemistry (Fig. 3).
Overall, 82.43% of 74 tumor specimens exhibited CA IX expression.
CA IX expression was detected in 93% (53 of 57) of CCRCC and in 60%
(6 of 10) of papillary RCC. One positive tumor was observed from
chromophobe RCCs and benign tumors but the overall small number of
specimens in these groups limits any evaluation. The proportion of
CA IX-stained pixels from the entire image as well as staining
intensity of CA IX-positive pixels were analyzed as an average from
10 images captured from each tissue section. Proportions of CA
IX-stained pixels were 6.30% in CCRCC, 0.24% in non-CCRCC and 0.25%
in BT (Table IV). The CA IX status
determined by IHC was significantly higher in CCRCC than in
non-CCRCC (p<0.001) and BT (p<0.001), respectively. The mean
value of CA IX staining intensity was 119.91 in CCRCC and was
significantly higher than in non-CCRCC (72.58, p=0.035) and in BT
(29.15, p=0.05). Within the CCRCC group, there was a
non-significant trend towards a decreased proportion of CA IX
staining as well as CA IX intensity in stage T3 when compared with
T1. CA IX staining in RCC samples was positively correlated with CA
IX tissue level measured by ELISA (Pearson’s correlation
coefficient = 0.66).
 | Table IVCA IX expression determined by
immunohistochemistry and patient clinical characteristics. |
Table IV
CA IX expression determined by
immunohistochemistry and patient clinical characteristics.
| CA IX-positive | CA IX-negative | Proportion of CA
IX-stained pixels (%) | Intensity of CA
IX-stained pixels |
|---|
| CCRCC (all
stages) | 53 | 4 | 6.30 | 119.91 |
| CCRCC stage T1 | 26 | 2 | 6.68 | 124.03 |
| CCRCC stage T3 | 22 | 2 | 5.33 | 115.20 |
| Non-CCRCC | 7 | 6 | 0.24 | 72.58 |
| BT | 1 | 3 | 0.25 | 29.15 |
| p-value | | | CCRCC vs.
non-CCRCC, <0.001
CCRCC vs. BT, <0.001
T1 vs. T3 CCRCC, 0.396 | CCRCC vs.
non-CCRCC, 0.035
CCRCC vs. BT, 0.050
T1 vs. T3 CCRCC, 0.422 |
Discussion
Despite the accumulating data obtained on the
biological and clinical features of kidney cancer, management of
tumor patients remains problematic. Use of more effective targeted
therapy compared to conventional therapy is crucial. However,
further progress can only be achieved through the identification
and validation of relevant biomarkers for the rational
stratification of patients and selection of proper treatment
strategy. Although CA IX is one of the most studied surface
antigens in RCC, attention has focused on the evaluation of CA IX
for CCRCC patients. Several studies demonstrated that a high CA IX
expression (unlike in non-renal tumors) correlates with better
clinical outcomes and is also able to predict response to high-dose
IL-2 therapy in advanced stage CCRCC (5,8). In
light of these facts, we performed a detection and quantification
of CA IX in blood as well as tissue specimens from 74 kidney cancer
patients to clarify its clinical importance.
The first method we used was RT-PCR detection of
circulating tumor cells (CTCs) in the peripheral blood. The spread
of neoplastic cells from primary tumor to distant sites is a
crucial step in the metastasis process and, thus, the detection of
CTCs may provide evidence about disease aggression. Bluemke and
colleagues screened the status of CTCs in the blood samples from
renal carcinoma patients and revealed that the presence of CTCs
correlated with the lymph node status and synchronous metastasis
(9). The ability to detect CTCs by
analyzing the expression of CA9 as a tumor-specific
biomarker in blood samples has previously been reported (10–13).
RT-PCR for CA9 is a highly specific and sensitive technique
for detecting CTCs shed from renal carcinomas. In this study,
almost 33% of the 74 patients tested by RT-PCR had
CA9-positive CTCs. The majority of the patients were
diagnosed with conventional RCC. Additionally, with higher tumor
stage, there was a trend towards reduced or even absent CA9
mRNA in blood specimens. Several studies, however, were not able to
correlate results obtained from RT-PCR to the clinical
characteristics of the patients. A possible explanation for this
lies in the specificity and sensitivity of this type of assay,
which is heavily dependent on the set of primers that are used.
