Introduction
Mesenchymal stem cells (MSCs) are non-hematopoietic
stromal cells that are a heterogeneous population of cells which
are able to proliferate in vitro as plastic-adherent cells.
They have fibroblast-like morphology, form colonies in vitro
and are capable of differentiating into bone, cartage and fat cells
(1). It has been shown that MSCs
have the propensity to migrate to sites of injury and disease
(2,3). Due to their multi-differentiation
potential and migratory capacity, MSCs are regarded as ideal
candidates for regenerative therapy or tissue engineering.
In addition to bone marrow, MSCs and MSC-like cells
have now been isolated from various sites, including adipose
tissue, amniotic fluid, periosteum and fetal tissues, and show
phenotypic heterogeneity (4–10).
With the recent developments of research, it has also been reported
that MSCs and MSC-like cells may be isolated from certain non-solid
tumors, such as lipoma and bone sarcoma, and solid tumors, such as
gastric and uterine cervix cancer (11–14).
The study of the correlation between MSCs or MSC-like cells and
tumors is ongoing. Whether MSCs inhibit or promote the growth or
development of tumors has been a controversial topic and there have
been many opposing reports in the past few years (15–17).
At the same time, the biological characteristics of MSCs and
MSC-like cells in different tissues have also been studied
extensively (18–21).
In this study, we attempted to locate and isolate
MSC-like cells from human esophageal squamous cell carcinoma
tissues (hEC-MSCs) and adjacent non-cancerous esophageal tissues
(hECN-MSCs) and compare their biological characteristics so as to
build a foundation for further study of the microenvironment and
pathological mechanisms of esophageal carcinoma in the future.
Materials and methods
Isolation of hEC-MSCs and hECN-MSCs
A total of 25 paired primary esophageal carcinoma
tissues and adjacent non-cancerous esophageal tissues were
collected from April 2010 to January 2011 at the Department of
Thoracic Surgery, the Affiliated Hospital of Jiangsu University,
China. There were 22 male and 3 female patients ranging from 49 to
73 years old (median, 60 years). None of the patients received
adjuvant radiation or chemotherapy prior to surgery. Specimens used
in this study were approved by the Ethical Committees of Jiangsu
University. Written informed consent was obtained from the patient.
The fresh esophageal carcinoma tissue and its paired non-cancerous
esophageal tissue were collected and washed with phosphate-buffered
saline (PBS). The tissues were cut into 1–3 mm3-sized
sections, then digestion with 0.1% collagenase type IV (Sigma, St.
Louis, MO, USA) was performed at 37°C for 30 min. The suspension
was centrifuged at 800 rpm for 5 min. Centrifugal precipitates were
washed twice, then resuspended in Dulbecco’s modified Eagle’s
medium (DMEM) containing 15% fetal bovine serum (FBS) on 35 mm
culture dishes, and incubated at 37°C with 5% CO2 for 30
min. After removing the non-adherent cells, the remaining cells
were left to incubate for 1–2 weeks (22). The adherent cells were then
trypsinized and passaged into a new culture flask at a ratio of 1:3
for further expansion. The cells were used for subsequent
experimental study at the third passage.
Cell growth curves
The cell growth curves were determined by cell
counting at a regular time every day. hEC-MSCs, hECN-MSCs and
MSC-like cells at the third passage from human bone marrow
(hBM-MSCs) (23) were seeded in
24-well plates at a density of 5×103 per well during the
logarithmic growth phase. The number of adherent cells per well was
counted on days 1–8 of culture, and the procedure was repeated
three times.
FACS analysis
For the FACS analysis, hEC-MSCs, hECN-MSCs and
hBM-MSCs were trypsinized. After being rinsed twice with PBS,
1×106 cells were incubated with specific PE-conjugated
antibodies against CD13, CD29, CD44, CD45, CD105 (for hEC-MSCs and
hECN-MSCs) and CD133, FITC-conjugated antibodies against CD34 and
HLA-DR (Becton-Dickinson, San Jose, CA, USA) in 250 μl PBS for 30
min. After being rinsed with PBS, the labeled cells were analyzed
using FACSCalibur. PE-IgG1 and FITC-IgG1 were used as controls.
