Introduction
Pancreatic cysts are commonly detected incidentally
in patients undergoing abdominal imaging for unrelated procedures.
Simple (retention) cysts, pseudocysts and serous cystadenomas lack
malignant potential. However, mucinous cystic neoplasms and
intraductal papillary mucinous neoplasm (IPMN) have a malignant
potential and require surgical treatment (1–4). Due
to the possibility of malignancy in specific pancreatic cysts, it
is important differentiate between benign and malignant lesions to
determine whether surgical resection or conservative management is
required.
Analysis of cystic fluid may be useful for
distinguishing between benign and malignant pancreatic lesions. To
date, several tests using cyst fluid to diagnose premalignant cyst
are in use. These include cytology, tumor markers [i.e.,
carcinoembryonic antigen (CEA) or carbohydrate antigen 19-9 (CA
19-9)], biochemical markers (i.e., amylase) and cyst fluid
viscosity. Among these tests, CEA has the highest diagnostic
accuracy for discriminating premalignant mucinous from nonmucinous
cysts (5,6). However, CEA cannot differentiate
between a premalignant cyst and a malignant lesion (7,8).
CA 19-9 is the most popular serum-based marker for
pancreatic cancer diagnosis and is important for the detection of
recurrent disease and surveillance of patients following surgery.
Previous studies have demonstrated that CA 19-9 cyst fluid analysis
may also be useful for differential diagnosis of pancreatic cysts,
particularly in pancreatic cystadenocarcinoma detection (9–11).
Current data are insufficient to reliably determine the clinical
value of CA 19-9 cyst fluid analysis, however, if a panel of tests
is performed in conjunction with clinical and radiological
observations, the identity of pancreatic cysts is predicted with a
high degree of reliability.
The third analyzed parameter, pancreatic cyst fluid
amylase, may be particularly useful for the identification of
pseudocysts. Distinguishing pseudocysts from malignant cystic
tumors is essential during selection of appropriate surgical
procedures. Pseudocysts may be managed by observation or, in
specific cases with endoscopic or surgical drainage. The amylase
content of pseudocysts is almost always high, whereas the level in
neoplastic cysts is generally low. However, cystic tumors of all
types may exhibit elevated amylase levels. Consequently, the
efficacy of amylase measurements in pancreatic cyst fluids is
limited, although low values indicate a neoplastic tumor (6,8,12,13).
The aim of the present study was to assess the
diagnostic utility and clinical value of CEA, CA 19-9 and amylase
analysis in pancreatic cyst fluid.
Materials and methods
Sample collection and classification
The present study included 52 patients (28 males and
24 females) with pancreatic cystic lesions. Patients underwent
fine-needle aspiration biopsy to collect cystic fluid for
cytological and biochemical analysis. Informed consent was obtained
from all patients. The study was approved by the ethical committee
of Lodz Medical University. Based on surgical histopathology,
cytology results and/or imaging follow-up (>18 months), cysts
were classified as benign (simple cysts, pseudocysts and serous
cystadenomas) or premalignant/malignant (mucinous cystadenomas,
IPMNs and cystadenocarcinomas) in 36 and 16 patients,
respectively.
Pancreatic cyst analysis
The following characteristics were analyzed: maximum
pancreatic cyst diameter, cyst number and location, wall thickness,
mural nodules, pancreatic duct communication and/or dilation and
presence of septations or calcifications. Following cyst fluid
aspiration, a portion of the specimen was sent to the chemistry
laboratory of the Department of Digestive Tract Diseases for CEA,
CA 19-9 and amylase analysis. The fluid was also examined by a
cytopathologist.
Analysis of patient characteristics
Age and gender of patients, presenting symptoms and
medical history of acute or chronic pancreatitis were also
assessed. Criteria for resection included premalignant/malignant
lesions identified by cytology or fluid analysis and suspicious
radiographical observations, including cyst size >3 cm and
intramural nodules, pancreatic duct dilation, peripheral
calcifications or associated mass. Resection was also advised for
symptomatic benign pancreatic cysts. All patients with
premalignant/malignant lesions underwent surgical treatment.
