Introduction
Astrocytoma is the most common primary tumour of the
central nervous system (1). Spinal
neoplasms in the adult population are mostly extradural (55%) and
intradural extra-medullary tumours (40%), whereas intramedullary
tumours account for 5% of all spinal cord tumours, excluding
metastatic lesions (2). Of these,
∼30% are tumours of low malignancy, including slow-growing
astrocytomas and ependymomas. Spinal cord astrocytomas are rare,
representing ∼1% of all primary central nervous system tumours and
6 to 8% of all spinal cord tumours (3). Few spinal cord astrocytomas are
anaplastic in nature; most are slow-growing lesions. The ratio of
low-grade to high-grade astrocytomas in the spinal cord is ∼3 to 1
(4,5). Glioblastomas (GBMs) represent ∼7.5% of
all intramedullary gliomas and 1 to 3% of all spinal cord tumours
(4,6). Moreover, GBM has a predilection of
development at the cervical or cervicothoracic region in >60% of
cases (1,7–9).
Clinical presentation is associated with the region of spinal cord
involved, irrespective of tumour type. Unlike their intracranial
counterpart, intramedullary GBMs have received scant attention in
the literature, with <200 cases reported. Even with aggressive
management, these tumours are generally associated with a dismal
outcome.
Case report
A 19-year-old male was transferred to our
institution in May 2010 with a 4-week history of progressive
weakness in both lower limbs, which progressed to paraparesis with
a left predominance and difficulty in initiating urination over a
week. Examination revealed spastic paraparesis (right/left: grade
4+ and 3+, respectively) and hypoesthesia below the T10 sensory
dermatome.
The study was approved by the Ethics Committee of
Hospital de Braga, Braga, Portugal. Informed consent was obtained
from the patient’s family.
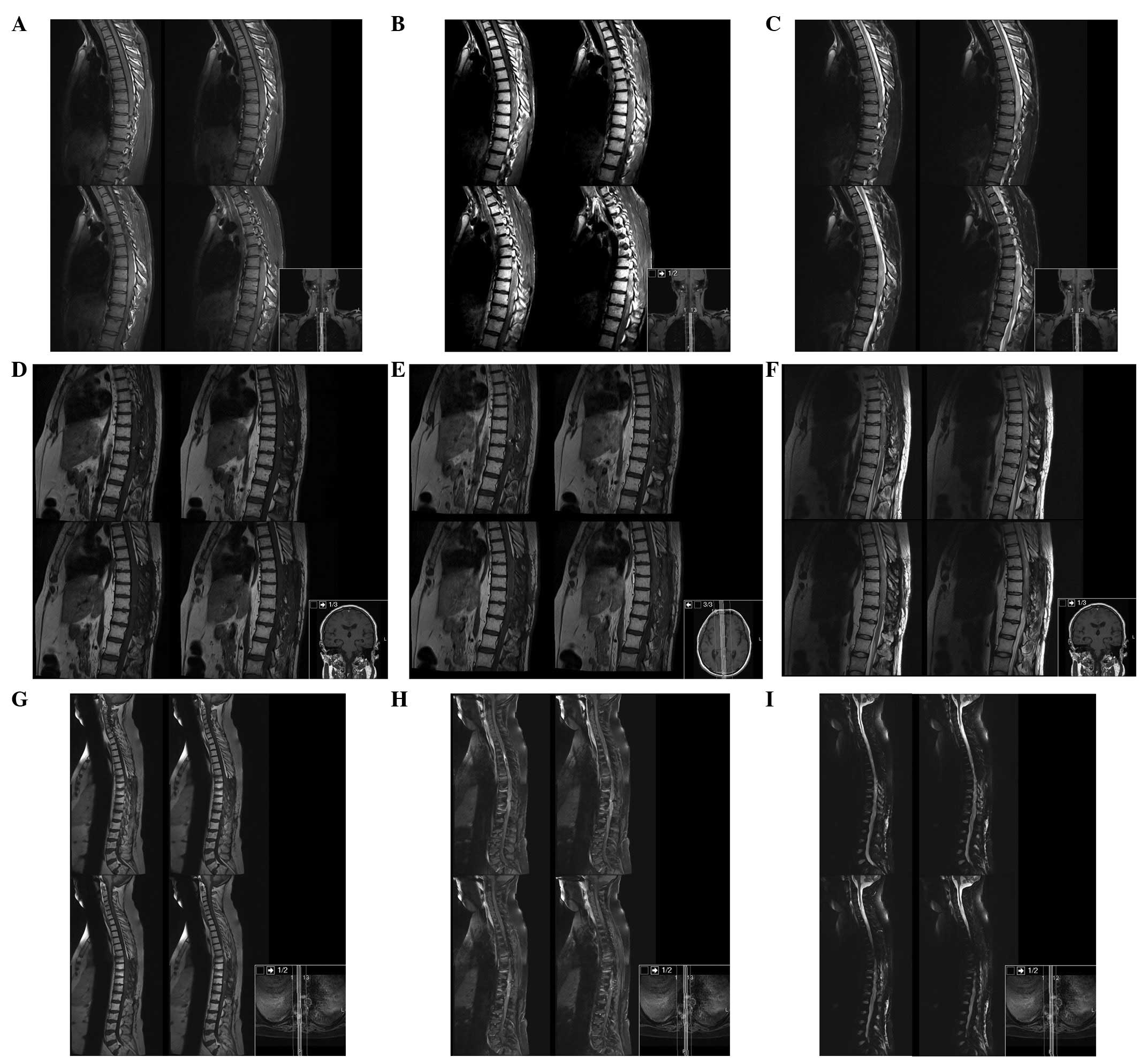
The patient underwent brain and spine magnetic
resonance imaging (MRI). The brain and cervical spine were negative
for masses and signal intensity alterations, whereas from T1 to L1
there was a marked spinal cord signal intensity and morphology
alteration, with notable spinal cord expansion between T6 and T11
and contrast enhancement between T6 and T9 (Fig. 1).
We performed a laminotomy and laminoplasty between
T6 and T11, and partial tumour removal under motor-evoked potential
monitoring. We were unable to distinguish the tumour margin from
the spinal cord and decided to partially remove the mass.
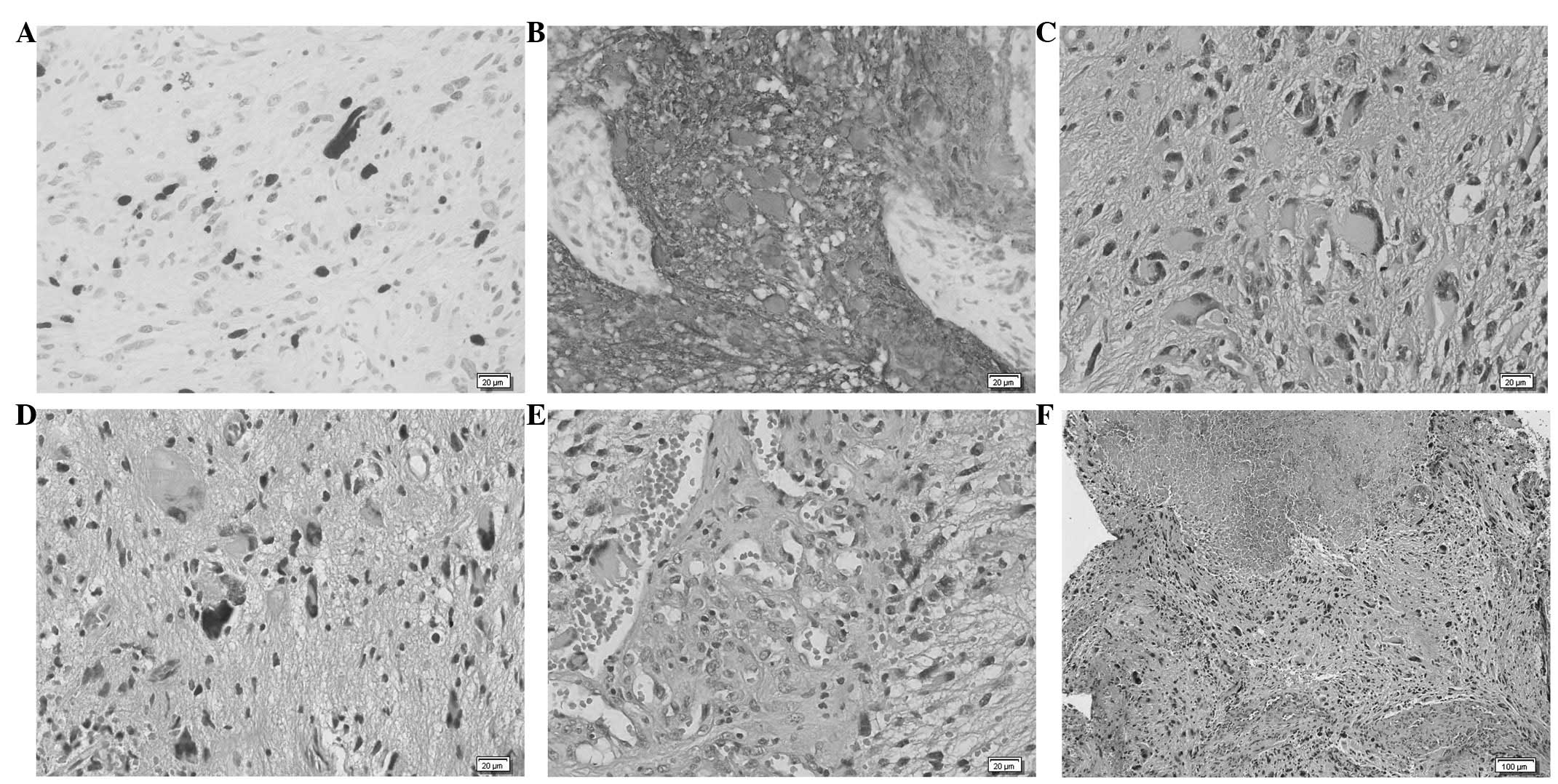
Histopathological study confirmed the diagnosis of
GBM (Fig. 2), with histological
findings of pleomorphism, atypical cells with high cellularity,
vascular proliferation and necrosis. As demonstrated by
immunohistochemistry, glial fibrillary acidic protein (GFAP) and
S100 Protein were consistently expressed by tumour cells. The
neoplasm also showed a high MIB1/Ki-67 labelling index.
Post-operatively the patient had transient
neurological deterioration with worsening of paraparesis, but with
intensive rehabilitation his condition returned to baseline.
Thoracoabdominal CT scan for extra-neuronal metastases was
negative. The patient was administered spinal radiotherapy between
T1 and L1 (45 Gy in 28 fractions) with chemotherapy with
temozolamide (completed 6 cycles).
Serial MRI at 3, 6 and 17 months was performed
(Fig. 3). Six months after surgery
the patient deteriorated, becoming completely paraplegic and losing
bladder function. Cranial and spine MRI revealed enlargement of the
residual tumour from T3 to T12 with cranial extension of oedema to
the obex, and subarachnoid metastatic deposits in C2, C4 and in the
pituitary stalk, with hydrocephalus. We proposed cranio-spinal
irradiation, but as the patient was stable and without signs or
symptoms of increased intracranial pressure (ICP) the patient and
his family decided to withhold this treatment and wait.
