Introduction
The occurrence of synchronous or metachronous second
primary malignancies (SPMs) is increased in patients with
neuroendocrine tumors (NETs) compared to the general population
(1,2). Since primary NETs of the breast are
extremely rare (3–8), evidence is lacking as to whether
patients with NE breast carcinomas (NEBC) could also suffer the
development of a second primary malignancy. In the present study,
for the first time, we report a case of pure NEBC accompanied with
synchronous abdominal non-Hodgkin’s lymphoma. We review the
diagnosis, clinicopathological features and histogenetic profiling
of NEBC and discuss the literature relevant to the clinical and
anatomopathological management of this case. Written informed
consent was obtained from the patient for publication of this case
report and accompanying images.
Case report
A 58-year-old woman presented with a lump in her
right breast. A physical examination revealed an extensive and
irregular mass located in the upper outer quadrant of the patient’s
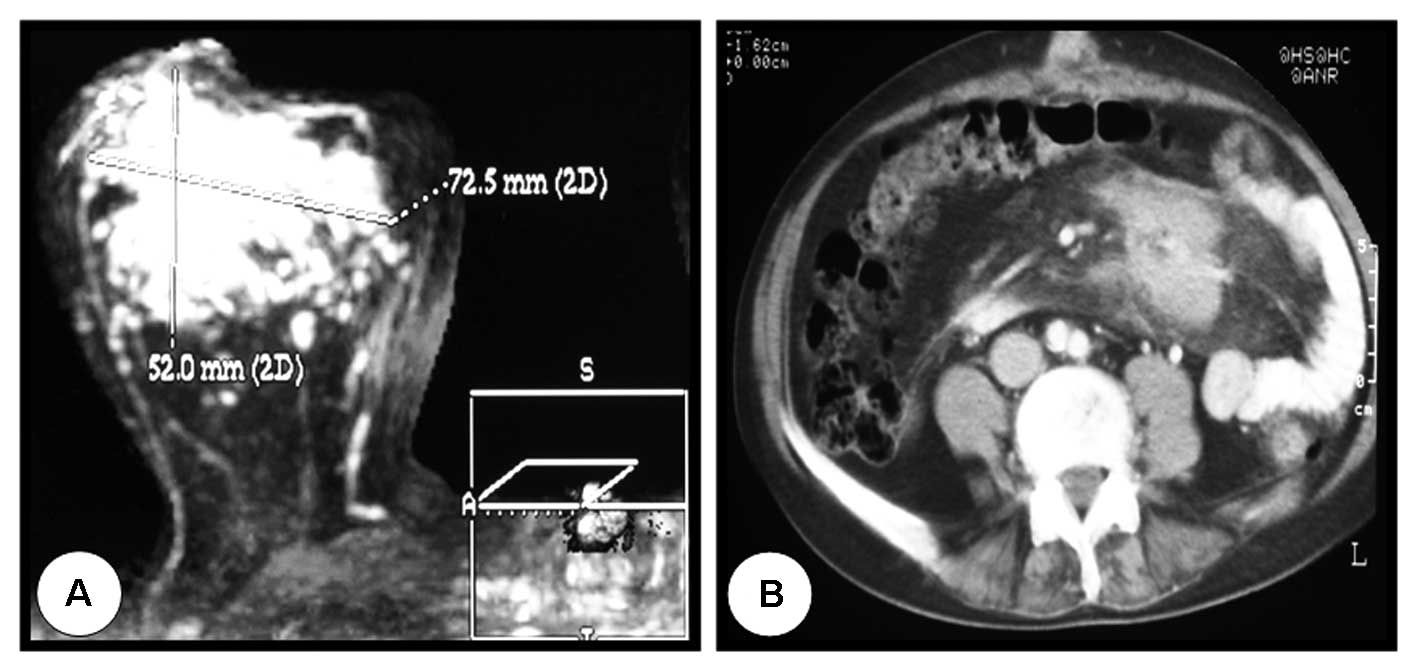
right breast. Mammography and magnetic resonance imaging (MRI)
showed a 7-cm mass that was highly suggestive of malignancy
(Fig. 1A), and pathology revealed
positive axillary lymph nodes. Core needle biopsy identified the
mass as an estrogen receptor (ER)-positive (ER+) infiltrating
ductal carcinoma. Further investigation using computed tomography
(CT) revealed an abdominal mesenteric mass of 6 cm, which was
biopsied and diagnosed 1.5 months later as a nodular, low-grade
(grade I) follicular lymphoma (Fig.
