Introduction
Liver regeneration is a tissue repair response of
the liver following damage due to various causes, including viral
infection, chemical intoxication and partial hepatectomy (PH).
Although the exact underlying mechanisms have not been fully
characterized, the process is acknowledged to be tightly regulated
through controlled delivery of ‘start and stop’ signals, including
numerous cytokines and growth factors, to maintain a constant
liver-to-body mass ratio (1–3). A
number of the growth factors involved in a regenerating liver are
known for their angiogenic properties (4). Among the various angiogenic factors
that have been identified, including basic fibroblast growth factor
(bFGF), vascular endothelial growth factor (VEGF) and
platelet-derived growth factor (PDGF), VEGF has been demonstrated
to be a major angiogenic factor following PH (5,6).
Thalidomide, α-N-phthalimido-glutarimide, was
initially marketed as a sedative and antinausea medicine in the
1950s, but was withdrawn due to teratogenicity (7). Unexpectedly, it has become the subject
of intensive investigation in oncology since its antiangiogenic
properties were first demonstrated in 1994 (8). In that study, the bFGF-induced
neovascularization in rabbit corneas was significantly reduced by
thalidomide. This drug has also been shown to inhibit VEGF-induced
angiogenesis (9,10). In addition to its antiangiogenic
effect, an immunomodulatory function is also a potential mechanism
of the anticancer activity of thalidomide. To date, the
effectiveness of thalidomide for treating neoplastic disorders has
been confirmed in diseases such as multiple myeloma (11) and Kaposi’s sarcoma (12). In addition, thalidomide has been
tentatively used for the treatment of advanced hepatocellular
carcinoma (13–16).
Antiangiogenic factors have been demonstrated to
reduce the formation of new blood vessels (17), resulting in slower tumor growth or
even tumor regression. Therefore, the combination of antiangiogenic
strategies with liver resection is a promising approach to treat
primary and metastatic liver cancers, such as hepatocellular
carcinoma and colorectal cancer. Post-hepatectomy liver failure
develops if liver regeneration is impaired, especially in
antiangiogenic condition. However, the effect of the antiangiogenic
agent on liver regeneration has not been fully clarified. In the
present study, we investigated the effect of thalidomide on VEGF
expression and liver regeneration in rats following 70% PH.
Materials and methods
Animals
Male Sprague-Dawley rats initially weighing 250–300
g were used. All animals were housed in a temperature and humidity
controlled environment, and they received humane care with free
access to standard chow and water throughout the study period. The
protocols in this study were submitted to and approved by the E-Da
Hospital (Taiwan) Institutional Animal Care and Use Committee
(IACUC-97007). All animal procedures were in compliance with our
institutional guidelines.
Experimental design
A total of 50 rats were subjected to 70% PH and
equally divided into two groups: the control and thalidomide
groups. Two days prior to PH, rats in the thalidomide group were
daily administered thalidomide (100 mg/kg, TTY BioPharm, Taipei,
Taiwan) in olive oil by intragastric administration. Control rats
received olive oil only. Animals in the two groups were equally
divided into 5 subgroups according to observation intervals, which
were 0, 48, 96, 144 and 192 h after PH.
PH
Liver regeneration was induced by 70% PH as
described by Higgins and Anderson (18). Animals were anesthetized with
ketamine (100 mg/kg, intraperitoneal injection). After a midline
laparotomy, the liver was exposed and the left and medial lobes
were ligated (4-0 silk) and resected. Glucose solution (5 ml; 5%;
37°C) was injected into the abdominal cavity and the abdominal
wound was closed in two layers with 4-0 silk. The resected liver
was termed ‘quiescent liver’ in this study.
Hepatic regeneration rate
The rate of liver regeneration was evaluated using
the formula of Kwon et al(19): Hepatic regeneration rate (%) = D/E ×
100, where D is the weight of the liver per 100 g of body weight at
death and E is the estimated liver weight per 100 g body weight
prior to hepatectomy, which was calculated from the weight of
resected liver (R); E = R/0.7.
Laser Doppler flowmetry analysis of
microcirculation
The principle of laser Doppler flowmetry combines
laser technology with the Doppler effect caused by the movement of
red blood cells in the microcirculation to estimate red blood cell
flux (20). The strength of this
technique is in observing changes in flow, either over time or over
an area of the exposed tissue. Before 70% PH and animal sacrifice,
the surface of the liver was scanned by a Moor LDI 2 imager (Moor
Instruments Ltd., Devon, UK) to assess the perfusion hemodynamics.