Fifteen patients with a final diagnosis of benign lesions also
underwent surgical resection due to symptoms or suspicious features
of imaging and/or cyst fluid analysis.
Statistical analysis
Statistical analysis comprised arithmetical mean,
median and standard deviation. Mann-Whitney or Fisher’s exact tests
were performed to determine differences between groups. P<0.05
was considered to indicate a statistically significant difference.
A receiver operating characteristic (ROC) curve depicting the
ability to discriminate between benign and premalignant/malignant
cysts was plotted for CEA, CA 19-9 and amylase and optimal cut-off
points were estimated.
Results
Patient characteristics
The mean age of patients included in the present
study was 55±3.2 years; there were 28 male (53.8%) and 24 female
(46.2%) subjects. A total of 36 cysts were classified as benign and
16 patients had premalignant/malignant cysts (8 mucinous
cystadenomas, 4 IPMNs and 4 cystadenocarcinomas). The majority of
patients were asymptomatic, whereas 23 patients (14 benign cyst and
9 premalignant/malignant lesions) presented abdominal pain, weight
loss and/or jaundice. Nine patients had a previous history of acute
pancreatitis, whereas chronic pancreatitis was confirmed in 11
patients.
Pancreatic cyst characteristics
The mean diameter of pancreatic cyst was 3.3 cm
(range, 1.5–8.1 cm). No statistically significant difference was
identified between benign and malignant cyst size (3.9±1.8 vs.
3.2±1.2 cm; P>0.05). Cyst localization was identified in the
pancreatic head and body or tail of pancreas in 29 (55.8%) and 23
(44.2%) patients, respectively. Cytology was assessed in all
patients and was reported as acellular, benign or atypical in 49
patients and positive for malignant cells in 3 patients.
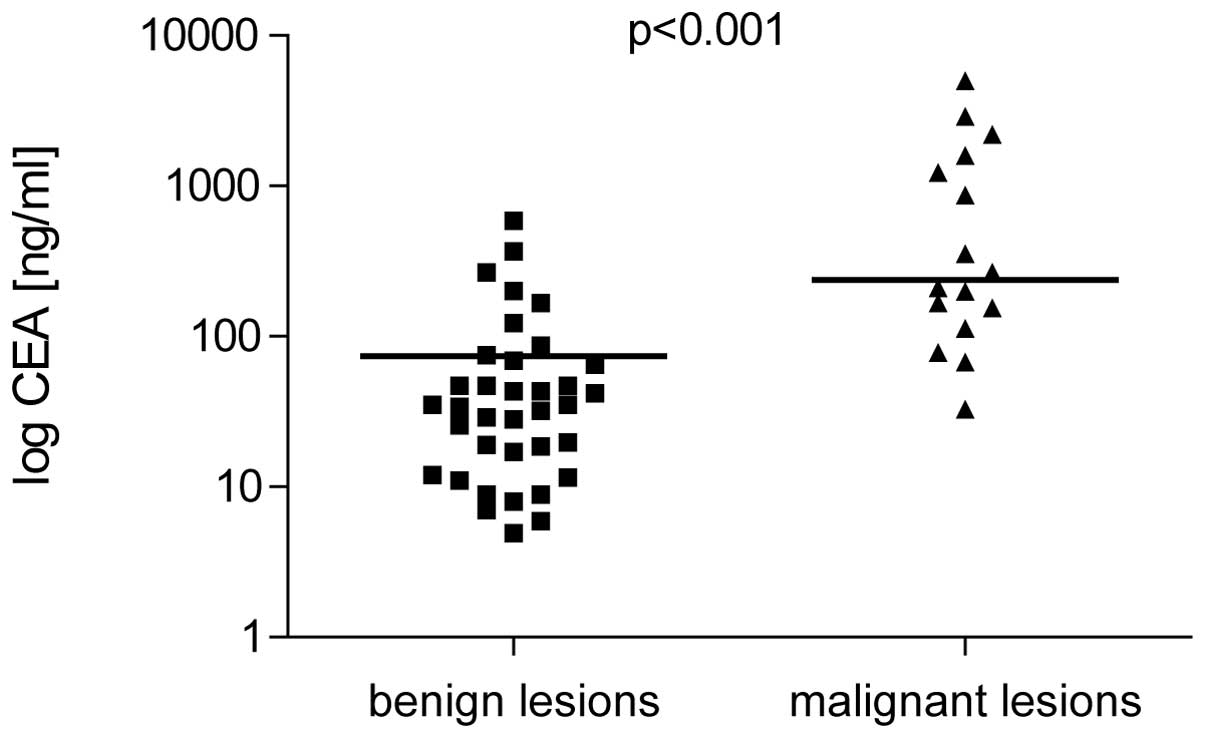
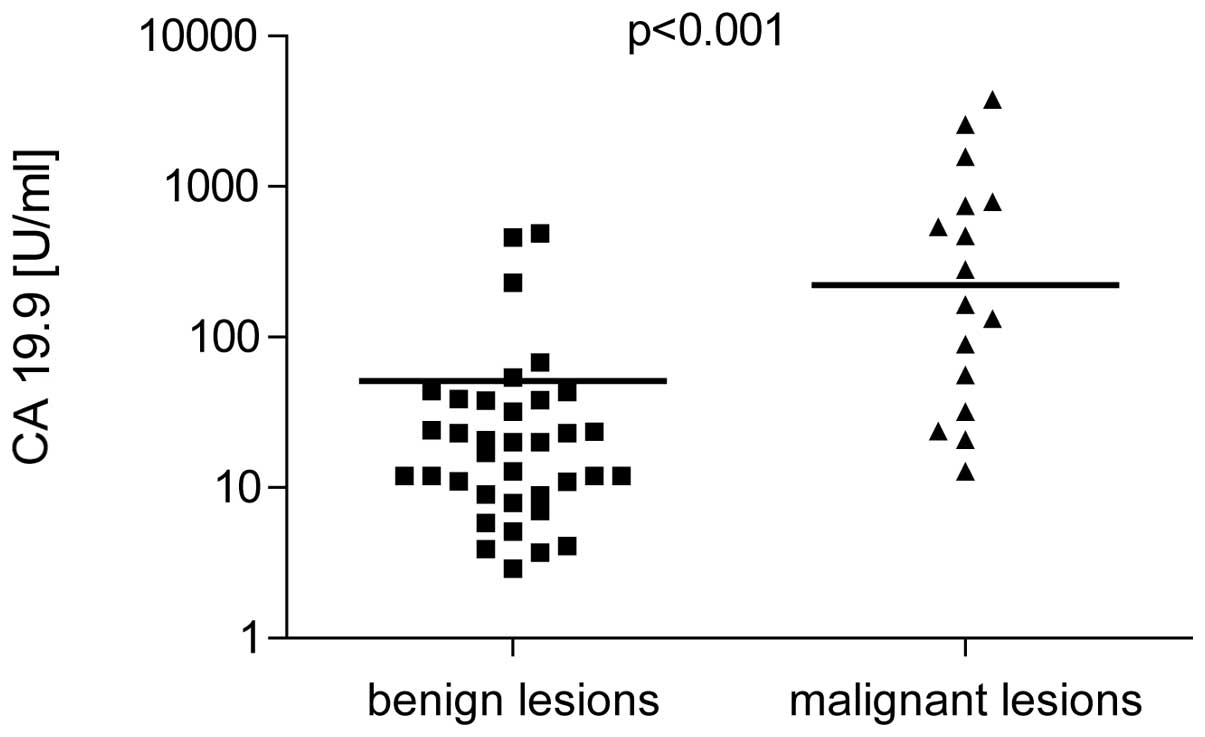
CEA and CA 19-9 levels
CEA and CA 19-9 were higher in patients with
malignant cysts (238±12.5 ng/ml and 222±31.5 U/ml, respectively)
compared with benign lesions (34.5±3.7 ng/ml and 18.5±1.9 U/ml;
P<0.001; Figs. 1 and 2). Sensitivity and specificity for CEA
(cut-off, 45 ng/ml) was 91.8 and 63.9% and for CA 19-9 (cut-off, 37
U/ml) was 81.3 and 69.4%, respectively. Positive predictive value
(PPV) of CEA was 53.6% and the negative predictive value (NPV) was
95.8% NPV and PPV of CA 19-9 were 54.2 and 89.3% respectively.