RT-PCR
To compare differentially expressed genes between
hEC-MSCs and hECN-MSCs, total RNA was extracted using TRIzol
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s
instructions, and cDNA was generated using Reverse transcriptase II
and Oligo-dT primer (Toyobo, Japan) according to the manufacturer’s
instructions. RT-PCR was performed with the following temperature
profile: a pre-denaturation step of 5 min at 94°C, followed by 35
cycles of 94°C for 30 sec, annealing temperature for 30 sec and
72°C for 30 sec and a final exposure to 72°C for 10 min. β-actin
was used as a control and the specific primers are listed in
Table I.
 | Table IPrimer sequences for the amplification
of target genes and β-actin. |
Table I
Primer sequences for the amplification
of target genes and β-actin.
| Genes | Primer sequence
(5′-3′) | Amplicon size
(bp) | Annealing temperature
(°C) |
|---|
| Oct-4 | For:
TATACACAGGCCGATGTGG | | |
| Rev:
GTGCATAGTCGCTGCTTGA | 397 | 60 |
| Nanog | For:
ATGCCTCACACGGAGACTG | | |
| Rev:
CTGCGTCACACCATTGCTA | 369 | 60 |
| Bmi-1 | For:
GCTGCCAATGGCTCTAATG | | |
| Rev:
AGGAGACTGCACTGGAGTACTG | 530 | 61 |
| TGF-β | For:
GGTGGAAACCCACAACGAAATC | | |
| Rev:
GCTAAGGCGAAAGCCCTCAAT | 385 | 60 |
| Vimentin | For:
CCAGGCAAAGCAGGAGTC | | |
| Rev:
GGGTATCAACCAGAGGGAGT | 380 | 59 |
| Snail | For:
CTGGGTGCCCTCAAGATG | | |
| Rev:
GTGGAGCAGGGACATTCG | 261 | 59 |
| Nucleostemin | For:
ACGCATGACCTGCCATAAGC | | |
| Rev:
ACCTGAGGACATCTGCAACC | 450 | 59 |
| Twist | For:
CGGGAGTCCGCAGTCTTA | | |
| Rev:
CACGCCCTGTTTCTTTGA | 497 | 57 |
| α-SMA | For:
CTGACTGAGCGTGGCTATTC | | |
| Rev:
CCACCGATCCAGACAGAGTA | 452 | 58 |
| E-cadherin | For:
CGCATTGCCACATACACTCT | | |
| Rev:
TTGGCTGAGGATGGTGTAAG | 435 | 60 |
| IGF-1 | For:
CTCCTCGCATCTCTTCTACC | | |
| Rev:
CTCCAGCCTCCTTAGATCAC | 235 | 57 |
| N-cadherin | For:
AGTCAACTGCAACCGTGTCT | | |
| Rev:
AGCGTTCCTGTTCCACTCAT | 281 | 60 |
| VEGF | For:
CCTTGCTGCTCTACCTCCAC | | |
| Rev:
ATCTGCATGGTGATGTTGGA | 280 | 61 |
| β-actin | For:
CACGAAACTACCTTCAACTCC | | |
| Rev:
CATACTCCTGCTTGCTGATC | 265 | 56 |
Immunohistochemistry
For immunohistochemical staining, the
streptavidin-biotin-peroxidase complex method was used to detect
the proteins. hEC-MSCs, hECN-MSCs and hBM-MSCs were fixed with 4%
paraformaldehyde at room temperature and endogenous peroxidase
activity was quenched and blocked with hydrogen peroxidase (30%
H2O2 and methanol, 1:50) for 30 min and 5%
BSA for 20 min. The cells were sequentially incubated with primary
antibodies for α-SMA, CK18 and VEGF (Boster, Wuhan, China) for 80
min, followed by biotin-labeled anti-rabbit or anti-mouse secondary
antibody (Boster) for 20 min at 37°C, and developed with
3,3′-diaminobenzidine (Boster). For negative controls, the
conditions were identical except that primary antibodies were
replaced by PBS.
Statistical analysis
Statistical analysis carried out using SPSS standard
version 13.0 software. Data are reported as the mean ± SD from at
least three independent tests. Significance of difference was
analyzed using the Student’s t-test. P<0.05 was considered to
indicate a statistically significant result.
Results
Morphology and growth characteristics of
hEC-MSCs and hECN-MSCs
hEC-MSCs and hECN-MSCs were successfully isolated by
collagenase digestion. A small quantity of long spindle-shaped
cells were found after 3–4 days of primary culture, and the cells
reached 80–90% confluence after 15–20 days of primary culture.
hEC-MSCs and hECN-MSCs had a similar morphologically and were long
and spindle-shaped (Fig. 1A). The
growth curves of hEC-MSCs and hECN-MSCs showed that hEC-MSCs grew
faster than hECN-MSCs in the same culture conditions after 5 days
of culture (P<0.05). hBM-MSCs were used as a control (Fig. 1B).