1B). To avoid undesirable treatment of the mesenteric mass with
conventional chemotherapy and since the patient had undergone
mitral valve replacement 7 years earlier, she was treated with
letrozole (2.5 mg/day) in a neoadjuvant setting for four months.
Following this therapeutic management, partial clinical response
was observed in the breast tumor with no change in the mesenteric
tumor. Four months later, the patient underwent total mastectomy
with axillary lymph node dissection. Histological examination
revealed two solid grade II neuroendocrine tumors (NETs) that
measured 5.5 and 0.9 cm, accompanied by severe lymphovascular
invasion and 14 positive metastatic lymph nodes out of 26. A
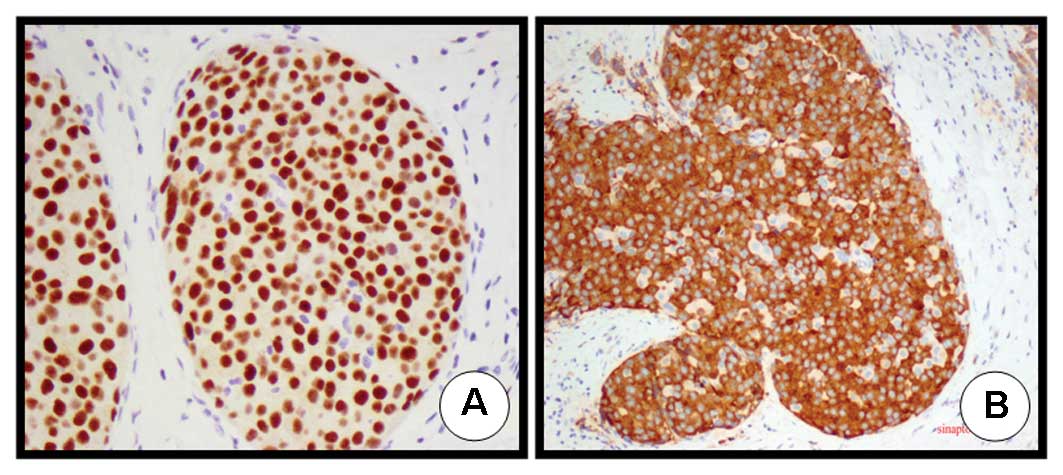
histological assessment revealed the following immunohistochemical
features: ER 3+ (Fig. 2A),
progesterone receptor (PR) 1+, HER2- 1+, p53 negative, E-cadherin
3+ and Ki67 3+. Staining was positive for both specific markers of
NETs (i.e., chromogranin and synaptophysin; Fig. 2B),
Discussion
NE features have been recognized in human breast
tumors for many years. Breast cancer-associated NE features may be
detected either as scattered cells immunoreactive for NE markers in
carcinomas of the usual type or as a special type of tumor in which
the vast majority of the cells display NE characteristics (3,4). In
1977, the first eight cases of breast tumors classified as NETs
based on the presence of argyrophilia and cytoplasmic dense core
granules were published (5). In
1989, Pagotti et al (6) reported
that approximately 8% of breast tumors displayed some degree of NE
differentiation in a consecutive series of 100 infiltrating breast
carcinomas. However, the actual prevalence of primary pure
NE-differentiated breast carcinoma (NEBC) was less than 1%. A
retrospective review of the mammograms of 1,845 histopathologically
proven breast cancer cases revealed five NEBCs (0.3%) (4). In 2003, the WHO classification of
breast tumors established that NEBC should exhibit morphological
features similar to those of NE tumors of the gastrointestinal
tract and lungs together with the immunohistochemical expression of
NE markers (i.e., chromogranin and synaptophysin) in more than 50%
of the tumor cell population (7).