Amyase levels
Mean amylase level in benign lesions (27,825.7±91.9
U/l) was identified as significantly higher compared with malignant
pancreatic cysts (8,359.2±32.7 U/l; P<0.05; Fig. 3). The highest levels of amylase were
observed in pseudocysts (41,778±131.5 U/l). However, the amylase
sensitivity and specificity for diagnosis of premalignant/malignant
lesions was lower than those of CEA and CA 19-9 (62.5 and 69.4%,
respectively). PPV and NPV of amylase was also lower, 47.6 and
80.6%, respectively.
ROC curve
ROC curve for the abilities of CEA, CA 19-9 and
amylase to distinguish between benign and malignant lesions is
plotted in Fig. 4. Area under the
curve was 0.892 [95% confidence interval (CI), 0.803–0.981] for
CEA, 0.873 (95% CI, 0.773–0.973) for CA 19-9 and 0.684 (95% CI,
0.508–0.861) for amylase.
Discussion
Pancreatic cysts are a heterogenous tumor group with
varied clinical presentation and malignant potential.
Distinguishing between benign inflammatory or serous lesions from
potentially malignant mucinous cystic tumors is vital for clinical
differential diagnosis of pancreatic cysts. Aspiration of
pancreatic fluid cysts for additional markers has been hypothesized
to be important for patient management.
The present study identified that the median cyst
fluid CEA and CA 19-9 levels in premalignant/malignant cysts was
significantly higher than in benign cysts (P<0.001). Sensitivity
for CEA and CA 19-9 was 91.8 and 81.3%, respectively, for mucinous
lesions. Previously, the combination of CEA fluid assessment and
K-ras mutation analysis levels was confirmed to maximize the
diagnostic yield of pancreatic cyst biopsy and improve sensitivity
and specificity of cyst classification (14). However, the current cost of DNA
mutational testing limits the availablity of this analysis in
specific laboratories.
To improve the efficacy of pancreatic cyst
diagnosis, additional tumor markers have been investigated,
including CA 19-9 and amylase. Analysis of CA 19-9 fluid levels for
the differential diagnosis of pancreatic cysts is controversial. CA
19-9 fluid levels are currently considered to be less specific
compared with CEA, particularly for detection of mucinous cysts
(15,16). However, a study performed by Wu
et al identified that CA 19-9 fluid assessment had higher
sensitivity and specificity compared with CEA for detection of
pancreatic cystadenocarcinomas (83.3 and 94.4 vs. 61.1 and 92.2%,
respectively) (9). Therefore, we
hypothesized that the combination of analyzed markers may improve
their accuracy for the differential diagnosis of pancreatic
cysts.
A previous study demonstrated that the sensitivity
of cyst fluid CEA combined with CA 19-9 measurement was higher than
single tumor marker examination (9). By contrast, Brugge et al
performed analysis of pancreatic cyst fluid in a large group of
patients and concluded that fluid CEA alone is most useful for
diagnosis of malignant pancreatic cysts. The combination of
additional tests, including CA 19-9 as well as CA 72-4, CA 125 and
CA 15-3, was not identified to be more accurate. Moreover, the
addition of cyst morphology or cytology to the CEA value did not
improve diagnostic accuracy (16).
In the present study, CA 19-9 levels, with a cut-off
value of 37 U/ml, were elevated in patients with malignant cysts
compared with benign lesions. Results are consistent with previous
studies reporting that low CA 19-9 fluid levels (less than 37 U/ml)
suggest benign lesions (13,17).
