Introduction
Multiple myeloma (MM) is a plasma-cell neoplasm that
is characterized by skeletal destruction, renal failure, anemia,
and hypercalcemia (1–4). The most common symptoms on
presentation are fatigue, bone pain and recurrent infection. MM is
the second most common hematological malignancy after non-Hodgkin's
lymphoma, and the incidence of MM in China is ∼1/100,000 (1). The median survival time following
diagnosis is ∼3 years.
As a first-line treatment, melphalan with prednisone
(MP) have remained the gold standard of therapy for a number of
decades. At present, high-dose therapy with autologous stem cell
transplantation (ASCT) has been demonstrated to improve
progression-free survival (PFS) and/or overall survival (OS) in
patients <65 years of age. MP-thalidomide and MP-bortezomib have
become standard therapies for patients who are non-transplant
candidates. The purpose of the present study was to analyze the
clinical characteristics and outcomes of MM patients who were
initially diagnosed and treated in our hospital.
Patients and methods
Patients
A total of 264 MM patients who fulfilled the
International Myeloma Working Group (IMWG) criteria at the Beijing
Chaoyang hospital in China were enrolled from January 1, 2006 to
December 31, 2011. In accordance with the protocol approved by the
Medical Ethics Committee at the Beijing Chaoyang Hospital,
retrospective analyses were carried out on the patients' data. All
264 MM patients were initially diagnosed and received therapy at
the Beijing Chaoyang Hospital. Physical examinations, image
diagnostic and laboratory tests, bone marrow aspirates and biopsies
were conducted to evaluate the disease condition of patients. Data
collected included age, gender, Durie Salmon (DS) and International
Staging System (ISS) stages, date of diagnosis, cytogenetic
abnormality, serum creatinine value, disease progression
information and survival status. All patients provided written,
informed consent prior to chemotherapy and ASCT.
Therapy and assessments
Induction therapy was performed following patient
diagnosis. The first-line induction regimens included thalidomide
and dexamethasone (TD); melphalan, prednisone and thalidomide
(MPT); thalidomide, amycin and dexamethasone (TAD); vincristine,
amycin and dexamethasone (VAD); vincristine, amycin, dexamethasone
and thalidomide (VADT); bortezomib and dexamethasone (BD);
bortezomib, thalidomide and dexamethasone (BTD); bortezomib,
epirubicin and dexamethasone (PAD) and bortezomib, epirubicin,
dexamethasone and thalidomide (PADT). Of the 264 patients, 212
(80.3%) patients did not receive ASCT and 52 (19.7%) patients
received ASCT after achieving remission. Thalidomide (100 mg/day)
was adminsitered to 89.6% patients as maintenance therapy, while
other patients received interferon as maintenance therapy due to
the presence of peripheral neuropathy and constipation.
Responses were assessed according to the IMWG
uniform response criteria (5).
Response criteria included a complete response (CR), a very good
partial response (VGPR), a partial response (PR) and progressive
disease (PD). PFS time was measured as the time period from the
start of treatment to disease progression or mortality. OS time was
defined as the time period from the initial diagnosis to mortality
by any cause.
Statistical analysis
Statistical analyses were performed using the
Statistical Package for the Social Sciences (SPSS) 15.0 software
(SPSS Inc., Chicago, IL, USA). PFS and OS were analyzed with the
Kaplan-Meier method. A log-rank test was utilized to assess the
differences between subgroups. P<0.05 was considered to indicate
a statistically significant difference.
Results
Clinical features
Of the 264 patients with MM, 146 (55.3%) were male
and 118 (44.7%) were female. Table
I shows the characteristics of the 264 patients prior to
treatment. The median patient age was 59 years (range, 28–84). The
majority of patients were ≤65 years (78.4%). The most common
monoclonal protein identified in myeloma was the IgG type (42%).
Additionally, 225 (85.2%) patients presented with DS stage III,
while 145 (54.9%) patients presented with ISS stage III. Renal
insufficiency was demonstrated in 28.0% of patients. High-risk
cytogenetic abnormality was detected in 49 patients using
fluorescence in situ hybridization (FISH). The FISH markers
included t(4;14), t(14;16) and del(17p), and were present in 12.2,
2.0 and 10.2% patients, respectively.
 | Table I.Characteristics of 264 newly diagnosed
MM patients. |
Table I.
