Introduction
Colorectal carcinoma is one of the most common
malignancies worldwide. Prior to the era of targeted therapy,
chemotherapy was the only option of systemic therapy for metastatic
colorectal carcinoma (mCRC). Chemotherapy regimens, comprising
5-fluorouracil (5-FU) plus leucovorin (LV) backbone with either
oxaliplatin or irinotecan, have improved both progression-free
survival (PFS) and overall survival (OS) (1–4).
Capecitabine, an oral antimetabolite that is metabolised
preferentially in tumor cells, in combination with oxaliplatin
demonstrates a similar efficacy to FOLFOX4 (oxaliplatin in
combination with 5-FU and LV) in the treatment of mCRC (5).
The development of targeted therapy has expanded
treatment options for patients with mCRC. Bevacizumab, a
recombinant humanized monoclonal antibody against vascular
endothelial growth factor (VEGF), used in conjunction with
chemotherapy has demonstrated efficacy as a first- and second-line
treatment (6–8). On the other hand, cetuximab, a
chimeric monoclonal antibody against epithelial growth factor
receptor (EGFR), is active as a first- and second-line treatment
when combined with chemotherapy and as a single agent in third-line
therapy (9–11). It has enhanced the effect of
treatment in patients with Kras wild-type mCRC (9,12) and
testing for the Kras status of the tumor specimen is recommended in
various international treatment guidelines.
With regard to chemotherapy, the efficacy is
independent of the sequence of use of individual chemotherapeutic
agents, provided that patients are treated with all the active
agents (13,14). However, the same principle of
maximum exposure and indiscriminate sequence of use of all agents
may not be applicable to the use of anti-VEGF and anti-EGFR
antibodies (15–17). In the case of cetuximab failure, the
option of either switching to a bevacizumab-containing regimen or
using cetuximab beyond progression are both practiced but lack
supporting evidence. As pre-clinical data have suggested that
acquired resistance to anti-EGFR antibody is associated with an
increased level of VEGF, the sequence of their use may have
practical implications (18–20).
In this retrospective study, the outcomes of patients who received
bevacizumab-containing regimens following cetuximab failure for
Kras wild-type mCRC were reviewed and presented.
Materials and methods
Study eligibility
All patients with mCRC who were treated with
bevacizumab-containing regimens between January 2006 and December
2011 were screened. Patients were eligible for review in our study
if they met the following criteria: i) Kras wild-type mCRC; ii)
chemotherapy and cetuximab received as immediate prior treatment;
iii) chemotherapy and bevacizumab received as the index line of
treatment; and iv) imaging conducted for response evaluation. Out
of the 50 patients that were screened, 18 patients satisfied the
criteria and were eligible for analysis.
Chemotherapy regimens
Patients were treated with bevacizumab at 5 mg/kg
every 2 weeks if combined with FOLFOX4 (1) or FOLFIRI (irinotecan plus 5-FU and LV)
(2), or at 7.5 mg/ kg every 3 weeks
if combined with XELOX (capecitabine plus oxaliplatin) (5), XELIRI (capecitabine plus irinotecan)
(21) or XELODA (capecitabine
alone) (22). No dose adjustment
was permitted for bevacizumab, while the dose of chemotherapeutic
agents was determined and adjusted at the discretion of the
treating oncologist, based on our departmental protocol.
Outcomes measures
Outcome measures included PFS (from the start of
bevacizumab treatment following cetuximab failure, to the first
recorded occurrence of physician-assessed disease progression, PD,
or death). The objective response rate (ORR) was evaluated by
imaging using response evaluation criteria in solid tumors (RECIST)
criteria every 8–12 weeks of treatment (23).
