Introduction
The Chinese herb, Jue-ming-zi, is the seed of the
plant Cassia tora L. (Leguminosae), and has been used as a
laxative and a tonic, as well as being a popular health tea drink.
The commercial products of Cassia tora L. include both
unroasted and roasted samples, and the laxative effect was found to
be higher in unroasted compared with roasted Cassia tora L
samples (1).
Pharmaceutical research has concentrated on the
beneficial activities of Cassia tora L. such as its
liver-protection, anti-aging, anticancer and antioxidant effects
(2–5). Cassia tora L. contains
anthraquinones, naphtho-pyrones, fatty acids, amino acids and
inorganic elements (6). Types of
Cassia tora L. with a high anthraquinone content, such as
chrysophanol, physcion and obtusin, may help to decrease blood
lipid levels (7).
The induction of apoptosis in cancer cells is
initially identified by morphological changes including cell
shrinkage, membrane blebbing, chromatin condensation and nuclear
fragmentation (8). Apoptosis is an
important defense against cancer. The process involves the
elimination of potentially harmful cells. Many diseases have been
associated with dysregulated apoptotic processes, ultimately
leading to the inhibition of cell death and the propagation of
diseases such as cancer (9).
Caspases are central components of the apoptotic
response. Caspase-9 is an apical isoform involved in
mitochondria-dependent apoptosis. This factor primarily activates
caspase-3, which then serves as a gateway for the activation of
downstream caspases (10). Nuclear
factor-κB (NF-κB) is involved in the inhibition of apoptosis,
stimulation of cell proliferation, inflammation, immune response
and tumorigenesis. Activation of NF-κB generally prevents
apoptosis. Expression of inducible nitric oxide synthase (iNOS) and
cyclooxygenase (COX)-2, two genes regulated by NF-κB, are induced
by inflammation and are frequently overexpressed in cancer cells.
Increased NF-κB activity that is localized in the nucleus is
particularly found in cells where there is abundant expression of
iNOS and COX-2 (11).
A previous epidemiological study demonstrated that
chronic inflammation predisposes individuals to various types of
cancer (12). Hallmarks of
inflammation-related cancers include the presence of inflammatory
cells and mediators in tumor tissues, tissue remodeling and
angiogenesis similar to that seen during chronic inflammatory
responses, and tissue repair. The study of mechanisms underlying
inflammation-related cancer has focused on the early stages of
cancer; however, inflammatory mediators and cells are also involved
in the migration, invasion and metastasis of malignant cells
(13). Metastasis is the leading
cause of mortality among cancer patients, and involves the spread
of cancer from a primary site and the formation of new tumors in
distant organs. Matrix metalloproteases (MMPs) are important in
numerous physiological and pathological processes including
embryonic development, morphogenesis, reproduction, tissue
remodeling, arthritis, cardiovascular disease and metastasis
(14). MMP activity is inhibited by
specific endogenous tissue inhibitors of metalloproteinases (TIMPs)
(15). To prevent the majority of
cancer types, improved treatments for metastasis are required
(16,17).
Previously, Cassia tora L. demonstrated
strong in vitro anticancer effects in JTC-26 human cervical
cancer cells (6). In the present
study, we further examined the anticancer and anti-metastatic
effects of Cassia tora L.; Cassia tora L. was
administered to human tongue carcinoma TCA8113 cells and the
molecular mechanisms underlying the anticancer effects of the
Cassia tora L. were studied. Changes in activities of
Cassia tora L. at different concentrations were evaluated
and their anti-metastatic effects were assessed in mice with tumors
propagated by 26-M3.1 colon carcinoma cells.
Materials and methods
Preparations of Cassia tora L
Cassia tora L. (Jue-ming-zi) was purchased
from Yunnan Baiyao Group Co. Ltd. (Kunming, China) and stored at
−80°C and freeze-dried to produce a powder. A 20-fold volume of
methanol was added to the powdered sample and extracted twice by
stirring overnight. The methanol extract was evaporated using a
rotary evaporator (N-1100; Eywla; Tokyo, Japan), concentrated and
then dissolved in dimethylsulfoxide (DMSO; Amresco, Solon, OH, USA)
to adjust to the stock concentration (20%, w/v).