Characteristics of 264 newly diagnosed
MM patients.
| Characteristic | Values |
|---|
| Age (years, median
(range)) | 59 (28–84) |
| ≤65 (median) (n,
%) | 56 (207, 78.4%) |
| >65 (median) (n,
%) | 72 (57, 21.6%) |
| Gender | |
| Male | 146, 55.3% |
| Female | 118, 44.7% |
| Ma component | |
| IgA | 52, 19.7% |
| IgD | 21, 8.0% |
| IgG | 111, 42.0% |
| Non-secretory | 13, 4.9% |
| K | 33, 12.5% |
| λ | 34, 12.9% |
| Durie-Salmon
stage | |
| I | 3, 1.1% |
| II | 36, 13.6% |
| III | 225, 85.2% |
| ISS stage | |
| I | 23, 8.7% |
| II | 96, 36.4% |
| III | 145, 54.9% |
| Renal function | |
| A (<2 mg/dl
serum creatinine) | 190, 72.0% |
| B (≥2 mg/dl serum
creatinine) | 74, 28.0% |
| FISH | |
| del(17p) | 5/49, 10.2% |
| t(14;16) | 1/49, 2.0% |
| t(4;14) | 6/49, 12.2.% |
Efficacy
A CR, VGPR or PR was achieved in 228/264 (86%)
patients. With a median follow-up time of 20 months for all
patients, the estimated median PFS time was 37.6 months and the
estimated median OS time was 61.0 months (Fig. 1). Of the 52 patients who received
ASCT, CR, VGPR and PR were achieved prior to ASCT in 28 (53.9%), 9
(17.3%) and 15 (28.9%) patients, respectively, and post-ASCT in 31
(59.6%), 7 (13.5%) and 14 (26.9%) patients, respectively. The CR
rate was not significantly higher post-ASCT compared with prior to
ASCT (P=0.55) (Table II). In the
non-ASCT group, the overall response rate (ORR) was 83.0%, with 48
(18.2%), 7 (2.7%) and 121 (45.8%) patients achieving a CR, VGPR and
PR, respectively.
 | Table II.Response rates of 52 patients with MM
before and after ASCT. |
Table II.
Response rates of 52 patients with MM
before and after ASCT.
| No. of patients (%)
|
|---|
| Response | CR | VGPR | PR | SD or PD |
|---|
| Prior-ASCT | 28 (53.85) | 9 (17.31) | 15 (28.85) | 0 (0) |
| Post-ASCT | 31 (59.62) | 7 (13.46) | 14 (26.92) | 0 (0) |
Of the 212 non-ASCT patients, 120 (56.6%) patients
received induction regimens without bortezomib and 92 (43.4%)
patients received bortezomib-based regimens. Among the non-ASCT
groups, patients who received bortezomib-based regimens
demonstrated a greater ORR compared with those who did not receive
bortezomib (92.3% vs. 75.8%; P<0.05) (Table III).
 | Table III.Effects of regimens and age on the
response rate of non-ASCT patients. |
Table III.
Effects of regimens and age on the
response rate of non-ASCT patients.
| Response (%)
|
|---|
| Variable | CR | VGPR or PR | SD or PD |
|---|
| Regimen | | | |
| Without
bortezomib | 11.67 | 64.17 | 24.17 |
| With
bortezomib | 36.96 | 55.43 | 7.61 |
| Age (years) | | | |
| ≤65 | 23.23 | 61.94 | 14.84 |
| >65 | 21.05 | 56.14 | 22.81 |
| ≤65 years | | | |
| Without
bortezomib | 11.90 | 64.29 | 23.81 |
| With
bortezomib | 36.62 | 59.15 | 4.23 |
| >65 years | | | |
| Without
bortezomib | 11.11 | 63.89 | 25.00 |
| With
bortezomib | 38.10 | 42.86 | 19.05 |
Among 155 patients aged ≤65 years, the 71 (45.8%)
patients who received bortezomib-based regimens demonstrated a
greater ORR compared with the 84 (54.2%) patients who received
regimens without bortezomib (93.0% vs. 76.2%, P<0.05).