The latter is a unique requirement for the accurate diagnosis of
primary pure NEBC. When utilizing the previous WHO classification
to determine the prevalence of NEBC in our institution (Dr Josep
Trueta University Hospital, Girona, Spain), we found that only 7
out of 1,368 infiltrating breast tumors fully satisfied the NEBC
criteria (0.5%) (8). This level of
NEBC incidence does not significantly differ from that reported in
earlier studies.
Although the prevalence of pure NEBC remains to be
definitively established when strictly following the WHO criteria,
there is an urgent need to establish NEBC-associated
clinico-histopathological features, prognostic factors and/or
imaging patterns that are distinct from those of other BC subtypes.
It has been reported that the presentation of NEBC is accompanied
by fairly well-circumscribed dense round or irregular masses with
spiculated or lobulated margins and homogeneous enhancement with a
time-intensity curve on MRI (3,4,9). The
NE histological features of pure NEBC are similar to those observed
in NE tumors at other body locations. One of the primary features
of NEBC is related to the presence of tumor cells in round solid
nests of spindle cells, plasmacytoid cells or large clear or
mucinous signet-ring cells with a peripheral palisading tendency.
Rarely, NEBCs exhibit polarized arrangements of tumor cells
containing eosinophilic granules around the lumina. Together, these
histological features form rosette-like structures in a
carcinoid-like pattern along with a cordonal arrangement of the
infiltrating tumor cells. However, the solid nests may also be
found in the solid type of in situ or infiltrating lobular
carcinoma, making an accurate diagnosis of NEBC more difficult. In
our case, this alternative diagnosis was excluded due to the
presence of rounded cells arranged in solid nests both in the first
biopsy and in the surgical specimen when assessing the palisading
cells for E-cadherin positivity and p63 negativity. Therefore, when
the diagnosis is suggestive of NEBC, the ultimate diagnosis should
be based on the immunohistochemical expression of one or both of
the NE markers synaptophysin and chromogranin in more than 50% of
the BC cell population (10). Both
markers were found in our case. It should be noted that we observed
a diffuse but strong staining for synaptophysin, whereas the
chromogranin staining was weak with a focal distribution. The weak
chromogranin staining may be related to the fact that most
diagnostic laboratories provide monoclonal antibodies raised
against isoform A of chromogranin. Accordingly, if an NEBC
primarily expresses isoform B of chromogranin, the tumor will be
scored as chromogranin-negative when using an antibody that
exclusively recognizes isoform A.
NETs are frequently associated with synchronous or
metachronous second primary malignancies (SPMs). It has been
reported that almost 15% of patients with NETs can be identified as
having an SPM (1,2). Prommegger et al (1) reviewed fourteen patients with NETs and
synchronous or metachronous SPMs from a series of 96 patients with
NETs to determine the primary site and characteristics of the NETs
and associated SPMs. Regardless of the localization of the NET
(i.e., appendix, ileum, duodenum, stomach, jejunum, pancreatic
tail, rectum or lung), the authors found that three months to five
years after diagnosis, NETs were highly associated with
gastrointestinal and genitourinary SPMs (i.e., SPMs of the colon,
stomach, bladder, ovary, pancreas, breast, lung, gastric MALT
lymphoma and liver). However, Prommegger et al (1) did not report any patients with NEBCs.
In our case, although we considered the possibility of metastatic
BC when analyzing the mesenteric biopsy, the immunohistochemical
profile clearly revealed a nodular, low-grade (grade I) follicular
lymphoma (CD20+, CD10+, BCL2+, CD23+, CD3−, CD5− and cyclin D1−).
It should be noted that although the occurrence of second tumors is
a well-recognized phenomenon in BC patients who have undergone
adjuvant chemotherapy and radiotherapy, presentation with second
synchronous non-breast primary malignancy is extremely rare. Tanaka
et al (11) reported a
significantly increased risk (30%) of the development of ovarian
cancer, thyroid cancer and non-Hodgkin’s lymphoma among BC patients
relative to the risk of the general population. However, this
sequence of events typically involves an interval of several years.