The Doppler shift is proportional to a blood flow-related variable
and is expressed in arbitrary perfusion units (PU).
Microcirculation density was quantified using software provided by
the manufacturer (Moor LDI system software V5).
Western blot analysis
Livers were homogenized by Ultrasonic cell disruptor
(Microson™ XL-2000; Misonix, Farmingdale, NY, USA) in tissue
protein extraction buffer (T-PER®, Pierce, Rockford, IL,
USA) containing protease inhibitors (Protease Inhibitor Cocktail
100X, Pierce) and the homogenate was centrifuged to obtain the
supernatant. Protein concentrations were determined and the samples
were subjected to sodium dodecyl sulfate/polyacrylamide gel
electrophoresis and transferred to a nitrocellulose membrane (ECL,
Amersham, Buckinghamshire, UK). After blocking and washing, blots
were incubated overnight at 4°C with rabbit affinity-purified
antibodies against proliferating cell nuclear antigen (PCNA;
dilution, 1:1,000; Epitomics, Burlingame, CA, USA), VEGF (dilution,
1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or
β-actin (dilution, 1:100; Sigma, St. Louis, MO, USA). The blots
were washed and incubated with horseradish peroxidase
(HRP)-conjugated secondary antibodies for 1 h. Finally, the signals
were detected using an enhanced chemiluminescence detection kit
(Amersham, Piscataway, NJ, USA). The chemiluminescent signal was
captured by a UVP BioSpectrum500 imaging system (UVP, Upland, CA,
USA).
Immunohistochemistry
Fresh liver samples were immediately immersed into
10% neutral formalin. Paraffin-embedded liver samples were cut into
5-μm sections. Liver sections were de-paraffinized,
rehydrated and placed in citrate buffer (10 mM, pH 6.0) and
microwaved twice for 7 min to improve staining by antigen
unmasking. After dewaxing and rehydration, liver sections were
placed in citrate buffer (10 mM, pH 6.0) and microwaved twice for 7
min to improve staining by antigen unmasking. The activity of
endogenous peroxidase was removed by incubation with 3%
H2O2 for 15 min at room temperature. VEGF was
identified by rabbit anti-rat VEGF polyclonal antibody (dilution,
1:500; SC-152, Santa Cruz Biotechnology, Inc.) followed by
HRP-conjugated goat anti-rabbit secondary antibody (Dako™ REAL™
EnVision Detection System, K5007; Carpinteria, CA, USA). Positive
signals were shown by 3,3′-diaminobenzidine (DAB) response.
Sections were then counterstained with hematoxylin.