The CA 19-9 cut-off value is most frequently utilized (9,10,17).
Increasing the cut-off value for CA 19-9 to support the diagnosis
of a malignant cyst has been previously demonstrated to increase
the specificity but decrease the sensitivity of the test. Frossard
et al reported that a CA 19-9 value greater than 50,000 U/ml
in the cyst fluid had an 86% sensitivity and 85% specificity for
distinguishing cystadenocarcinoma from other cystic lesions.
However, this high cut-off value had a sensitivity of only 15% for
detection of mucinous cysts. The authors concluded that this high
threshold for CA 19-9 is suitable for the detection of malignancies
but is insensitive for premalignant lesions (15).
In the present study, sensitivity of the third
analyzed parameter, amylase, was 62.5% and the specificity was
69.4%, which was lower than those of CEA and CA 19-9. Previous
studies on the clinical efficacy of amylase for differential
diagnosis of pancreatic cysts are inconsistent. However, the
parameter may be useful for confirmation of pseudocyst diagnosis,
particularly in patients with a medical history of pancreatitis.
Snozek et al reported that CEA and amylase fluid levels less
than 30 ng/ml and more than 8500 U/l, respectively, were observed
in 91% of pseudocysts (12). In
addition, Attasaranya et al demonstrated that the median
level of amylase was higher in pseudocysts compared with all other
cystic lesions (19,834 vs. 882 U/l, respectively), however, this
difference was identified to be at the limit of statistical
significance (P=0.05). The authors reported a high sensitivity
(100%) and specificity (63.6%) of cyst fluid amylase at a cut-off
of 5,000 U/l for differentiating pseudocysts from all other
pancreatic cysts (8).
Increased amylase fluid levels are not specific for
pseudocysts and has been observed in additional cysts, including
mucinous cystadenomas and IPMN (6,18,19).
Le Borgne et al, following surgical resection of 398
pancreatic cystic tumors, observed that 6% of mucinous cystadenomas
and 10% of cyst-adenocarcinomas were associated with pancreatic
ducts (19). Park et al
identified that 54% of noninflammatory cysts, including mucinous
cystic neoplasms had an increased level of amylase. However, lower
amylase levels were identified in malignant mucinous cysts than
benign mucinous cysts (6).
At present, the efficacy of amylase level analysis
for the differentiation of benign from premalignant/malignant cysts
has not been determined. However analysis of amylase may be useful
for patients with a medical history of pancreatitis where there is
a greater probability of a pseudocyst. Further evaluation of this
efficacy, based on long-term prospective studies in patients with
pancreatic cysts, must be performed.
In conclusion, the present study indicates that
analysis of pancreatic cyst fluid may be a safe and useful adjunct
for the differential diagnosis of pancreatic cystic lesions. This
analysis may distinguish inflammatory and benign neoplastic cysts
from premalignant/malignant pancreatic lesions. Results appear
promising, not only for CEA, but also for CA 19-9, however, the
clinical value of these markers must be confirmed.
Acknowledgements
This study was supported by the
Medical University of Lodz and the Polish Sociaty for the Digestive
Tract Neoplasms Prevention.
References
|
1.
|
Testini M, Gurrado A, Lissidini G, Venezia
P, Greco L and Piccinni G: Management of mucinous cystic neoplasms
of the pancreas. World J Gastroenterol. 16:5682–5692. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Baiocchi GL, Portoliani N, Missale G, et
al: Intraductal papillary mucinous neoplasm of the pancreas (IPMN):
clinico-pathological correlations and surgical indications. World J
Surg Oncol. 7:8–25. 2010.PubMed/NCBI
|
|
3.
|
Leung KK, Ross WA, Evans D, Fleming J, Lin
E, Tamm EP and Lee JH: Pancreatic cystic neoplasm: the role of cyst
morphology, cyst fluid analysis and expectant management. Ann Surg
Oncol. 16:2818–2824. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Michaels PJ, Brachtel EF, Bounds BC,
Brugge WR and Pitman MB: Intraductal papillary mucinous neoplasm
(IPMN) of the pancreas: cytopathologic analysis and correlation
with histologic grade. Cancer (Cancer Cytopathol). 108:163–173.