Targeted adverse events were recorded in accordance
with two prospective observational cohort studies, the BRiTE and
BEAT study (24,25). Adverse events included
gastrointestinal perforation (GIP; perforation, intra-abdominal
abscess and fistula), arterial thromboembolic events (ATEs;
myocardial infarction, cerebrovascular accident, transient ischemic
attack and unstable angina), postoperative bleeding or
wound-healing complications (POWHCs), grade III/IV bleeding and
hypertension requiring additional anti-hypertensives. Toxicity
grading was based on the National Cancer Institute (NCI) Common
Toxicity Criteria for Adverse Events (CTCAE), version 3.0 (26). Adverse events attributed to
bevacizumab were identified up to 90 days after permanent
discontinuation of the drug.
Statistical analysis
The primary endpoint of our analysis was median
progression-free survival (mPFS) and ORR. Survival rates were
estimated using the Kaplan-Meier method and survival curves were
compared using the log-rank test. The Fisher’s exact test was used
to compare response rates. P<0.05 was considered to indicate a
statistically significant difference. Analyses were conducted using
the Statistical Package for Social Sciences (SPSS) 19.0 for Windows
(SPSS, Inc.; Chicago, IL, USA).
Results
Patient characteristics
The median patient age was 56.5 years. Metastatic
disease was identified at initial diagnosis in 15 (83.3%) patients,
while 10 (55.6%) patients presented with metastases involving more
than 1 organ. The liver was the most common site of metastasis and
5 patients exhibited liver-only metastasis (Table I). Following cetuximab failure, 8
and 10 patients received second- and third-line
bevacizumab-containing regimens, respectively. Bevacizumab was
administered with irinotecan-based chemotherapy in 13 patients and
oxaliplatin-based chemotherapy in 5 patients. The median time
period from cetuximab failure to the start of bevacizumab treatment
was 6.8 weeks (range, 1–60) and the median number of cycles of