Cancer cell preparation
Human tongue carcinoma TCA8113 cells obtained from
the Shanghai Institute of Biochemistry and Cell Biology (SIBCB;
Shanghai, China) were used in the experiments. The cells were
cultured in Roswell Park Memorial Institute (RPMI)-1640 medium
(Gibco Co., Birmingham, MI, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco Co.) and 1% penicillin-streptomycin (Gibco Co.),
at 37°C in a humidified atmosphere containing 5% CO2
(model 311 S/N29035; Forma; Waltham, MA, USA). The medium was
replaced two or three times each week.
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The anticancer effects of Cassia tora L. were
assessed by an MTT assay. Human tongue carcinoma TCA8113 cells were
seeded in a 96-well plate at a density of 2×104 cells/ml
and a volume of 180 μl/well. Cassia tora L. solution
(20 μl) was added at concentrations of 0.25, 0.5 and 1.0
mg/ml, and then the cells were incubated at 37°C in 5%
CO2 for 48 h. An MTT solution (200 μl; 5 mg/ml;
Amresco) was added and the cells were cultured for a further 4 h
under the same conditions. Following removal of the supernatant,
150 μl DMSO was added to each well and mixed for 30 min.
Subsequently, the absorbance of each well was measured with an
enzyme-linked immunosorbant assay (ELISA) reader (model 680;
Bio-Rad; Hercules, CA, USA) at 540 nm (18).
Measuring RNA expression using reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from human tongue carcinoma
TCA8113 cells using TRIzol reagent (Invitrogen Life Technologies;
Carlsbad, CA, USA) according to the manufacturer’s instructions.
The RNA was digested with RNase-Free DNase (Roche; Basel,
Switzerland) for 15 min at 37°C and purified using an RNeasy kit
(Qiagen; Hilden, Germany) according to the manufacturer’s
instructions. cDNA was synthesized from 2 μg of the total
RNA by incubation at 37°C for l h with AMV reverse transcriptase
(GE Healthcare, Little Chalfont, UK) and random hexanucleotides
,according to the manufacturer’s instructions. The sequences of the
primers used to specifically amplify the genes of interest are
listed in Table I. Amplification
was performed in a thermal cycler (Eppendorf; Hamburg, Germany).
The PCR products were separated in 1.0% agarose gels and visualized
by ethidium bromide (EtBr) staining (19).
 | Table ISequences of reverse
transcription-polymerase chain reaction (RT-PCR) primers used in
this study. |
Table I
Sequences of reverse
transcription-polymerase chain reaction (RT-PCR) primers used in
this study.
| Gene name | Sequence |
|---|
| Bax | Forward: 5′-AAG CTG
AGC GAG TGT CTC CGG CG-3′
Reverse: 5′-CAG ATG CCG GTT CAG GTA CTC AGT C-3′ |
| Bcl-2 | Forward: 5′-CTC GTC
GCT ACC GTC GTG ACT TGG-3′
Reverse: 5′-CAG ATG CCG GTT CAG GTA CTC AGT C-3′ |
| Caspase-3 | Forward: 5′-CAA ACT
TTT TCA GAG GGG ATC G-3′
Reverse: 5′-GCA TAC TGT TTC AGC ATG GCA-3′ |
| Caspase-9 | Forward: 5′-GGC CCT
TCC TCG CTT CAT CTC-3′
Reverse: 5′-GGT CCT TGG GCC TTC CTG GTA T-3′ |
| NF-κB | Forward: 5′-CAC TTA
TGG ACA ACT ATG AGG TCT CTG G-3′
Reverse: 5′-CTG TCT TGT GGA CAA CGC AGT GGA ATT TTA GG-3′ |
| IκB-α | Forward: 5′-GCT GAA
GAA GGA GCG GCT ACT-3′
Reverse: 5′-TCG TAC TCC TCG TCT TTC ATG GA-3′ |
| iNOS | Forward: 5′-AGA GAG
ATC GGG TTC ACA-3′
Reverse: 5′-CAC AGA ACT GAG GGT ACA-3′ |
| COX-2 | Forward: 5′-TTA AAA
TGA GAT TGT CCG AA-3′
Reverse: 5′-AGA TCA CCT CTG CCT GAG TA-3′ |
| MMP-2 | Forward: 5′-CTT CTT
CAA GGA CCG GTT CA-3′
Reverse: 5′-GCT GGC TGA GTA CCA GTA-3′ |