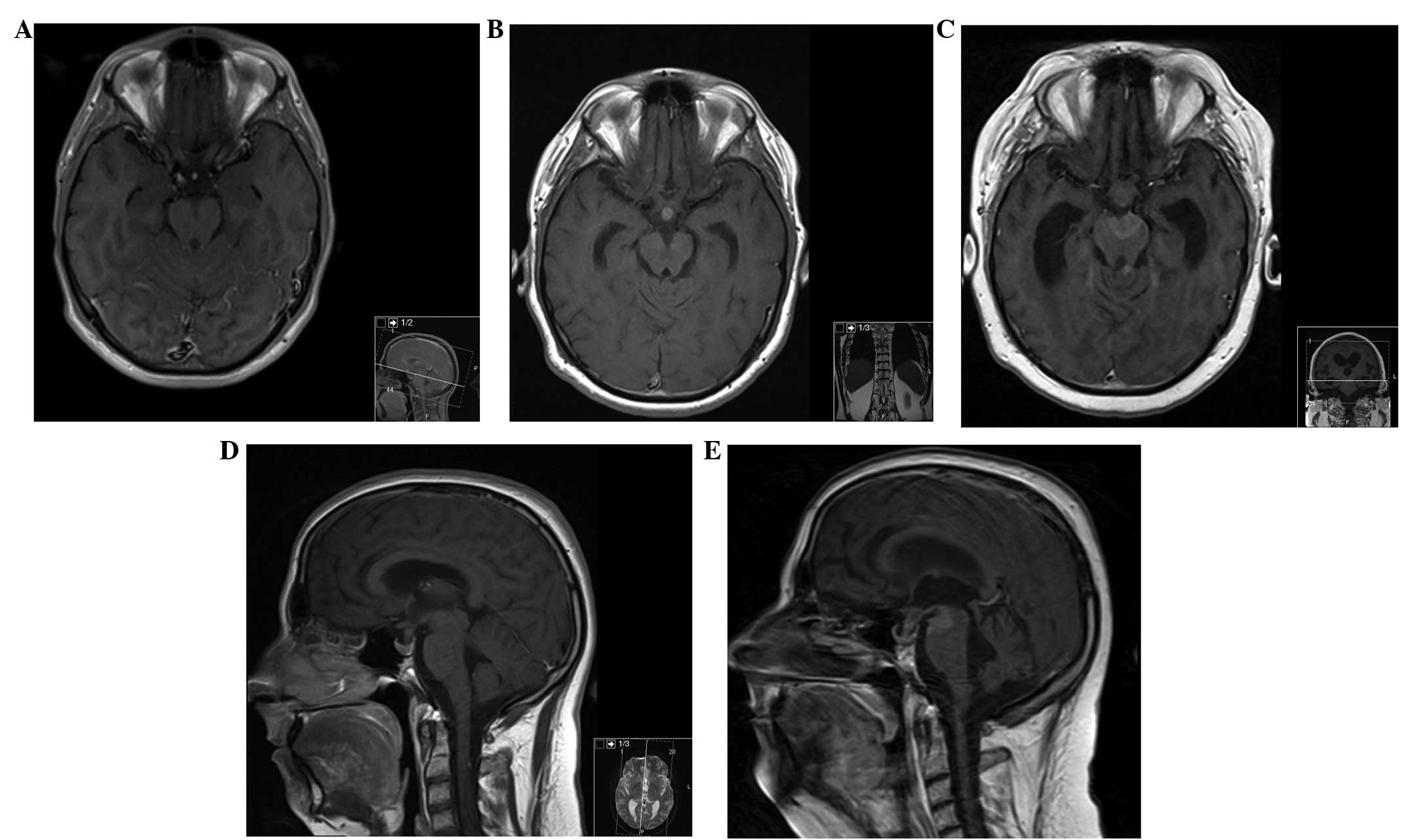 | Figure 3MRI of the brain. (A) Axial section,
contrast-enhanced T1-weighted MRI of the brain, at presentation,
demonstrating no lesions. (B and D) Axial and sagittal section,
respectively, contrast-enhanced T1-weighted MRI of the brain, at 6
months after initial presentation, showing a metastatic deposit in
the pituitary stalk. (C) Axial and (E) sagittal section
contrast-enhanced T1-weighted MRI of the brain, at 17 months after
initial presentation, demonstrating new metastatic deposits in the
interpeduncular cistern and in the left superior cerebellar
peduncle. MRI, magnetic resonance imaging. |
Neuroaxis MRI performed 17 months after surgery
revealed an enlargement of the enhancement mass from T3 to T12 with
less perilesional oedema and a new metastatic deposit in the
interpeduncular cistern and left superior cerebellar peduncle.
On the follow-up 20 months after surgery, the
patient presented with a left third cranial nerve paralysis and
bilateral mydriasis without signs of increased ICP or mental status
change. CT scan showed larger ventricles and enlargement of the
pituitary stalk and left superior cerebellar peduncle metastatic
deposits. Given the disease progression and the Karnofsky score
(40%), we did not consider further treatment and the patient
succumbed to the disease 1 month later.