2006.
|
|
5.
|
Bhutani MS, Gupta V, Guha V, Gheonea DI
and Saftoiu A: Pancreatic cyst fluid analysis - a review. J
Gastrointestin Liver Dis. 20:175–180. 2011.PubMed/NCBI
|
|
6.
|
Park WG, Mascarenhas R, Palaez-Luna M, et
al: Diagnostic performance of cyst fluid carcinoembryonic antigen
and amylase in histologically confirmed pancreatic cysts. Pancreas.
40:42–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Sawhney MS, Devarajan S, O’Farrel P, et
al: Comparison of carcinoembryonic antigen and molecular analysis
in pancreatic cyst fluid. Gastrointest Endoscopy. 69:1106–1110.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Attasaranya S, Pais S, LeBlanc J, McHenry
L, Sherman S and DeWitt J: Endoscopic ultrasound-guided fine needle
aspiration and cyst fluid analysis for pancreatic cancer. J
Pancreas. 8:553–563. 2007.PubMed/NCBI
|
|
9.
|
Wu H, Cheng NS, Zhang YG, Luo HZ, Yan LN
and Li J: Improved early diagnosis of cystadenocarcinoma of the
pancreas. Hepatobiliary Pancreat Dis Int. 6:87–91. 2007.PubMed/NCBI
|
|
10.
|
Aljebreen AM, Romagnuolo J, Perini R and
Sutherland F: Utility of endoscopic ultrasound, cytology and fluid
carcinoembryonic antigen and CA 19-9 levels in pancreatic cystic
lesions. World J Gastroenterol. 13:3962–3966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wu H, Yan LN, Cheng NS, Zhang YG and Ker
CG: Role of cystic fluid in diagnosis of the pancreatic cystadenoma
and cystadeno-carcinoma. Hepatogastroenterology. 54:1915–1918.
2007.PubMed/NCBI
|
|
12.
|
Snozek CL, Mascarenhas RC and O’Kane DJ:
Use of cyst fluid CEA, Ca19-9 and amylase for evaluation of
pancreatic lesions. Clin Biochem. 42:1585–1588. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Obeso G, Murphy E, Brugge W and Deshpande
V: Pseudocyst of the pancreas: the role of cytology and special
stains for mucin. Cancer Cytopathol. 117:101–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Talar-Wojnarowska R, Pazurek M, Durko Ł,
et al: A comparative analysis of K-ras mutation and
carcinoembryonic antigen in pancreatic cyst fluid. Pancreatology.
(In press).
|
|
15.
|
Frossard JL, Amouyal P, Amouyal G, et al:
Performance of endosonography-guided fine needle aspiration and
biopsy in the diagnosis of pancreatic cystic lesions. Am J
Gastroenterol. 98:1516–1524. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Brugge WR, Lewandrowski K,
Lee-Lewandrowski E, et al: Diagnosis of pancreatic cystic
neoplasms: a report of the cooperative pancreatic cyst study.
Gastroenterology. 126:1330–1336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Van der Waaij LA, van Dullemen HM and
Porte RJ: Cyst fluid analysis in the differential diagnosis of
pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc.
62:383–389. 2005.PubMed/NCBI
|
|
18.
|
Maire F, Voitot H, Aubert A, et al:
Intraductal papillary mucinous neoplasms of the pancreas:
performance of pancreatic fluid analysis for positive diagnosis and
the prediction of malignancy. Am J Gastroenterol. 103:2871–2877.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Le Borgne J, de Calan L and Partensky C:
Cystadenomas and cystadenocarcinomas of the pancreas: a
multiinstitutional retrospective study of 398 cases. French
Surgical Association. Ann Surg. 230:152–161. 1999.PubMed/NCBI
|