bevacizumab was 6.5 (range, 4–12).
 | Table I.Baseline patient characteristics. |
Table I.
Baseline patient characteristics.
| Characteristic | No. patients
(n=18) |
|---|
| Median age
(range) | 56.5 (42–72) |
| Gender | |
| Male | 9 |
| Female | 9 |
| ECOG performance
status | |
| 0 | 3 |
| 1 | 15 |
| Primary tumor
site | |
| Colon | 13 |
| Rectum | 5 |
| Number of metastatic
sites | |
| 1 | 8 |
| >1 | 10 |
| Site of
metastasis | |
| Liver | 15 |
| Lymph node | 8 |
| Lung | 5 |
| Locoregional | 4 |
| Peritoneum | 3 |
| Prior
chemotherapy | |
|
Fluoropyrimidine | 18 |
| Oxaliplatin | 17 |
| Irinotecan | 6 |
Treatment efficacy
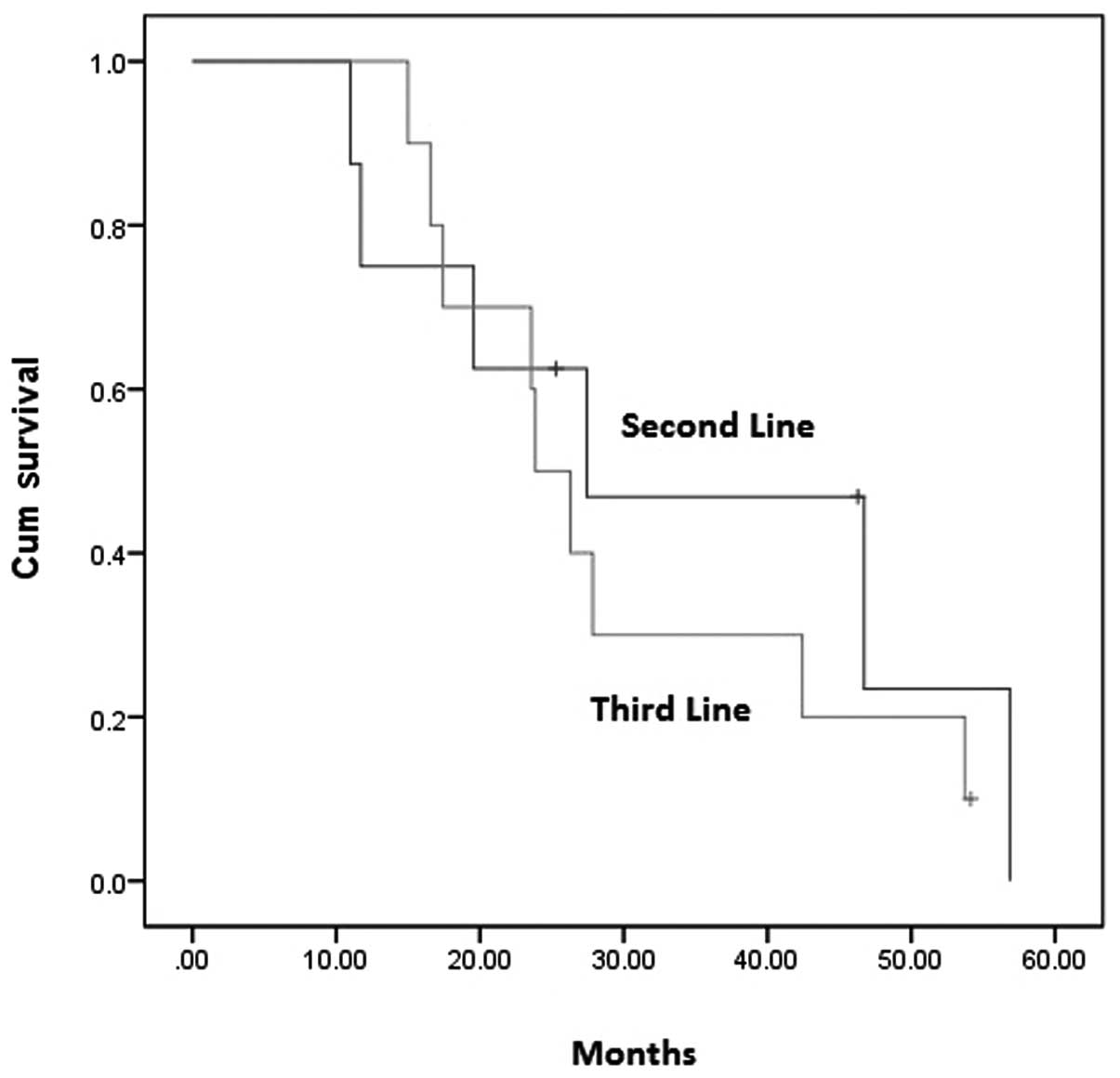
After a median follow-up of 12.1 months, the mPFS
for the total group of patients was 26.3 weeks (95% CI, 19.5–33.0)
with an ORR of 38.9%. For the 8 patients who received
bevacizumab-containing regimens as a second-line treatment, 1
complete response (CR) and 3 partial responses (PR) were observed,
producing an ORR of 50%. The mPFS was 27.4 weeks (95% CI,
2.0–52.8). For the 10 patients who received the third-line
treatment, 3 PRs were observed and thus the ORR was 30%. The mPFS
was 23.9 weeks (95% CI, 19.7–28.1). No statistically significant
difference in PFS (P= 0.552) and ORR (P= 0.63) was observed between
patients who received bevacizumab as a second- or third-line
treatment following cetuximab failure (Figs. 1 and 2).
Toxicity related to bevacizumab
Two patients presented with worsened hypertension
that was controlled by an additional anti-hypertensive drug. One
patient was found to have intestinal perforation 78 days after the
last dose of bevacizumab. Another patient had bevacizumab suspended
for 6 weeks before planned resection of liver and pelvic
metastases. Complete resection was achieved and pathological
examination confirmed a partial response. Additionally, no
post-operative complications were observed for this patient. In the
whole cohort of patients, no ATEs or grade III/IV bleeding were
observed.