Bortezomib-based regimens, as opposed to regimens without
bortezomib, also demonstrated a greater ORR in patients >65
years (81.0 vs. 75%; P<0.05) (Table
III).
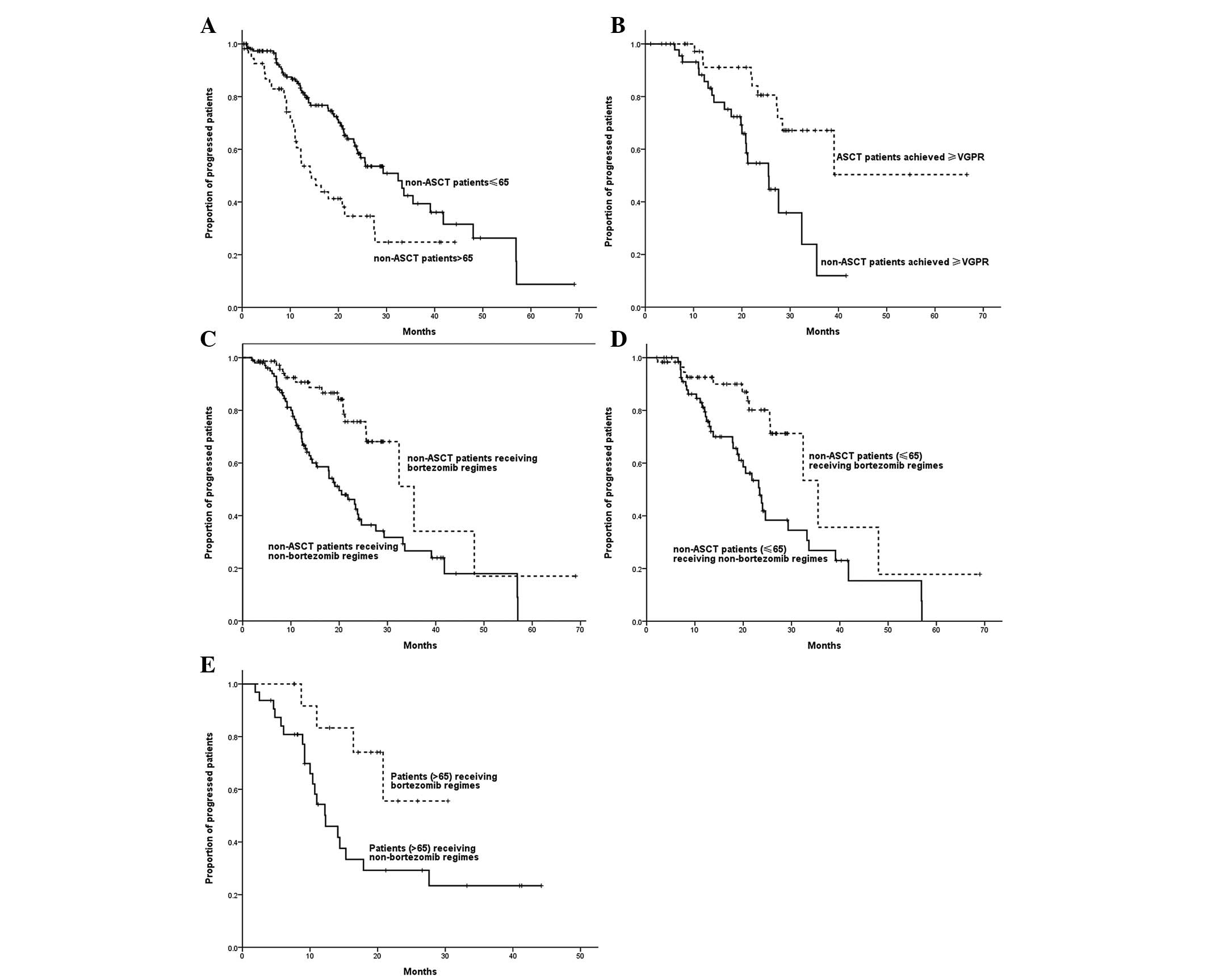
Subgroup analysis of PFS
A significant correlation was observed between age
and PFS time, with the PFS time of patients >65 years being
shorter than that of patients ≤65 years (14.4 vs. 32.4 months;
P=0.001). With a median follow-up time of 20 months, for patients
who achieved CR/VGPR following induction therapy, the estimated
median PFS time of patients who received ASCT was longer than that
of patients who did not receive ASCT (not reached vs. 35.5 months;
P=0.002). Non-ASCT patients who received bortezomib-based regimens
demonstrated a longer PFS time compared with those who received
regimens without bortezomib (37.0 vs. 25.6 months; P= 0.001). Among
patients ≤65 years, those who received bortezomib-based regimens
demonstrated a longer PFS time than those who received regimens
without bortezomib (35.5 vs. 23.4 months; P=0.003).