Expression of surface antigens
FACS analysis demonstrated that hEC-MSCs and
hECN-MSCs expressed the typical MSC surface antigens. hBM-MSCs
served as a control (Fig. 2).
Gene expression in hEC-MSCs and
hECN-MSCs
RT-PCR results showed that hEC-MSCs and hECN-MSCs
expressed stem cell-related genes, including Oct-4, Nanog, Bmi-1
and Nucleostemin, and stroma cell-related genes, including TGF-β,
Vimentin, Twist, α-SMA and Snail. However, the expression levels of
VEGF, Bmi-1, Nanog and Oct-4 were higher in hEC-MSCs than in
hECN-MSCs between the three pairs of hEC-MSCs and hECN-MSCs.
Neither hEC-MSCs nor hECN-MSCs expressed E-cadherin. hBM-MSCs
served as a control and β-actin was used as a housekeeping gene
(Fig. 3).
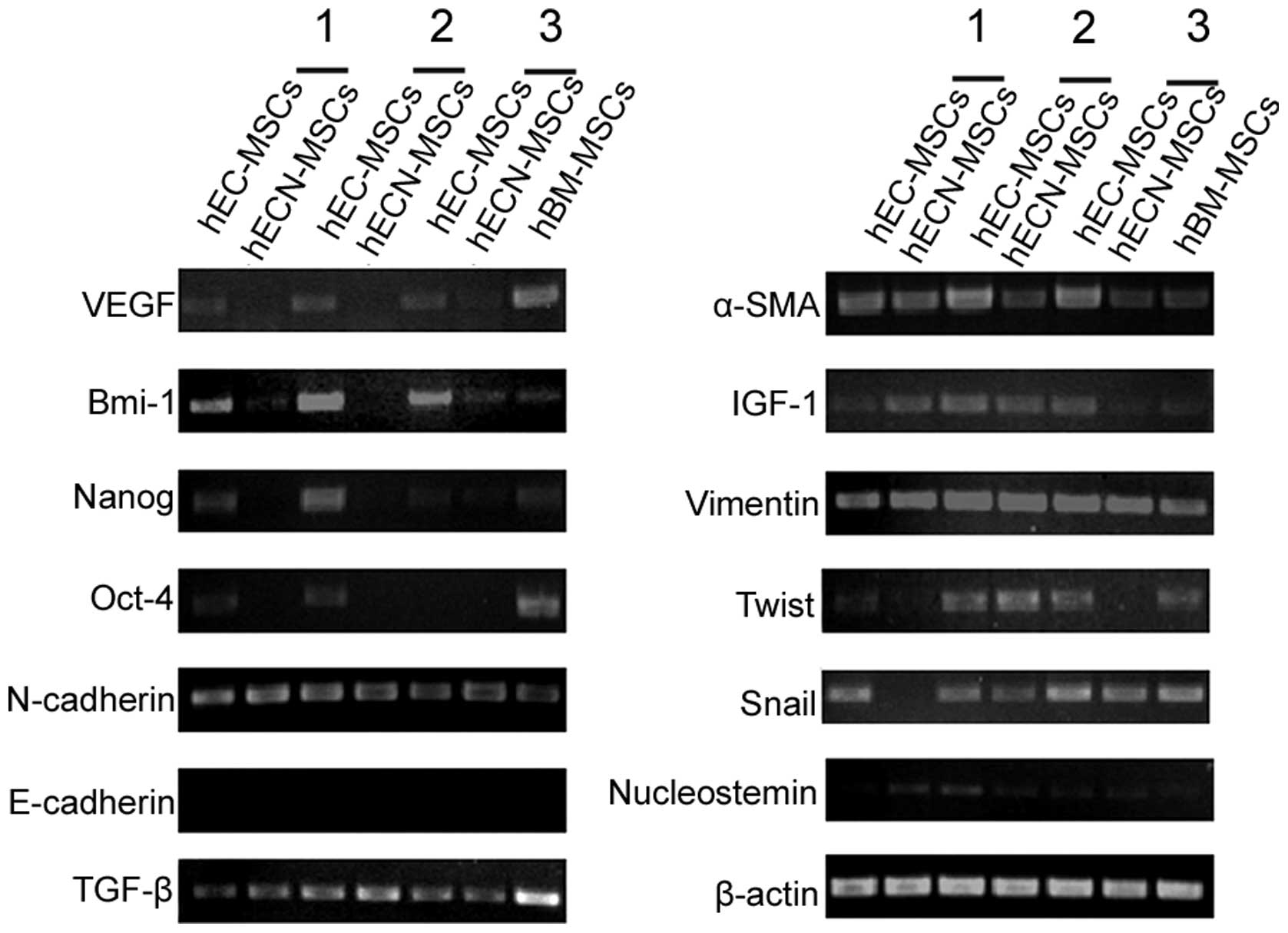 | Figure 3Gene expression in mesenchymal stem
cells (MSCs) from human esophageal carcinoma (hEC-MSCs) and
adjacent non-cancerous tissues (hECN-MSCs). RT-PCR was applied to
compare the expression levels of VEGF, Bmi-1, Nanog, Oct-4,
N-cadherin, E-cadherin, TGF-β, α-SMA, IGF-1, Vimentin, Twist, Snail
and Nucleostemin between three pairs of hEC-MSCs and hECN-MSCs.