Thus, the first prerequisite for acquisition of
correct results is in primer design which reflects the exon-intron
structure of the CA9 gene (14). Furthermore, Barathova et al
demonstrated that the CA9 gene expression involves
alternative splicing and thus, the alternatively spliced (AS)
variant of CA9 mRNA lacking exons 8–9 exists. In contrast to
full-length (FL) CA9 mRNA, the AS variant is detected in
normal tissues and independently from the levels of hypoxia
(15). Therefore, it may produce
false-positive results and affect the clinical value of CA IX as a
marker of hypoxia (16,17). Additional steps, including
preference of small volume of blood samples (200 μl) prior
to enrichment from larger blood volumes and rapid RNA isolation
following homogenization of blood samples with InstaPure reagent,
are crucial steps for obtaining significant results in this study.
McKiernan et al assessed CA9-expressing cells in 49%
of a total of 37 RCC patients. Using the primers designated against
exons 1 and 5, they detected the two variants of CA9 mRNA
and, therefore, they did not reveal any correlation between
CA9 expression and age, gender or tumor grade (10). The same set of primers with
contrasting results was used in a further two studies. The first
one revealed only 19% positivity for CA9 in a group of 59
patients with RCC (11). However,
almost 32% positivity for CA9 in peripheral blood samples
was achieved by Gilbert et al(12). Considering that these authors used
similar methodology and comparable patient groups, there is no
explanation for the discrepancies. However, the second study
provides evidence that the estimated 5-year disease-free survival
for patients with detectable CA9 mRNA in the peripheral
blood was 39.5% in comparison with 88.1% for CA9-negative
patients.
Several studies have examined the potential clinical
utility of s-CA IX in the serum from RCC patients for prognostic
and predictive applications (18–23).
s-CA IX is a shorter form of CA IX (50/54 kDa) which is released
into the culture medium or into the body fluids via a
metalloprotease-dependent process regulated by TACE/ADAM17
(24). Zavada et al first
described the detection of s-CA IX in the serum and urine of
patients with RCC (18). In this
study, two different capture antibodies were employed to compare
the results. Initially, we used the commercially available CA IX
ELISA kit from R&D. s-CA IX in serum samples was then
quantified using V/10 as a capture antibody (7). In both cases, we observed a
significant correlation between serum CA IX and the histological
subtype of RCC. Although lower mean values of s-CA IX were
obtained, introduction of the V/10 antibody enabled us to reveal a
significant association between the s-CA IX level and tumor stage
in CCRCC patients. Our results are in accordance with the study of
Li et al, who first reported a significant association
between serum CA IX and stage, tumor grade and size in patients
with CCRCC. In their study of 91 patients, the mean value of serum
CA IX in patients with metastatic CCRCC (216.68±67.02 pg/ml) was
significantly higher than that in localized CCRCC (91.65±13.29
pg/ml) (19). Similar CA IX values
for CCRCC patients (126.1 pg/ml) with the same commercial ELISA kit
for CA IX were reported by Zhou et al(20). However, the serum CA IX levels
measured by ELISA did not correlate with the tissue levels of CA IX
determined by ELISA or immunohistochemistry. By contrast, a
significant trend for an association between serum CA IX and tumor
expression assessed with immunohistochemistry was described by
Papworth et al(21). Their
results showed higher serum CA IX in CCRCC compared to other RCC
types and a positive correlation of CA IX with tumor stage was
revealed. However, these authors did not find a significant
prognostic association between serum CA IX and the recurrence-free
survival of CCRCC patients.
In addition to the blood-based assays, WB and
immunohistochemistry may be used to determine the status of CA IX
in tissue samples. Previously, several studies have demonstrated
the expression of CA IX in kidney tumors by WB (18,25).