The synchronous presentation of BC and an SPM, that is, a
malignancy diagnosed within a six-month period, is an exceptional
phenomenon, particularly when considering the synchronous
association of BC and lymphoma. Although there are a few
publications describing this specific association, all of these
publications report that the lymphoma was either located in the
breast itself or in the axillary nodes (11–13).
The exceptionality of our case is that the lymphoma occurred in the
abdominal cavity and, in addition, that the lymphoma was
synchronous with a very rare subtype of BC (i.e., NEBC). These
findings strongly support the notion that we should confirm or
reject a differential diagnosis of a SPM. Awareness of this may
greatly improve the staging and treatment of both diseases - which
may be different - when there is a diagnostic suspicion of
metastatic disease in patients with NEBC.
Prognostic factors in NEBC do not differ from those
classically considered for other BC subtypes. Histological grade,
mucinous differentiation and the expression of ER and PR have been
suggested as reliable features that are indicative of the clinical
outcome of NEBC (14,15). In agreement with earlier studies,
the tumor in our case was classified as grade II (moderately
differentiated) according to modified Scarff-Bloom-Richardson
histological grading criteria. Although the focal amount of
mucinous differentiation was not sufficient for the tumor to be
considered a mucinous carcinoma, it may correlate with a good
prognosis, whereas the presence of the small cell NE subtype has
been reported to negatively impact the prognosis of NE tumors. The
NEBC case described herein was positive for both ER and PR.
Regarding treatment, anthracycline-based chemotherapy is the first
choice, and maintenance hormone therapy has been generally
prescribed for the management of patients with ER/PR-positive NEBC
(16). For our patient, we
initially used letrozole as hormone therapy in a neoadjuvant
setting (2.5 mg/day for four months) due to the tumor size (7 cm)
and to avoid the undesirable treatment of the mesenteric mass with
conventional chemotherapy. Letrozole was also selected because the
patient had been treated with anthracycline-based regimens when she
underwent mitral valve replacement 7 years earlier.
We cannot confirm whether there was a direct
correlation between the two primary malignancies observed in our
patient or whether they were the result of independent events
(17). Using array and metaphase
comparative genomic hybridization (CGH) with synchronous primary
breast tumors, Ghazani et al (18)
recently suggested that synchronous BC may represent a special
subtype of breast tumor in which, at least in certain cases, one
tumor gives rise to the other. Although we are aware that the more
widespread clinical use of this technology will require the use of
standardized methods for the routine analysis of clinical
specimens, CGH arrays should be considered as a valuable tool that
may offer a definitive answer during the clinicopathological
follow-up of NETs and associated SPMs (19). Since primary symptoms are caused by
SPMs in a significant subgroup of NET patients (1), it is reasonable to suggest that NETs
and NEBCs should be cautiously considered to be index tumors.
Therefore, risk-adapted clinicopathological follow-up with
systematic investigation is strongly recommended.
Acknowledgements
The work in the laboratory of Javier
A. Menendez is supported by the Instituto de Salud Carlos III
(Ministerio de Sanidad y Consumo, Fondo de Investigación Sanitaria
(FIS), Spain, Grants CP05-00090 and PI06-0778 and RD06-0020-0028),
the Fundación Científica de la Asociación Española Contra el Cáncer
(AECC, Spain), and by the Ministerio de Ciencia e Innovación
(SAF2009-11579, Plan Nacional de I+D+ I, MICINN, Spain).
References
|
1.
|
Prommegger R, Ensinger C, Steiner P,
Sauper T, Profanter C and Margreiter R: Neuroendocrine tumors and
second primary malignancy, a relationship with clinical impact?
Anticancer Res. 24:1049–1051. 2004.PubMed/NCBI
|
|
2.
|
Berruti A, Saini A, Leonardo E, Cappia S,
Borasio P and Dogliotti L: Management of neuroendocrine
differentiated breast carcinoma. The Breast. 13:527–529. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Frachon S, Pasquier D, Treilleux I,
Seigneurin D, Ringeisen F, Rosier P, Bolla M and Boutonnat J:
Breast carcinoma with predominant neuroendocrine differentiation.