Human bone marrow MSCs (hBM-MSCs) served as a control and β-actin
was used as a housekeeping gene. |
Immunohistochemical staining of hEC-MSCs
and hECN-MSCs
The results of immunohistochemical staining showed
that the protein expression levels of CK18 and VEGF were higher in
hEC-MSCs than in hECN-MSCs, while the expression level of α-SMA was
similar between hEC-MSCs and hECN-MSCs (Fig. 4).
Discussion
As essential components of tumor stroma cells, MSCs
play an important part in the tumor microenvironment which
modulates tumor growth and development in a number of ways. MSCs
are able to become tumor-associated fibroblasts (TAFs) or
carcinoma-associated fibroblasts (CAFs) which contribute to
fibrovascular network expansion and tumor progression (24,25).
They are also capable of differentiating into pericytes and mural
cells around tumor blood vessels, which contribute to the formation
of a mature vasculature in tumors (26).
MSCs in tumor tissue are generally believed to be
stem cells from bone marrow (27).
Strong evidence demonstrates that MSCs are able to home to injury
sites in a number of pathological conditions, including
inflammation, tissue repair and neoplasia (2,3,28).
During progression and development of tumors, MSCs can be recruited
in large numbers to the tumor site (29).
In the present study, we successfully isolated five
paired hEC-MSCs and hECN-MSCs from 25 paired primary esophageal
squamous cell carcinoma tissues and adjacent non-cancerous
esophageal tissues. The cells presented a typical long spindle
shape. The cell growth rate of hEC-MSCs was significantly higher
than that of paired hECN-MSCs. Our results indicated that hEC-MSCs
and hECN-MSCs expressed CD13, CD29 and CD44, weakly expressed
CD105, but did not express CD34, CD45, CD133 and HLA-DR, which was
also similar to hBM-MSCs according to our previous studies
(23).
To explore the role of hEC-MSCs and hECN-MSCs in
tumor development and progression, gene expression was compared
between them by RT-PCR. Among the 13 known genes, Oct-4, Nanog,
Bmi-1 and Nucleostemin were associated with stem cells, while
N-cadherin, TGF-β, Vimentin, Twist, α-SMA and Snail were associated
with stromal cells. Previous studies have shown that the expression
of Oct-4 and Nanog was at a higher level in certain epithelial
malignant tumors compared with corresponding cancer-adjacent
tissues and normal tissues (30,31).
Similarly, Bmi-1 was reported to have low expression or even no
expression in cancer-adjacent and normal tissues, but its
expression was significantly higher in tumor tissues, and was
associated with poor prognosis in patients (32,33).
VEGF promotes the formation of new blood vessels in tumors, which
plays an important role in the growth, invasion and metastasis of
tumors (34,35). In our study, the expression levels
of VEGF, Bmi-1, Nanog and Oct-4 were notably higher in hEC-MSCs
than in hECN-MSCs.
Moreover, the results of immunohistochemistry showed
that the protein expression levels of α-SMA and VEGF were
correlated with the corresponding gene expression levels. The
expression level of CK18 in hEC-MSCs was also higher than in
hECN-MSCs. CK18 is a cytokeratin, and is a specific marker of
epithelial cells. Research has shown that the expression level of
CK18 is closely correlated with the malignant degree of cells. It
was lower in normal epithelial cells than in tumor epithelial
cells, and the expression level increases with the degree of
malignancy (36,37).