Our study has shown that the majority of CCRCC patients expressed
CA IX protein in tumor tissue. By contrast, tissue specimens from
normal kidney and BTs were completely CA IX-negative. Despite the
fact that WB reliably distinguishes CCRCC (as strongly CA
IX-positive samples) from non-RCC tissues, it is clear that this
method is not really suitable as a diagnostic test for a large
amount of tissue samples. Furthermore, it is a time-consuming assay
which is at best only semi-quantitative. Consequently, we performed
ELISA to determine the level of CA IX expression in tumor tissues.
Zhou et al have recently demonstrated that ELISA provides an
objective and quantitative method for measurement of CA IX in renal
tissues (20). Moreover, the tissue
CA IX levels determined by ELISA highly correlated with the CA IX
detected by immunohistochemistry. In agreement with Zhou et
al, we found that CCRCC tissues demonstrated significantly
higher CA IX levels than non-CCRCC and benign tumors. By contrast,
we demonstrated a significantly higher CA IX expression for CCRCC
in stage T1 (8278.05 pg/100 μg of total proteins) when
compared with stage T3 tumors (4478.82 pg). Findings of the present
study showed a trend to a higher CA IX expression in tumors larger
than 4 cm in stage T1 and decreased positivity for T3 tumors larger
that 7 cm. However, no statistically significant difference was
observed between them. These results are crucial particularly from
the point of view of clinical application of ELISA in tissue. CA IX
levels determined from smaller quantity of tumor tissue (when
compared with IHC) may therefore serve as a method for
stratification of RCC patients.
Immunohistochemistry, in contrast to WB, has become
the gold standard for measuring the CA IX expression in kidney
tumors and thus, several studies have reported a prognostic
significance of CA IX in RCC (5,8,26,27).
However, the evaluation of CA IX in RCC tumors may be directly
affected by the primary antibody or by the size of the tumor
sample. Inasmuch as a small piece of tissue (for example as used in
tissue microarrays) is not representative, we preferred an analysis
of the whole tissue sections of RCC tumors. In this study, we
confirmed that CA IX expression in tissue sections was correlated
with the histological subtype of RCC. Therefore, we observed that
93% of CCRCC patients demonstrated CA IX expression. Additionally,
we found that as the stage of CCRCC tumors increases, the staining
for CA IX decreases. Our results are in accordance with Bui and
colleagues, who first reported that diffuse membranous CA IX
staining may be found in more than 90% of CCRCC patients (5). Diffuse intratumoral distribution is a
result of VHL inactivation and thus, constitutive activation of
HIF-1 (28). By contrast, in
non-renal solid malignancies, the hypoxic activation of HIF-1α
results in focal CA IX expression predominantly in the perinecrotic
regions (3). In our study, we
confirmed that CA IX is typically more highly expressed in clear
cell RCCs than in non-CCRCC tumors and BTs. Moreover, the staining
pattern of CA IX in papillary and chromophobe RCCs was not
homogenous as in the case of CCRCC but rather fitted more to the
hypoxia-induced pathway. Additionally, the staining intensity for
CA IX was significantly higher in CCRCC than in non-CCRCC
groups.
In conclusion, we have analyzed tissue as well as
blood samples from a group of 74 kidney patients. Blood-based
assays were performed for the detection of CA9 mRNA using
RT-PCR or for s-CA IX protein shed from the existing renal tumor.
In the current study, we describe the first use of specific primers
for the full-length form of CA9 to detect the presence of
CTCs in the peripheral blood of kidney cancer patients. The main
finding of our study is that RT-PCR defined CA9 expression
in CTCs as able to stratify patients with clear cell RCC into
groups according to the tumor stage, with decreased or absent
CA9 expression being associated with worse prognosis.
Therefore, RT-PCR detection of CA9 expression in the
peripheral blood may indicate future prospects for the non-invasive
diagnosis of RCC. Together with the quantification of CA IX in
serum and tissue samples via ELISA, it may serve as a basis for
optimal management of tumor patients and may be important for
monitoring the progression and recurrence of the disease.