Discussion
Intramedullary GBM is a rare disease entity. It
develops primarily from the spinal cord or as a secondary
metastasis from the brain, which covers up to 25% of the total
occurrences. Intramedullary GBM has a predilection to develop from
the cervical region in primary cases, and has a tendency to develop
at a young age (<30 years old). Despite the best treatment
(surgery and adjuvant therapy), the estimated survival barely
exceeds 6 to 16 months (1,8–11).
Certain patients with malignant spinal cord
astrocytomas develop hydrocephalus (1,7), which
is thought to be due to increased protein concentration in the
cerebrospinal fluid (CSF), occlusion of the CSF channel in the
subarachnoid space at the skull base and brain surface,
arachnoiditis and bleeding of spinal cord tumours (6,7).
Seeding of an intracranial GBM along the spine
occurs in 25% of cases, but the reverse process is extremely
uncommon (1,3). Patients with malignant spinal cord
astrocytomas may develop disseminated disease, mostly via the
leptomeningeal route (1,6); however, no definite evidence has been
elucidated. Sites of intracranial metastases include the
subarachnoid space, ventricles, cerebellum, hypothalamus, brain
stem, thalamus and septum pellucidum. Surgical manipulation of GBM
has not been shown to increase the tumour seeding into the CSF
(3). Continuous spread to
contiguous regions is rare, with the most common sites metastases
being extra-neuronal, including the lungs, lymph nodes, bone, liver
and pleura (12). In our case,
metastases were observed in the subarachnoid space in C2 and C4,
pituitary stalk, interpeduncular cistern and left superior
cerebellar peduncle.
All current therapeutic measures have produced
disappointing results and few data concerning their real value are
available, with survival times between 6 and 16 months with a mean
survival period of 12 months after diagnosis (1,4,7–10)
Radical surgery is suggested for confirmation of the diagnosis and
for cytoreduction of the tumour as an adjunct to radiotherapy and
chemotherapy.
Most authors suggest focal spine radiotherapy and
chemo-therapy with temozolamide, while others recommend a more
aggressive approach with whole-brain irradiation in addition to
focal spine irradiation, even if there is no evidence of
intracranial dissemination. Others suggest intrathecal
administration of interferon-β via an Ommaya reservoir in
conjunction with cranio-spinal irradiation (3,7).
MRI is considered the gold standard imaging modality
to diagnose intramedullary tumours (13,14),
and gadolinium-enhanced MRI of the entire neuroaxis is advocated to
rule out metastasis, evaluate treatment efficacy and detect relapse
(15).
To the best of our knowledge, only 16 cases of
spinal GBM involving the conus medullaris have been previously
reported (1,4,13–19,20–24),
making this case the first with spinal and intracranial metastasis
with hydrocephalus and the third most longest survival (21
months).
In conclusion, primary spinal GBM is an extremely
rare entity. Despite aggressive treatment with radical surgery,
radiotherapy and chemotherapy, this disease progresses rapidly with
a poor prognosis and a short survival time. We advocate an
aggressive management of the different complications as they arise
(progression, metastasis, hydrocephalus) to extend the patient’s
survival as long as possible with the best quality of life.
Improvement of current modes of treatment and new treatment options
(chemotherapy protocols, gene therapy) are required to improve
survival and ensure better quality of life.
Abbreviations:
|
GBM
|
glioblastoma
|
|
CSF
|
cerebrospinal fluid
|
|
MRI
|
magnetic resonance imaging
|
|
ICP
|
intracranial pressure
|
References
|
1
|
Cohen AR, Wisoff JH, Allen JC and Epstein
F: Malignant astrocytomas of the spinal cord. J Neurosurg.
70:50–54. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balériaux DL: Spinal cord tumors. Eur
Radiol. 9:1252–1258. 1999.
|
|
3
|
Johnson D and Schwarz S: Intracranial
metastases from malignant spinal-cord astrocytoma. Case report. J
Neurosurg. 66:621–625. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Medhkour A and Chan M: Extremely rare
glioblastoma multiforme of the conus medullaris with holocord and
brain stem metastases, leading to cranial nerve deficit and
respiratory failure: A case report and review of the literature.