Statistical analysis
Student’s t-test was used to compare sample means
with paired or unpaired controls, as appropriate. Results are
expressed as means ± SEM. P<0.05 was considered to indicate a
statistically significant result.
Results
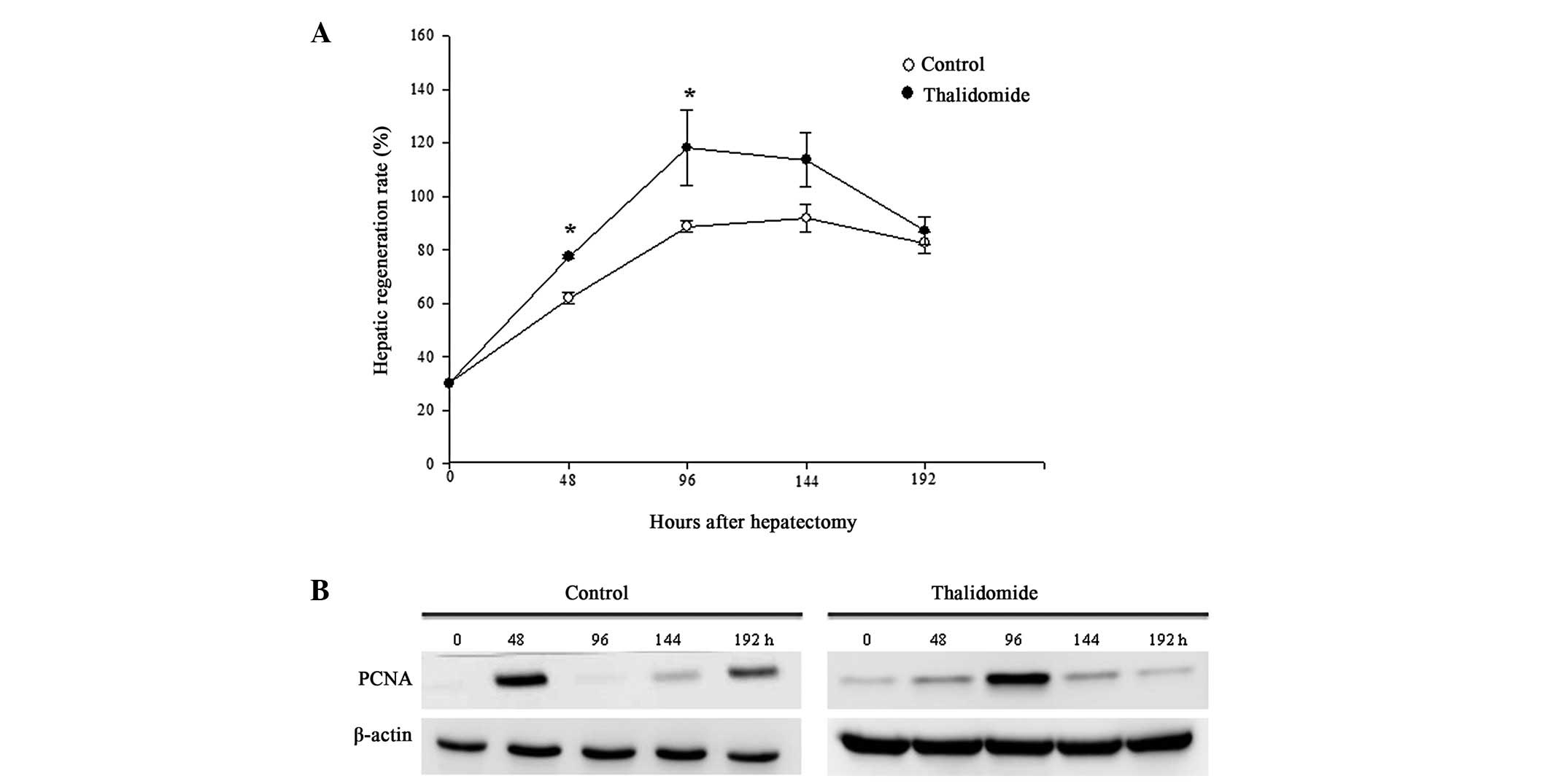
Liver regeneration rate
All the experimental rats survived the 70% liver
resection and thalidomide treatment. Following hepatectomy, the
restituted liver mass in the two groups markedly increased with a
peak at 96 h and then declined (Fig.
1A). Unexpectedly, the calculated regeneration rate in the
thalidomide group at the first two post-hepatectomy time-points (48
and 96 h) was significantly higher than that in the control rats,
while no difference between the groups was found at the subsequent
time-points.
Expression of PCNA
PCNA is a protein marker for DNA synthesis and is
commonly used as an indicator for cell proliferation. At resting
state, weak expression of PCNA was detected in both groups.
Following hepatectomy, the expression level of PCNA increased
significantly and reached a peak at 48 h in the control group, 96 h
in the thalidomide group and declined abruptly thereafter (Fig. 1B).
Hepatic microcirculation
To quantify the circulatory effect of thalidomide on
liver regeneration, hepatic blood flow was assessed by laser
Doppler flowmetry before PH (0 h, quiescent liver) and sacrifice
(regenerating liver). Prior to PH, the hepatic microcirculation in
rats treated with thalidomide for 2 days was comparatively less
than that in their corresponding controls (Fig. 2). However, no significant difference
in blood flow in the remnant liver between the control and
thalidomide groups was detected at any studied time-point.
Western blot analysis of VEGF
Prior to liver resection (0 h), a low expression
level of VEGF was detected in control rats and rats treated with
thalidomide for 48 h (Fig. 3).
Hepatectomy induced marked expression of VEGF, which peaked at
48–96 h and declined rapidly in the two groups. No significant
difference in the expression level between the groups at any
studied time-point was found.
Immunohistochemical staining of VEGF
Positive VEGF immunoreactivity was mainly localized
in the cytoplasm of hepatocytes. Prior to PH, faint staining was
observed in the two groups. At 48 h after PH, VEGF was mainly
expressed in the periportal area in both groups (data not shown).