In conclusion, our study showed that MSC-like cells
may be successfully isolated from human esophageal carcinoma and
adjacent non-cancerous esophageal tissues. The study may lay the
foundations for further research on the esophageal carcinoma
microenvironment and its mechanism of development.
Acknowledgements
This study was funded by the Startup
Foundation for Advanced Talents, Jiangsu University (No. 9JDG037)
and the National Natural Science Foundation of China (No.
31071421).
References
|
1
|
Horwitz EM, Le Blanc K, Dominici M, et al:
Clarification of the nomenclature for MSC: The International
Society for Cellular Therapy position statement. Cytotherapy.
7:393–395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Natsu K, Ochi M, Mochizuki Y, Hachisuka H,
Yanada S and Yasunaga Y: Allogeneic bone marrow-derived mesenchymal
stromal cells promote the regeneration of injured skeletal muscle
without differentiation into myofibers. Tissue Eng. 10:1093–1112.
2004. View Article : Google Scholar
|
|
3
|
Rojas M, Xu J, Woods CR, Mora AL, Spears
W, Roman J and Brigham KL: Bone marrow-derived mesenchymal stem
cells in repair of the injured lung. Am J Respir Cell Mol Biol.
33:145–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
In’t Anker PS, Scherjon SA, Kleijburg-van
der Keur C, et al: Amniotic fluid as a novel source of mesenchymal
stem cells for therapeutic transplantation. Blood. 102:1548–1549.
2003.PubMed/NCBI
|
|
5
|
Nakahara H, Dennis JE, Bruder SP,
Haynesworth SE, Lennon DP and Caplan AI: In vitro differentiation
of bone and hypertrophic cartilage from periosteal-derived cells.
Exp Cell Res. 195:492–503. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zuk PA, Zhu M, Ashjian P, et al: Human
adipose tissue is a source of multipotent stem cells. Mol Biol
Cell. 13:4279–4295. 2002.PubMed/NCBI
|
|
7
|
Kim SM, Lim JY, Park SI, et al: Gene
therapy using TRAIL-secreting human umbilical cord blood-derived
mesenchymal stem cells against intracranial glioma. Cancer Res.
68:9614–9623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bieback K and Kluter H: Mesenchymal
stromal cells from umbilical cord blood. Curr Stem Cell Res Ther.
2:310–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Flynn A, Barry F and O’Brien T: UC
blood-derived mesenchymal stromal cells: an overview. Cytotherapy.
9:717–726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gucciardo L, Lories R, Ochsenbein-Kölble
N, Done’ E, Zwijsen A and Deprest J: Fetal mesenchymal stem cells:
Isolation, properties and potential use in perinatology and
regenerative medicine. BJOG. 116:166–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin TM, Chang HW, Wang KH, et al:
Isolation and identification of mesenchymal stem cells from human
lipoma tissue. Biochem Biophys Res Commun. 361:883–889. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gibbs CP, Kukekov VG, Reith JD, et al:
Stem-like cells in bone sarcomas: implications for tumorigenesis.
Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu X, Zhang X, Wang S, et al: Isolation
and comparison of mesenchymal stem-like cells from human gastric
cancer and adjacent non-cancerous tissues. Cancer Res Clin Oncol.
137:495–504. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun X, Cai H, Qian H, Zhu W, Yan Y, Xu H
and Xu W: Mesenchymal stem cells isolated from human uterine cervix
cancer tissues. Cell Biol Int. 35:119–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khakoo AY, Pati S, Anderson SA, et al:
Human mesenchymal stem cells exert potent antitumorigenic effects
in a model of Kaposi’s sarcoma. J Exp Med. 203:1235–1247.
2006.PubMed/NCBI
|
|
16
|
Xu WT, Bian ZY, Fan QM, Li G and Tang TT:
Human mesenchymal stem cells (hMSCs) target osteosarcoma and
promote its growth and pulmonary metastasis. Cancer Lett.
281:32–41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahmed N, Abubaker K, Findlay J and Quinn
M: Epithelial mesenchymal transition and cancer stem cell-like
phenotypes facilitate chemoresistance in recurrent ovarian cancer.