Surg Neurol. 63:576–582. 2005. View Article : Google Scholar
|
|
5
|
Stein BM: Surgery of intramedullary spinal
cord tumors. Clin Neurosurg. 26:529–542. 1979.PubMed/NCBI
|
|
6
|
Ciappetta P, Salvati M, Capoccia G, Artico
M, Raco A and Fortuna A: Spinal glioblastoma: report of seven cases
and review of the literature. Neurosurgery. 28:302–306. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asano N, Kitamura K, Seo Y, et al: Spinal
cord glioblastoma multiforme with intracranial dissemination - case
report. Neurol Med Chir (Tokyo). 30:489–494. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grisold W, Pernetzky G and Jellinger K:
Giant-cell glioblastoma of the thoracic cord. Acta Neurochir
(Wien). 58:121–126. 1981. View Article : Google Scholar
|
|
9
|
Guidetti B, Mercuri S and Vagnozzi R:
Long-term results of the surgical treatment of 129 intramedullary
spinal gliomas. J Neurosurg. 54:323–330. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alvisi C, Cerisoli M and Giulioni M:
Intramedullary spinal gliomas: long-term results of surgical
treatments. Acta Neurochir (Wien). 70:169–179. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kopelson G and Linggood RM: Intramedullary
spinal cord astrocytoma versus glioblastoma: the prognostic
importance of histological grade. Cancer. 50:732–735. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Russell DS and Rubinstein LJ:
Glioblastoma. Pathology of Tumours of the Nervous System. 5th
edition. Edward Arnold; London: pp. 426–452. 1998
|
|
13
|
Bonde V, Balasubramaniam S and Goel A:
Glioblastoma multiforme of the conus medullaris with holocordal
spread. J Clin Neurosci. 15:601–603. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stecco A, Quirico C, Giampietro A, Sessa
G, Boldorini R and Carriero A: Glioblastoma multiforme of the conus
medullaris in a child: description of a case and literature review.
AJNR Am J Neuroradiol. 26:2157–2160. 2005.PubMed/NCBI
|
|
15
|
Mori K, Imai S, Shimizu J, Taga T, Ishida
M and Matsusue Y: Spinal glioblastoma multiforme of the conus
medullaris with holocordal and intracranial spread in a child: a
case report and review of the literature. Spine J. 12:e1–e6. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Andrews AA, Enriques L, Renaudin J and
Tomiyasu U: Spinal intramedullary glioblastoma with intracranial
seeding. Report of a case. Arch Neurol. 35:244–245. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eden KC: Dissemination of a glioma of the
spinal cord in the leptomeninges. Brain. 61:298–310. 1938.
View Article : Google Scholar
|
|
18
|
Kawanishi M, Kuroiwa T, Nagasawa S, Ohta
T, Oketa M and Onomura T: A case of spinal glioblastoma with
intracranial dissemination. No Shinkei Geka. 21:1109–1112. 1993.(In
Japanese).
|
|
19
|
O’Connell JE: The subarachnoid
dissemination of spinal tumours. J Neurol Neurosurg Psychiatry.
9:55–62. 1946.
|
|
20
|
Santi M, Mena H, Wong K, Koeller K, Olsen
C and Rushing EJ: Spinal cord malignant astrocytomas.
Clinicopathologic features in 36 cases. Cancer. 98:554–561. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scarrow AM, Rajendran P and Welch WC:
Glioblastoma multiforme of the conus medullaris. Clin Neurol
Neurosurg. 102:166–167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shirato H, Kamada T, Hida K, et al: The
role of radiotherapy in the management of spinal cord glioma. J
Radiat Oncol Biol Phys. 33:323–328. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Strik HM, Effenberger O, Schäfer O, Risch
U, Wickboldt J and Meyermann R: A case of spinal glioblastoma
multiforme: immunohistochemical study and review of the literature.
J Neurooncol. 50:239–243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tashiro K, Tachibana S and Tsura M:
Clinicopathological studies of spinal cord neoplasm with
disseminating intracranial metastasis possibly producing akinetics
mutism. No To Shinkei. 28:1311–1318. 1976.(In Japanese).
|