Additional studies with larger cohorts of patients, along with
relevant follow-up are required to confirm the clinical relevance
and utility of the CA IX as a biomarker.
Abbreviations:
|
CA IX
|
carbonic anhydrase IX protein
|
|
CA9
|
carbonic anhydrase IX gene
|
|
HIF
|
hypoxia-inducible factor
|
|
RCC
|
renal cell carcinoma
|
|
CCRCC
|
clear cell RCC
|
|
PRCC
|
papillary RCC
|
|
CHRCC
|
chromophobe RCC
|
|
BT
|
benign tumor
|
|
RT-PCR
|
reverse transcription polymerase chain
reaction
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
WB
|
Western blotting
|
|
IHC
|
immunohistochemistry
|
Acknowledgements
This study was supported by the grants
from the Slovak Scientific Grant Agency (VEGA 2/0216/09, VEGA
2/0184/09 and VEGA 2/0152/12), from the 7th Framework program of
the EU (Collaborative project METOXIA), and from the Research &
Development Operational Program funded by the ERDF (project ITMS
26240220062).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Gnarra JR, Tory K, Weng Y, et al:
Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat
Genet. 7:85–90. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wykoff CC, Beasley NJ, Watson PH, et al:
Hypoxia-inducible expression of tumor-associated carbonic
anhydrases. Cancer Res. 60:7075–7083. 2000.PubMed/NCBI
|
|
4
|
Svastova E, Hulikova A, Rafajova M, et al:
Hypoxia activates the capacity of tumor-associated carbonic
anhydrase IX to acidify extracellular pH. FEBS Lett. 577:439–445.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bui MH, Seligson D, Han KR, et al:
Carbonic anhydrase IX is an independent predictor of survival in
advanced renal clear cell carcinoma: implications for prognosis and
therapy. Clin Cancer Res. 9:802–811. 2003.PubMed/NCBI
|
|
6
|
Pastorekova S, Zavadova Z, Kostal M,
Babusikova O and Zavada J: A novel quasi-viral agent, MaTu, is a
two-component system. Virology. 187:620–626. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zat’ovicova M, Tarabkova K, Svastova E, et
al: Monoclonal antibodies generated in carbonic anhydrase
IX-deficient mice recognize different domains of tumour-associated
hypoxia-induced carbonic anhydrase IX. J Immunol Methods.
282:117–134. 2003.
|
|
8
|
Atkins M, Regan M, McDermott D, et al:
Carbonic anhydrase IX expression predicts outcome of interleukin 2
therapy for renal cancer. Clin Cancer Res. 11:3714–3721. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bluemke K, Bilkenroth U, Meye A, et al:
Detection of circulating tumor cells in peripheral blood of
patients with renal cell carcinoma correlates with prognosis.
Cancer Epidemiol Biomarkers Prev. 18:2190–2194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McKiernan JM, Buttyan R, Bander NH, et al:
The detection of renal carcinoma cells in the peripheral blood with
an enhanced reverse transcriptase-polymerase chain reaction assay
for MN/CA9. Cancer. 86:492–497. 1999. View Article : Google Scholar
|
|
11
|
de la Taille A, Katz A, Cao Y, et al:
Blood-based RT-PCR assays of MN/CA9 or PSMA: clinical application
in renal cancer patients. Urology. 56:393–398. 2000.PubMed/NCBI
|
|
12
|
Gilbert SM, Whitson JM, Mansukhani M, et
al: Detection of carbonic anhydrase-9 gene expression in peripheral
blood cells predicts risk of disease recurrence in patients with
renal cortical tumors. Urology. 67:942–945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohlmann CH, Ozgur E, Schrader AJ, et al:
Detection of circulating tumor cells in patients with renal cell
carcinoma by reverse transcriptase polymerase chain reaction for
G250/MNCA-9: results of a prospective trial. Urol Oncol.
24:287–293. 2006. View Article : Google Scholar
|
|
14
|
Opavsky R, Pastorekova S, Zelnik V, et al:
Human MN/CA9 gene, a novel member of the carbonic anhydrase family:
structure and exon to protein domain relationships. Genomics.