Discussion
To our knowledge, this is the first study concerning
the outcomes of bevacizumab-containing regimens following cetuximab
failure in patients with Kras wild-type mCRC.
The treatment outcomes of patients treated with
bevacizumab-containing regimens as the second-line therapy were
comparable with a previously reported phase III trial (8). Patients treated with
bevacizumab-containing regimens as the third-line therapy
demonstrated a median PFS of 23.9 weeks and an ORR of 30%, which
were both superior to those found previously. Emmanouilides et
al studied the outcomes of 19 patients who had received
bevacizumab with 5-FU plus LV as a third-line treatment in a
prospective study. The median time to progression was 16 weeks but
no objective response was documented (27). In a retrospective analysis by Kang
et al, bevacizumab was combined with either FOLFOX or
FOLFIRI in a third-line or later treatment after failure of 5-FU,
oxaliplatin and irinotecan. The median PFS was 5.3 months and the
overall response rate was 9.5% (28). The results of these two studies were
similar to those of a TRC-0301 study in which the median PFS was
3.5 months and the response rate was 4% (29). Vincenzi et al conducted a
phase II study using bevacizumab and 5-FU plus LV as the
fourth-line setting in 48 patients who failed cetuximab,
oxaliplatin, irinotecan and 5-FU treatment. The response rate was
only 6.25%; however, 30.4% of patients achieved a stable disease
status (30). In all the
aforementioned studies, the Kras status of tumors was not noted and
prior cetuximab exposure was only documented in the study by
Vincenzi et al. Although 17 patients in the present study
failed oxaliplatin, 5-FU and cetuximab treatment, only 6 patients
in our study as compared with all patients in the aforementioned
studies failed oxaliplatin, irinotecan and 5-FU treatment. While
‘chemo-refractoriness’ differed among patients in the present study
and those quoted previously, the potential additional benefit of
using bevacizumab following cetuximab failure should not be
overlooked.
It has been demonstrated that the exact sequence of
chemotherapeutic agents used in mCRC chemotherapy did not affect
the outcome (14), provided
patients were exposed to all active agents (13). However, there is no real evidence
for applying the same principle to the use of anti-EGFR and
anti-VEGF antibodies. Both the CAIRO2 and PACCE trials demonstrated
inferior results with the addition of anti-EGFR antibody to
bevacizumab-containing regimens (15,16).
These two phase III randomized controlled studies were unable to
confirm why administering more did not lead to improvement with
regard to the use of targeted therapies; however, the results did
call for the investigation of an optimal sequence of use of these
targeted therapies. Notably, a study by Norguet et al
investigated the effect of prior exposure to bevacizumab on the
efficacy of subsequent cetuximab treatment (17). In the present study, patients with
prior exposure to bevacizumab were associated with a significantly
inferior outcome with subsequent cetuximab treatment. Taken
together, it is necessary to identify patients who may benefit most
from a specific sequence of use of anti-EGFR and anti-VEGF
antibodies.
An enhanced treatment effect was demonstrated in
patients with Kras wild-type tumor treated with cetuximab (9,12). All
patients in the present study had Kras wild-type tumor and were
treated with cetuximab. As demonstrated in pre-clinical studies,
prolonged exposure of cancer cells to EGFR-blocking antibodies
gives rise to resistant cells that have increased VEGF expression.
Thus, cancer cells may become more dependent on the VEGF pathway
when they acquire resistance to the EGFR inhibitor (18–20).
It could be postulated that the superior outcomes of the patients
in the present study were partly due to the selection of patients
with Kras wild-type tumor; patients were treated with bevacizumab
at a time when the cancer cells had become more dependent on the
VEGF pathway upon acquiring resistance to the EGFR inhibitor.