Bortezomib-based regimens also led to a longer PFS time in patients
>65 years (not reached vs. 12.3 months; P=0.026) (Fig. 2).
Subgroup analysis of OS time
The OS time for DS stage III was shorter than that
of DS stage I or II patients, although no significant difference
was observed (P=0.209). No significant difference was detected in
the estimated median OS time among patients with ISS stage I, II or
III (not reached vs. not reached vs. 48.0 months, respectively;
P=0.051). The OS time of patients with high-risk cytogenetic
abnormality, del(17p), t(14; 16) and t(4; 14), was significantly
shorter compared with that of other patients (30.2 months vs. not
reached; P=0.029). Patients who had a baseline serum creatinine
level <2 mg/dl demonstrated a greater OS time compared with that
of patients with a baseline serum creatinine level ≥2 mg/dl (63.4
vs. 38.6 months; P=0.025). Among all patients who achieved a
CR/VGPR, those who received ASCT demonstrated a greater OS time
compared with non-ASCT patients (not reached vs. 63.4 months;
P=0.031). In relapsed patients, those who received regimens
containing bortezomib in the initial treatment phase demonstrated
the same median survival time following relapse compared with those
who received regimens that did not contain bortezomib (26.5 vs.
10.5 months; P=0.271) (Fig. 3).
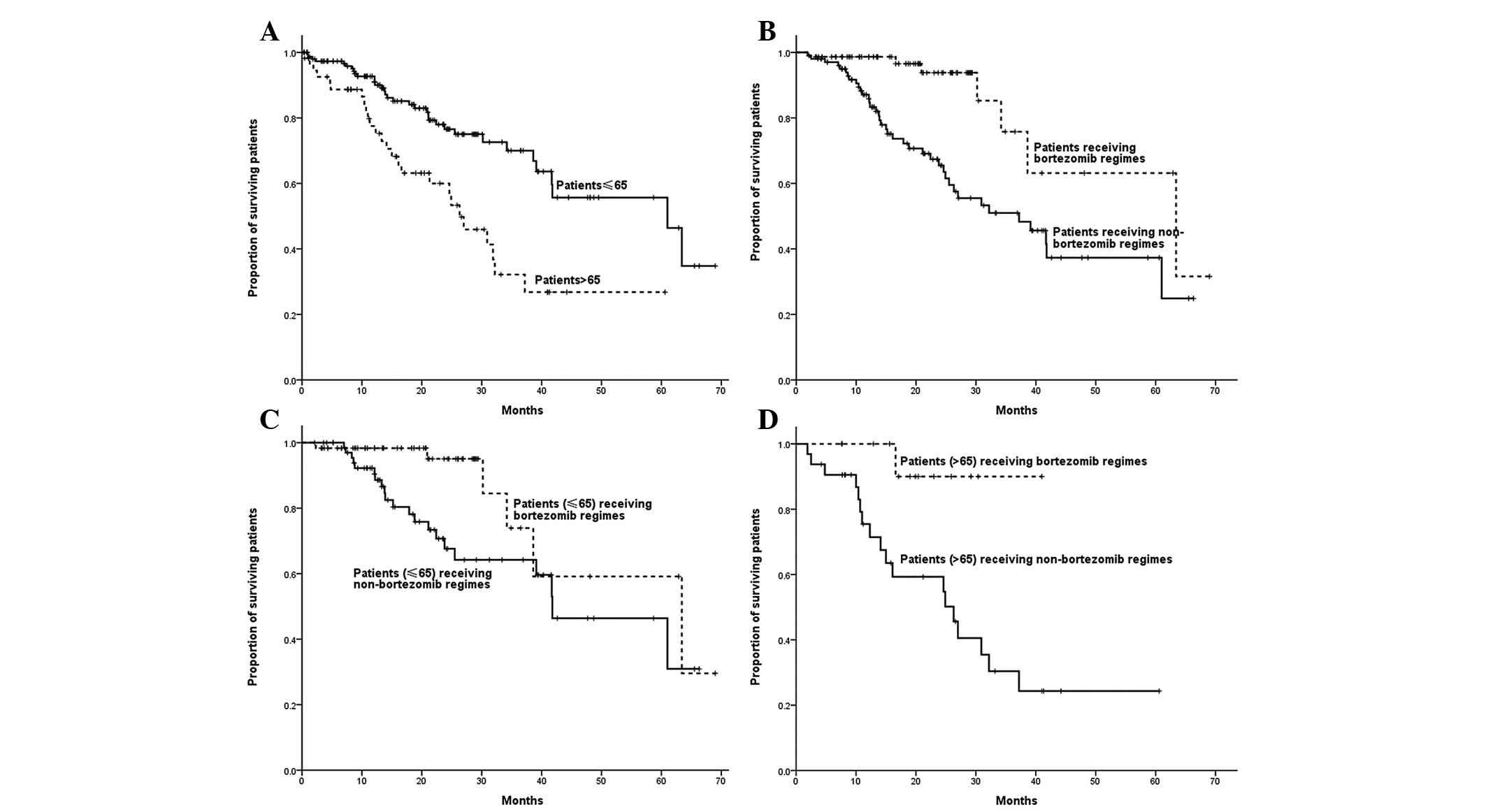
Among non-ASCT patients, age was correlated with OS
time, with the OS time of patients >65 years being shorter than
that of patients ≤65 years (26.3 vs. 61.0 months; P=0.001). The OS
time of patients who received bortezomib-containing regimens was
significantly longer than that of patients who received regimens