Curr Cancer Drug Targets. 10:268–278. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moon JH, Kwak SS, Park G, et al: Isolation
and characterization of multipotent human keloid-derived
mesenchymal-like stem cells. Stem Cells Dev. 17:713–724. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bruno S, Bussolati B, Grange C, et al:
Isolation and characterization of resident mesenchymal stem cells
in human glomeruli. Stem Cells Dev. 18:867–879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mitrano TI, Grob MS, Carrión F, et al:
Culture and characterization of mesenchymal stem cells from human
gingival tissue. J Periodontol. 81:917–925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schüring AN, Schulte N, Kelsch R, Röpke A,
Kiesel L and Götte M: Characterization of endometrial mesenchymal
stem-like cells obtained by endometrial biopsy during routine
diagnostics. Fertil Steril. 95:423–426. 2011.PubMed/NCBI
|
|
22
|
Zhou ZW, Hu JB, Shi SB, Zhu XZ, Wang XH
and Xu WR: Isolation of mesenchymal stem-like cells from human
esophageal carcinoma and identification of their biological
characteristics. Basic Clin Med. 32:798–803. 2012.
|
|
23
|
Xu W, Zhang X, Qian H, et al: Mesenchymal
stem cells from adult human bone marrow differentiate into a
cardiomyocyte phenotype in vitro. Exp Biol Med. 229:623–631.
2004.PubMed/NCBI
|
|
24
|
Spaeth EL, Dembinski JL, Sasser AK, et al:
Mesenchymal stem cell transition to tumor-associated fibroblasts
contributes to fibrovascular network expansion and tumor
progression. PLoS One. 4:e49922009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mishra P J, Mishra P J, Humeniuk R, et al:
Carcinoma-associated fibroblast-like differentiation of human
mesenchymal stem cells. Cancer Res. 68:4331–4339. 2008.PubMed/NCBI
|
|
26
|
Rajantie I, Ilmonen M, Alminaite A,
Ozerdem U, Alitalo K and Salven P: Adult bone marrow-derived cells
recruited during angiogenesis comprise precursors for
periendothelial vascular mural cells. Blood. 104:2084–2086. 2004.
View Article : Google Scholar
|
|
27
|
Bergfeld SA and DeClerck YA: Bone
marrow-derived mesenchymal stem cells and the tumor
microenvironment. Cancer Metastasis Rev. 29:249–261. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hall B, Andreeff M and Marini F: The
participation of mesenchymal stem cells in tumor stroma formation
and their application as targeted-gene delivery vehicles. Handb Exp
Pharmacol. 180:263–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kidd S, Spaeth E, Dembinski JL, et al:
Direct evidence of mesenchymal stem cell tropism for tumor and
wounding micro-environments using in vivo bioluminescent imaging.
Stem Cells. 27:2614–2623. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Attasi Y, Mowla SJ, Ziaee SA, et al:
Oct-4, an embryonic stem cell marker, is highly expressed in
bladder cancer. Int J Cancer. 120:1598–1602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chiou SH, Yu CC, Huang CY, et al: Positive
correlations of Oct-4 and Nanog in oral cancer stem-like cells and
high-grade oral squamous cell carcinoma. Clin Cancer Res.
14:4085–4095. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Pan K, Zhang HK, et al: Increased
polycomb-group oncogene Bmi-1 expression correlates with poor
prognosis in hepatocellular carcinoma. J Cancer Res Clin Oncol.
134:535–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim JH, Yoon SY, Kim CN, et al: The Bmi-1
oncoprotein is overexpressed in human colorectal cancer and
correlates with the reduced p16INK4a/p14ARF proteins. Cancer Lett.
203:217–224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maeda K, Chung YS, Ogawa Y, et al:
Prognostic value of vascular endothelial growth factor expression
in gastric carcinoma. Cancer. 77:858–863. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Takanami I, Tanaka F, Hashizume T and
Kodaira S: Vascular endothelial growth factor and its receptor
correlate with angiogenesis and survival in pulmonary
adenocarcinoma. Anticancer Res. 17:2811–2814. 1997.PubMed/NCBI
|
|
36
|
Xu W, Zhang MW, Huang J, Wang X, Xu SF, Li
Y and Wang SJ: Correlation between CK18 gene and gastric carcinoma
micrometastasis. World J Gastroenterol. 11:6530–6534.
2005.PubMed/NCBI
|
|
37
|
Nanda KD, Ranganathan K, Devi U and Joshua
E: Increased expression of CK8 and CK18 in leukoplakia, oral
submucous fibrosis, and oral squamous cell carcinoma: An
immunohistochemistry study. Oral Surg Oral Med Oral Pathol Oral
Radiol. 113:245–253. 2012. View Article : Google Scholar : PubMed/NCBI
|


