In the present study, the toxicity related to
bevacizumab was infrequent and manageable. Two patients required
administration of one additional anti-hypertensive drug for the
treatment of worsened hypertension during the course of
bevacizumab. This proportion of patients was similar to those
observed in landmark studies with an incidence of 4–11% for grade
III/IV hypertension (6–8). The patient who did not survive due to
a bowel perforation was unlikely to have suffered the perforation
as a result of bevacizumab; the event occurred 78 days after the
last dose of bevacizumab when the patient was receiving
hypofractionated palliative radiotherapy to the pelvis. Therefore,
there were no patient fatalities due to bevacizumab-related
toxicity during the active phase of bevacizumab treatment.
In conclusion, the use of bevacizumab-containing
regimens following cetuximab failure in patients with Kras
wild-type mCRC has modest activity and acceptable toxicity. A small
sample size and retrospective nature were the major limitations of
the present study. However, the results remain informative. Unlike
concurrent use of bevacizumab and cetuximab, and likely the
sequential use of cetuximab following bevacizumab, the use of
bevacizumab-containing regimens following cetuximab failure may
represent an optimal sequence of targeted therapies and warrants
further research in prospective studies.
References
|
1.
|
de Gramont A, Figer A, Seymour M, et al:
Leucovorin and fluorouracil with or without oxaliplatin as
first-line treatment in advanced colorectal cancer. J Clin Oncol.
18:2938–2947. 2000.
|
|
2.
|
Douillard JY, Cunningham D, Roth AD, et
al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: a multicentre randomised trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Goldberg RM, Sargent DJ, Morton RF, et al:
A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan, and oxaliplatin combinations in patients with
previously untreated metastatic colorectal cancer. J Clin Oncol.
22:23–30. 2004. View Article : Google Scholar
|
|
4.
|
Saltz LB, Cox JV, Blanke C, et al:
Irinotecan plus fluorouracil and leucovorin for metastatic
colorectal cancer. N Engl J Med. 343:905–914. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Cassidy J, Clarke S, Díaz-Rubio E, et al:
Randomized phase III study of capecitabine plus oxaliplatin
compared with fluorouracil/folinic acid plus oxaliplatin as
first-line therapy for metastatic colorectal cancer. J Clin Oncol.
26:2006–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Saltz LB, Clarke S, Díaz-Rubio E, et al:
Bevacizumab in combination with oxaliplatin-based chemotherapy as
first-line therapy in metastatic colorectal cancer: a randomized
phase III study. J Clin Oncol. 26:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Giantonio BJ, Catalano PJ, Meropol NJ, et
al: Bevacizumab in combination with oxaliplatin, fluorouracil, and
leucovorin (FOLFOX4) for previously treated metastatic colorectal
cancer: results from the Eastern Cooperative Oncology Group Study
E3200. J Clin Oncol. 25:1539–1544. 2007. View Article : Google Scholar
|
|
9.
|
Van Cutsem E, Köhne CH, Hitre E, et al:
Cetuximab and chemo-therapy as initial treatment for metastatic
colorectal cancer. N Engl J Med. 360:1408–1417. 2009.PubMed/NCBI
|
|
10.
|
Cunningham D, Humblet Y, Siena S, et al:
Cetuximab mono-therapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Jonker DJ, O’Callaghan CJ, Karapetis CS,
et al: Cetuximab for the treatment of colorectal cancer. N Engl J
Med. 357:2040–2048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Bokemeyer C, Bondarenko I, Hartmann JT, et
al: Efficacy according to biomarker status of cetuximab plus
FOLFOX-4 as first-line treatment for metastatic colorectal cancer:
the OPUS study. Annals of Oncology. 22:1535–1546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Grothey A, Sargent D, Goldberg RM and
Schmoll HJ: Survival of patients with advanced colorectal cancer
improves with the availability of fluorouracil-leucovorin,
irinotecan, and oxaliplatin in the course of treatment. J Clin
Oncol. 22:1209–1214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Tournigand C, André T, Achille E, et al:
FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: a randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Tol J, Koopman M, Cats A, et al:
Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal
cancer. N Engl J Med. 360:563–572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Hecht JR, Mitchell E, Chidiac T, et al: A
randomized phase IIIB trial of chemotherapy, bevacizumab, and
panitumumab compared with chemotherapy and bevacizumab alone for
metastatic colorectal cancer. J Clin Oncol. 27:672–680. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Norguet E, Dahan L, Gaudart J, Gasmi M,
Ouafik Lh and Seitz JF: Cetuximab after bevacizumab in metastatic
colorectal cancer: Is it the best sequence? Dig Liver Dis.