At 96 h, the positive immunoreactivity was limited to the
pericentral area in the control group (Fig. 4A–C), while observed in peri-central
and periportal hepatocytes in the thalidomide group (Fig. 4D–F). At the subsequent time-points,
markedly weaker expression of VEGF was observed and mostly located
in the pericentral area in the two groups (data not shown).
Discussion
The present study demonstrated that thalidomide
delayed the PH-induced hepatic cell proliferation but did not
impair the overall liver regeneration. In addition, the PH-induced
upregulation of VEGF was not inhibited by thalidomide.
The maximal expression of PCNA, a marker for DNA
synthesis, was observed to occur at 48 h post-hepatectomy in the
control group of this study. However, the peak for hepatocyte
proliferation during liver regeneration in the rat as determined by
Ki-67 or 5-bromodeoxyuridine (5-BrdU) labeling is at 24 h (1). There are at least two possible
explanations for this discrepancy. One is that 24 h was not one of
the selected time-points in our study. The other is the
methodologies used in different studies. The approach we employed
in this study was detection of the overall PCNA expression in liver
homogenate, which is derived from various types of cells with
different proliferation rates. By contrast, Ki-67 staining is used
to determine the growth of a specific cell population, such as
hepatocytes in the liver. In the present study, the maximal PCNA
expression detected in the thalidomide group was 96 h after liver
resection, a significant delay as compared with control rats;
nevertheless, the overall restoration of liver mass was not
affected.
The significance for the observed transient greater
liver regeneration rate in the thalidomide group requires further
investigation. However, we speculated that it may be due to the
non-specific effect of thalidomide, such as increasing the water
content in liver tissue based on the transient watery appearance of
thalidomide-treated liver (our unpublished observation). If so, the
liver regeneration rate may thus be overestimated.
Laser Doppler flowmetry is a technique for the
non-invasive blood flow monitoring and is considered to be a
suitable technique for the analysis of hepatic microcirculation
(20–22). In the present study, thalidomide
impaired hepatic micro-circulation in the quiescent, but not
regenerating, liver. The reduced blood flow in the
thalidomide-treated quiescent liver may be associated with the
inhibitory effect of thalidomide on the release of tumor necrosis
factor (TNF)-α and nitric oxide, two potent vasodilators, as
suggested by a previous study in which thalidomide ameliorated the
portal pressure and hyper-dynamic circulation in partially portal
vein-ligated rats by reducing TNF-α and nitric oxide production
(23). After PH, this inhibitory
effect of thalidomide was eliminated by rapid release of TNF-α
(1), resulting in similar hepatic
microcirculation in the two groups.
VEGF is an important factor in the early phase of
liver regeneration (24). In this
study, treatment with thalidomide for 48 h before PH did not affect
the expression of VEGF, as evidenced by the insignificant
difference in the expression level between control and thalidomide
groups at 0 h. Following PH, VEGF was markedly upregulated and the
expression profile during the regenerative process was similar in
the two groups. These observations suggest that thalidomide exerts
no significant effect on the expression of VEGF either in quiescent
liver or in PH-induced regenerating liver. The immunohistochemical
result showing that VEGF was predominantly expressed in the
periportal hepatocytes at 48 h post-PH is consistent with a
previous study (5). At 96 h, the
positive staining was observed only in the pericentral area in the
control group, while observed in the periportal and pericentral
areas in the thalidomide group. This time-dependent alteration in
the main expression site in the liver suggests a waved pattern for
the expression of VEGF, which advances from the periportal to
pericentral area. Although the significance for the observed
difference in the expression areas at 96 h between groups requires
further studies for clarification, we hypothesize that that it may
reflect the slower angiogenesis in thalidomide-treated rats.
In conclusion, our results demonstrate that
thalidomide exerts no significant effect on the expression of VEGF
and does not impair the overall PH-induced restoration of liver
mass, providing supportive evidence that thalidomide may be used as
an adjunct treatment modality for liver cancers.
Acknowledgements
The authors would like to thank the
E-Da Hospital, Taiwan for financially supporting this research
under Contract No. EDAHP97014.
References
|
1
|
Michalopoulos GK and DeFrances MC: Liver
regeneration. Science. 276:60–66. 1997. View Article : Google Scholar
|
|
2
|
Mohammed FF and Khokha R: Thinking outside
the cell: proteases regulate hepatocyte division. Trends Cell Biol.