without bortezomib (63.4 vs. 37.2 months; P=0.001). Patients ≤65
years who received bortezomib-based regimens demonstrated a longer
OS compared with those who received regimens without bortezomib
(63.4 vs. 41.8 months; P=0.021). Bortezomib-based regimens, as
opposed to regimens without bortezomib, led to a longer OS time in
patients >65 years (not reached vs. 26.3 months; P=0.017)
(Fig. 4).
Discussion
The present study retrospectively analysed the
clinical data of 264 MM patients at the Beijing Chaoyang Hospital
in China. The data revealed that MM frequently occurred in the
elderly population. In the majority of western countries, the
median age of patients with MM is ∼65 years. However, in the
present study, the median age was only 59 years. One possibility is
that ethnic diversity may account for the difference. However, the
median age of MM patients may have increased as the Chinese life
expectancy has steadily increased. Basic investigations are
required to identify the reason for the difference. In this study,
the majority of patients were in either DS or ISS stage III, which
differed from other studies. This difference reinforced the
requirement for greater medical attention and higher sensitivity
tests to identify more patients in early stage. In this study, the
most common monoclonal protein was also IgG concordant with a study
(4). Moreover, the prevalence of
renal insufficiency was 28%, which is in accordance with the
majority of studies. Three common high-risk cytogenetic
abnormalities, t(4;14), t(14;16) and del(17p), were detected by
FISH in 49 patients; the occurences demonstrated were similar to
those of other studies.s (6–7).