Ann Pathol. 24:278–283. 2004.PubMed/NCBI
|
|
4.
|
Günhan-Bilgen I, Zekioglu O, Ustün EE,
Memis A and Erhan Y: Neuroendocrine differentiated breast
carcinoma: imaging features correlated with clinical and
histophatological findings. Eur Radiol. 13:788–793. 2003.PubMed/NCBI
|
|
5.
|
Cubilla AL and Woodruff JM: Primary
carcinoid tumor of the breast: A case report of eight patients. Am
J Surg Pathol. 1:283–292. 1977. View Article : Google Scholar
|
|
6.
|
Pagotti M, Marci L, Finzi G, Capella C,
Eusebi V and Bussolati G: Neuroendocrine differentiation in
carcinoma of the breast: A study of 51 cases. Semin Diagn Pathol.
6:174–188. 1989.PubMed/NCBI
|
|
7.
|
Tavassoli FA and Devilee P: World Health
Organization Classification of Tumours. Tumours of the Breast and
Female Genital Organs. IARC Press; Lyon: 2003
|
|
8.
|
López-Bonet E, Alonso-Ruano M, Barraza G,
Vazquez-Martin A, Bernadó L and Menendez JA: Solid neuroendocrine
breast carcinomas: Incidence, clinico-pathological features and
immunohistochemical profiling. Oncol Rep. 20:1369–1374.
2008.PubMed/NCBI
|
|
9.
|
Fujimoto Y, Yagyu R, Murase K, Kawajiri H,
Ohtani H, Arimoto Y, Yamamura T, Inoue T and Moritani S: A case of
solid neuroendocrine carcinoma of the breast in a 40-year-old
woman. Breast Cancer. 14:250–253. 2007.PubMed/NCBI
|
|
10.
|
Sapino A, Papotti M, Righi L, Cassoni P,
Chiusa L and Bussolati G: Clinical significance of neuroendocrine
carcinoma of the breast. Ann Oncol. 12:115–117. 2001. View Article : Google Scholar
|
|
11.
|
Tanaka H, Tsukuma H, Koyama H, Kinoshita
Y, Kinoshita N and Oshima A: Second primary cancers following
breast cancer in the Japanese female population. Jpn J Cancer Res.
92:1–8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Cox J, Lunt L and Webb L: Synchronous
presentation of breast carcinoma and lymphoma in the axillary
nodes. Breast. 15:246–252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Frei KA, Bonel HM, Forrer P, Alleman J and
Steiner RA: Primary breast lymphoma, contralateral breast cancer,
and bilateral Brenner tumors of the ovary. Obstet Gynecol.
100:1079–1082. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Sapino A and Bussolati G: Is detection of
endocrine cells in breast adenocarcinoma of diagnostic and clinical
significance? Histopathology. 40:211–214. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zekioglu O, Erhan Y, Ciriş M and
Bayramoglu H: Neuroendocrine differentiated carcinomas of the
breast: a distinct entity. Breast. 12:251–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Adegbola T, Connolly CE and Mortimer G:
Small cell neuroendocrine carcinoma of the breast: a report of
three cases and review of literature. J Clin Pathol. 58:775–778.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Frey BM, Morant R, Senn HJ, Fisch T and
Schmid U: Simultaneous occurrence of breast carcinoma and malignant
lymphoma. Case observations and literature review. Schweiz Med
Wochenschr. 124:1010–1016. 1994.(In German).
|
|
18.
|
Ghazani AA, Arneson N, Warren K, Pintilie
M, Bayani J, Squire JA and Done SJ: Genomic alterations in sporadic
synchronous primary breast cancer using array and metaphase
comparative genomic hybridization. Neoplasia. 9:511–520. 2007.
View Article : Google Scholar
|
|
19.
|
Wa CV, DeVries S, Chen YY, Waldman FM and
Hwang ES: Clinical application of array-based comparative genomic
hybridization to define the relationship between multiple
synchronous tumors. Mod Pathol. 18:591–597. 2005. View Article : Google Scholar
|