43:917–919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Bianco R, Rosa R, Damiano V, et al:
Vascular endothelial growth factor receptor-1 contributes to
resistance to anti-epidermal growth factor receptor drugs in human
cancer cells. Clin Cancer Res. 14:5069–5080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Naumov GN, Nilsson MB, Cascone T, et al:
Combined vascular endothelial growth factor receptor and epidermal
growth factor receptor (EGFR) blockade inhibits tumor growth in
xenograft models of EGFR inhibitor resistance. Clin Cancer Res.
15:3484–3494. 2009. View Article : Google Scholar
|
|
20.
|
Viloria-Petit A, Crombet T, Jothy S, et
al: Acquired resistance to the antitumor effect of epidermal growth
factor receptor-blocking antibodies in vivo. Cancer Res.
61:5090–5101. 2001.PubMed/NCBI
|
|
21.
|
Patt YZ, Lee FC, Liebmann JE, et al:
Capecitabine plus 3-weekly irinotecan (XELIRI regimen) as
first-line chemotherapy for metastatic colorectal cancer: phase II
trial results. Am J Clin Oncol. 30:350–357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Cassidy J, Twelves C, Van Cutsem E, et al:
First-line oral capecitabine therapy in metastatic colorectal
cancer: a favorable safety profile compared with intravenous
5-fluorouracil/leucovorin. Annals of Oncology. 13:566–575. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: Revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
24.
|
Kozloff M, Yood MU, Berlin J, et al:
Clinical outcomes associated with bevacizumab-containing treatment
of metastatic colorectal cancer: the BRiTE observational cohort
study. Oncologist. 14:862–870. 2009. View Article : Google Scholar
|
|
25.
|
Van Cutsem E, Rivera F, Berry S, et al:
Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX,
FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the
BEAT study. Ann Oncol. 20:1842–1847. 2009.PubMed/NCBI
|
|
26.
|
Trotti A, Colevas AD, Setser A, et al:
CTCAE v3.0: development of a comprehensive grading system for the
adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar
|
|
27.
|
Emmanouilides C, Pegram M, Robinson R,
Hecht R, Kabbinavar F and Isacoff W: Anti-VEGF antibody bevacizumab
(Avastin) with 5FU/LV as third line treatment for colorectal
cancer. Tech Coloproctol. 8:s50–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Kang B, Kim T, Lee JL, et al: Bevacizumab
plus FOLFIRI or FOLFOX as third-line or later treatment in patients
with meta-static colorectal cancer after failure of 5-fluorouracil,
irinotecan, and oxaliplatin: a retrospective analysis. Med Oncol.
26:32–37. 2009. View Article : Google Scholar
|
|
29.
|
Chen HX, Mooney M, Boron M, et al: Phase
II multicenter trial of bevacizumab plus fluorouracil and
leucovorin in patients with advanced refractory colorectal caner:
an NCI Treatment Referral Center Trial TRC-0301. J Clin Oncol.
24:3354–3360. 2006. View Article : Google Scholar
|
|
30.
|
Vincenzi B, Santini D, Russo A, et al:
Bevacizumab in association with de Gramont 5-fluorouracil/folinic
acid in patients with oxaliplatin-, irinotecan-, and
cetuximab-refractory colorectal cancer: a single-center phase 2
trial. Cancer. 115:4849–4856. 2009. View Article : Google Scholar
|