A key finding of this study was that patients >65
years demonstrated an inferior outcome compared with non-ASCT
patients ≤65 years, as measured by the OS time. The reason for the
reduced efficacy in elderly patients is likely to be
multifactorial. Elderly patients may have been less heavily
pretreated with respect to prior therapy, and patients >65 years
did not undergo ASCT. Herein, we excluded the function of ASCT;
however, age remained an influence on patient survival. It may be
that older patients and physicians were less accepting of high-dose
chemotherapy. Thus, one possibility for the poorer outcome in
elderly patients is the lower average daily dose that older
patients received. Another possible explanation for the age effect
is that the biology of malignant plasma cells and the bone marrow
microenvironment may be different in older, compared to younger,
patients. There are conflicting studies concerning the impact of
patient age on prognosis in newly diagnosed MM. Certain studies
have demonstrated that elderly patients have an inferior survival
compared with younger patients, whereas others have reported no
effect of age on survival (8).
Although the DS system has previously been
demonstrated to be an effective staging tool for patients with
multiple myeloma, a number of studies have revealed that it is not
capable of indicating a significant survival difference between
stages I, II, and III (9). As only
1% of patients in the current study were in DS stage I, DS stages I
and II were combined, and compared with DS stage III. A similar
result was found in that DS stages I/II and III were not able to
significantly differentiate patients, although the OS time for DS
stage III was shorter than that of DS stage I/II patients. A
combination of serum β2-microglobulin and serum albumin provided
the simplest, most powerful and reproducible three-stage
classification, i.e., the ISS staging system (10). No difference in the median OS time
of patients with ISS stage I, II or III was observed. Therefore,
our study was not able to confirm the prognostic utility. It is
possible that novel drugs modified the prognostic value of the ISS
stage. Up to 20% of newly diagnosed MM patients demonstrated renal
impairment complications. Renal insufficiency, in particular
dialysis dependency, was an independent poor prognostic factor in
MM, while the majority of patients were unable to achieve dialysis
independence. Renal impairment (serum creatinine level ≥2 mg/dl)
and a poor OS time were evident in 28% of MM patients, whereas
renal impairment was a poor prognostic factor.
Cytogenetic status was the most important prognostic
factor in patients with MM. Patients with hyperdiploidy or
immunoglobulin heavy chain (IgH) translocation t(11;14)
demonstrated good or average survival times, respectively. Poor
cytogenetic features were: 17p deletion, chromo-some 1q gains,
t(4;14) and t(14;16)(11–12). In this study, three common high-risk
cytogenetic abnormalities were detected in 49 patients by FISH. The
results demonstrated that patients with high-risk cytogenetic
abnormalities had a poor OS time compared with other patients.
High-dose melphalan with autologous stem cell
support has been an integral part of myeloma therapy, either as
salvage therapy or as consolidation of an initial remission. The
response to therapy is a crucial prognostic factor in patients with
MM. Patients who achieve CR demonstrated an event-free survival
(EFS) and an OS time significantly longer than those who remained
in the PR stage. Improvement in the depth of response has been
associated with a significantly longer EFS and OS time (13–15).
In this study, ASCT was not able to significantly increase the CR
rate of patients. It is possible that the majority of patients had
achieved at least a VGPR prior to ASCT and we were not able to
detect the clearing effect of ASCT on minimal residual disease. In
order to exclude the influence of the response to therapy on PFS
and OS times, we compared the PFS and OS times of ASCT patients who
achieved CR/VGPR prior to ASCT with those of the non-ASCT patients
who also achieved CR/VGPR. ASCT was able to improve the PFS
(P=0.002) and OS (P=0.031) times. High-dose melphalan with
autologous stem cell support was able to benefit MM patients.
The boronic dipeptide, bortezomib, targets the
proteasome to prevent intracellular protein degradation (16–17).
It causes cell cycle arrest, anti-angiogenic effects, induction of
the stress response and apoptosis of multiple myeloma cells
mediated by caspase-8/9. In the present study, patients who
received bortezomib-containing regimens had greater PFS and OS
times. Among non-ASCT patients, the OS time of patients receiving
bortezomib-containing regimens was significantly longer than that
of patients receiving regimens without bortezomib (P=0.001). Among
non-ASCT patients, both ≤65 and >65 years, patients who received
bortezomib-based regimens demonstrated a longer OS time compared
with those who received regimens without bortezomib. In relapsed
patients, those who received regimens containing bortezomib in the
initial stages of treatment did not demonstrate an increased median
survival time post-relapse compared with those who did not receive
regimens containing bortezomib (P=0.271). However, the number of
relapsed patients was limited, and significant differences in OS
time may be observed with an increased sample size. These results
demonstrated that bortezomib is effective in MM patients.