33:480–487. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barathova M, Takacova M, Holotnakova T, et
al: Alternative splicing variant of the hypoxia marker carbonic
anhydrase IX expressed independently of hypoxia and tumour
phenotype. Br J Cancer. 98:129–136. 2008. View Article : Google Scholar
|
|
16
|
Malentacchi F, Simi L, Nannelli C, et al:
Alternative splicing variants of carbonic anhydrase IX in human
non-small cell lung cancer. Lung Cancer. 64:271–276. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malentacchi F, Vinci S, Melina AD, et al:
Splicing variants of carbonic anhydrase IX in bladder cancer and
urine sediments. Urol Oncol. Sep 25–2010.(Epub ahead of print).
|
|
18
|
Zavada J, Zavadova Z, Zat’ovicova M, Hyrsl
L and Kawaciuk I: Soluble form of carbonic anhydrase IX (CA IX) in
the serum and urine of renal carcinoma patients. Br J Cancer.
89:1067–1071. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li G, Feng G, Gentil-Perret A, Genin C and
Tostain J: Serum carbonic anhydrase 9 level is associated with
postoperative recurrence of conventional renal cell cancer. J Urol.
180:510–513; discussion. 513–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou GX, Ireland J, Rayman P, Finke J and
Zhou M: Quantification of carbonic anhydrase IX expression in serum
and tissue of renal cell carcinoma patients using enzyme-linked
immunosorbent assay: prognostic and diagnostic potentials. Urology.
75:257–261. 2010. View Article : Google Scholar
|
|
21
|
Papworth K, Sandlund J, Grankvist K,
Ljungberg B and Rasmuson T: Soluble carbonic anhydrase IX is not an
independent prognostic factor in human renal cell carcinoma.
Anticancer Res. 30:2953–2957. 2010.PubMed/NCBI
|
|
22
|
Pena C, Lathia C, Shan M, Escudier B and
Bukowski RM: Biomarkers predicting outcome in patients with
advanced renal cell carcinoma: results from sorafenib phase III
Treatment Approaches in Renal Cancer Global Evaluation Trial. Clin
Cancer Res. 16:4853–4863. 2010. View Article : Google Scholar
|
|
23
|
Wind TC, Messenger MP, Thompson D, Selby
PJ and Banks RE: Measuring carbonic anhydrase IX as a hypoxia
biomarker: differences in concentrations in serum and plasma using
a commercial enzyme-linked immunosorbent assay due to influences of
metal ions. Ann Clin Biochem. 48:112–120. 2011. View Article : Google Scholar
|
|
24
|
Zatovicova M, Sedlakova O, Svastova E, et
al: Ectodomain shedding of the hypoxia-induced carbonic anhydrase
IX is a metalloprotease-dependent process regulated by TACE/ADAM17.
Br J Cancer. 93:1267–1276. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liao SY, Aurelio ON, Jan K, Zavada J and
Stanbridge EJ: Identification of the MN/CA9 protein as a reliable
diagnostic biomarker of clear cell carcinoma of the kidney. Cancer
Res. 57:2827–2831. 1997.PubMed/NCBI
|
|
26
|
Sandlund J, Oosterwijk E, Grankvist K,
Oosterwijk-Wakka J, Ljungberg B and Rasmuson T: Prognostic impact
of carbonic anhydrase IX expression in human renal cell carcinoma.
BJU Int. 100:556–560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Patard JJ, Fergelot P, Karakiewicz PI, et
al: Low CAIX expression and absence of VHL gene mutation are
associated with tumor aggressiveness and poor survival of clear
cell renal cell carcinoma. Int J Cancer. 123:395–400. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wiesener MS, Munchenhagen PM, Berger I, et
al: Constitutive activation of hypoxia-inducible genes related to
overexpression of hypoxia-inducible factor-1alpha in clear cell
renal carcinomas. Cancer Res. 61:5215–5222. 2001.PubMed/NCBI
|