15:555–563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fausto N, Laird AD and Webber EM: Liver
regeneration. 2 Role of growth factors and cytokines in hepatic
regeneration. FASEB J. 9:1527–1536. 1995.PubMed/NCBI
|
|
4
|
Drixler TA, Vogten MJ, Ritchie ED, et al:
Liver regeneration is an angiogenesis-associated phenomenon. Ann
Surg. 236:703–712. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taniguchi E, Sakisaka S, Matsuo K,
Tanikawa K and Sata M: Expression and role of vascular endothelial
growth factor in liver regeneration after partial hepatectomy in
rats. J Histochem Cytochem. 49:121–130. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leung DW, Cachianes G, Kuang WJ, Goeddel
DV and Ferrara N: Vascular endothelial growth factor is a secreted
angiogenic mitogen. Science. 246:1306–1309. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McBride WG: Thalidomide embryopathy.
Teratology. 16:79–82. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
D’Amato RJ, Loughnan MS, Flynn E and
Folkman J: Thalidomide is an inhibitor of angiogenesis. Proc Natl
Acad Sci USA. 91:4082–4085. 1994.
|
|
9
|
Kenyon BM, Browne F and D’Amato RJ:
Effects of thalidomide and related metabolites in a mouse corneal
model of neovascularization. Exp Eye Res. 64:971–978. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kruse FE, Joussen AM, Rohrschneider K,
Becker MD and Völcker HE: Thalidomide inhibits corneal angiogenesis
induced by vascular endothelial growth factor. Graefes Arch Clin
Exp Ophthalmol. 236:461–466. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singhal S, Mehta J, Desikan R, et al:
Antitumor activity of thalidomide in refractory multiple myeloma. N
Engl J Med. 341:1565–1571. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Little RF, Wyvill KM, Pluda JM, et al:
Activity of thalidomide in AIDS-related Kaposi’s sarcoma. J Clin
Oncol. 18:2593–2602. 2000.
|
|
13
|
Hsu C, Chen CN, Chen LT, et al: Low-dose
thalidomide treatment for advanced hepatocellular carcinoma.
Oncology. 65:242–249. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pinter M, Wichlas M, Schmid K, et al:
Thalidomide in advanced hepatocellular carcinoma as antiangiogenic
treatment approach: a phase I/II trial. Eur J Gastroenterol
Hepatol. 20:1012–1019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schwartz JD, Sung M, Schwartz M, et al:
Thalidomide in advanced hepatocellular carcinoma with optional
low-dose interferon-alpha2a upon progression. Oncologist.
10:718–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patt YZ, Hassan MM, Lozano RD, et al:
Thalidomide in the treatment of patients with hepatocellular
carcinoma: a phase II trial. Cancer. 103:749–755. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Higgins GM and Anderson RM: Experimental
pathology of the liver - restoration of the liver of the white rat
following partial surgical removal. Arch Pathol. 7:187–202.
1931.
|
|
19
|
Kwon AH, Uetsuji S, Yamamura M, Hioki K
and Yamamoto M: Effect of administration of fibronectin or
aprotinin on liver regeneration after experimental hepatectomy. Ann
Surg. 211:295–300. 1990.PubMed/NCBI
|
|
20
|
Garcia N Jr and Sanyal AJ: Laboratory
assessment of hepatic hemodynamics. Clin Liver Dis. 5:591–615.
2001. View Article : Google Scholar
|
|
21
|
Arvidsson D, Svensson H and Haglund U:
Laser-Doppler flowmetry for estimating liver blood flow. Am J
Physiol. 254(4 Pt 1): G471–G476. 1988.PubMed/NCBI
|
|
22
|
Sun Z, Klein AS, Radaeva S, et al: In
vitro interleukin-6 treatment prevents mortality associated with
fatty liver transplants in rats. Gastroenterology. 125:202–215.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lopez-Talavera JC, Cadelina G, Olchowski
J, Merril W and Groszmann RJ: Thalidomide inhibits tumor necrosis
factors alpha, decreases nitric oxide synthesis, and ameliorates
the hyperdynamic circulatory syndrome in portal-hypertensive rats.
Hepatology. 23:1616–1621. 1996.
|
|
24
|
Bockhorn M, Goralski M, Prokofiev D, et
al: VEGF is important for early liver regeneration after partial
hepatectomy. J Surg Res. 138:291–299. 2007. View Article : Google Scholar : PubMed/NCBI
|