This study presented the clinical characteristics of
MM patients who were initially diagnosed and received treatment at
the Beijing Chaoyang Hospital, and the effects of the majority of
common regimens on newly diagnosed patients. Age was demonstrated
to influence the OS of patients. Neither the DS nor the ISS stage
were able to provide an exact prognosis, while renal function
insufficiency was a poor prognostic factor and high risk
cytogenetic abnormality indicated a poor prognosis. ASCT and
bortezomib-based regimens were able to prolong PFS and OS
times.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (General Program;
Grant No. 81172252).
References
|
1.
|
Raab MS, Podar K, Breitkreutz I,
Richardson PG and Anderson KC: Multiple myeloma. Lancet.
374:324–339. 2009. View Article : Google Scholar
|
|
2.
|
Rajkumar SV: Multiple myeloma: 2011 update
on diagnosis, risk-stratification, and management. Am J Hematol.
86:57–65. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kyle RA and Rajkumar SV: Multiple myeloma.
Blood. 111:2962–2972. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kyle RA, Gertz MA, Witzig TE, et al:
Review of 1027 patients with newly diagnosed multiple myeloma. Mayo
Clin Proc. 78:21–33. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Durie BG, Harousseau JL, Miguel JS, et al:
International uniform response criteria for multiple myeloma.
Leukemia. 20:1467–1473. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Becker N: Epidemiology of multiple
myeloma. Recent Results Cancer Res. 183:25–35. 2011. View Article : Google Scholar
|
|
7.
|
Tuscano JM: Multiple myeloma: epidemiology
and therapeutic options. Manag Care. 17:9–15. 2008.PubMed/NCBI
|
|
8.
|
Mileshkin L, Biagi JJ, Mitchell P, et al:
Multicenter phase 2 trial of thalidomide in relapsed/refractory
multiple myeloma: adverse prognostic impact of advanced age. Blood.
102:69–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Choi JH, Yoon JH and Yang SK: Clinical
value of new staging systems for multiple myeloma. Cancer Res
Treat. 39:171–174. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Greipp PR, San Miguel J, Durie BG, et al:
International staging system for multiple myeloma. J Clin OncoI.
23:3412–3420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Avet-Loiseau H, Attal M, Moreau P, et al:
Genetic abnormalities and survival in multiple myeloma: the
experience of the Intergroupe Francophone du Myélome. Blood.
109:3489–3495. 2007.PubMed/NCBI
|
|
12.
|
Gutiérrez NC, Castellanos MV, Martín ML,
et al: Prognostic and biological implications of genetic
abnormalities in multiple myeloma undergoing autologous stem cell
transplantation: t(4;14) is the most relevant adverse prognostic
factor, whereas RB deletion as a unique abnormality is not
associated with adverse prognosis. Leukemia. 21:143–150. 2007.
|
|
13.
|
Nadal E, Giné E, Bladé J, et al: High-dose
therapy/autologous stem cell transplantation in patients with
chemosensitive myeloma: predictors of complete remission. Bone
Marrow Transplant. 33:61–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Lahuerta JJ, Mateos MV, Martínez-López J,
et al: Influence of pre- and post-transplantation responses on
outcome of patients with multiple myeloma: sequential improvement
of response and achievement of complete response are associated
with longer survival. J Clin Oncol. 26:5775–5782. 2008. View Article : Google Scholar
|
|
15.
|
van de Velde HJ, Liu X, Chen G, et al:
Complete response correlates with long-term survival and
progression-free survival in high-dose therapy in multiple myeloma.
Haematologica. 92:1399–1406. 2007.PubMed/NCBI
|
|
16.
|
Delforge M: Bortezomib for previously
untreated multiple myeloma. Expert Opin Pharmacother. 12:2553–2564.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Terpos E, Roussou M and Dimopoulos MA:
Bortezomib in multiple myeloma. Expert Opin Drug Metab Toxicol.
4:639–654. 2008. View Article : Google Scholar
|