| MMP-9 | Forward: 5′-TGG GCT
ACG TGA CCT ATG AC-3′
Reverse: 5′-GCC CAG CCC ACC TCC ACT CC-3′ |
| TIMP-1 | Forward: 5′-GTC AGT
GAG AAG CAA GTC GA-3′
Reverse: 5′-ATG TTC TTC TCT GTG ACC CA-3′ |
| TIMP-2 | Forward: 5′-TGG GGA
CAC CAG AAG TCA AC-3′
Reverse: 5′-TTT TCA GAG CCT TGG AGG AG-3′ |
| GAPDH | Forward: 5′-CGG AGT
CAA CGG ATT TGG TC-3′
Reverse: 5′-AGC CTT CTC CAT GGT CGT GA-3′ |
Protein extraction and western blot
analysis
Total cell lysates were obtained with an extraction
buffer as previously described by Choi et al(20). Protein concentrations were
determined using a protein assay kit (Bio-Rad). For western blot
analysis, aliquots of the lysate containing 30–50 μg of
protein were separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and then electrotransferred onto a
nitrocellulose membrane (Schleicher and Schuell; Keene, NH, USA).
The membranes were subjected to immunoblot analysis and the
proteins were visualized by an enhanced chemiluminescence (ECL)
method (GE Healthcare). The cell lysates were separated by 12%
SDS-PAGE, transferred onto a polyvinylidene fluoride membrane (GE
Healthcare), blocked with 5% skimmed milk and incubated with the
primary antibodies (dilution, 1:1,000). Antibodies against Bax,
Bcl-2, iNOS and COX-2 were obtained from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Following incubation with horse-radish
peroxidase-conjugated secondary antibody at room temperature,
immunoreactive proteins were detected using an ECL assay kit (GE
Healthcare) according to the manufacturer’s instructions. Bands in
the blot were visualized using a LAS3000 luminescent image analyzer
(Fujifilm Life Science; Tokyo, Japan).
Measuring lung metastasis following
Cassia tora L. treatment in BALB/c mice bearing 26-M3.1 colon
carcinoma cell tumors
26-M3.1 colon carcinoma cells were obtained from
Professor Yoon, Department of Food and Nutrition, Yuhan University
(Bucheon, South Korea). These highly metastatic cells were
maintained as monolayers in EMEM (Gibco Co.) supplemented with 7.5%
FBS, a vitamin solution, sodium pyruvate, non-essential amino acids
and L-glutamine (Gibco Co.). The cultures were maintained in a
humidified atmosphere of 5% CO2 at 37°C. Experimental
lung metastasis was induced by injecting colon 26-M3.1 cells into
the lateral tail vein of 6-week-old female Balb/c mice
(Experimental Animal Center of Chongqing Medical University;
Chongqing, China) (21). Cassia
tora L. solution (50, 100 or 200 mg/kg) was subcutaneously
injected into the mice, which were then intravenously inoculated
with 26 M-3.1 cells (2.5×104/mouse) after 2 days. The
mice were sacrificed after 2 weeks and the lungs were fixed in
Bouin’s solution (saturated picric acid: formalin: acetic acid;
15:5:1; v/v/v). The rate of metastasis was assessed by counting the
number of lung tumor colonies using a digital camera (Canon D550;
Canon, Inc.; Tokyo, Japan). protocol for the animal experiments was
approved by the Animal Ethics Committee of Chongqing Medical
University.
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences in the mean values of individual groups were assessed
with a one-way analysis of variance (ANOVA) with a Duncan’s
multiple range test. P<0.05 was considered to indicate a
statistically significant difference. SAS software, version 9.1
(SAS Institute Inc.; Cary, NC, USA) was used for statistical
analyses.
Results
In vitro anticancer effect of Cassia
tora L. on TCA8113 cells
The anticancer effect of Cassia tora L. on
TCA8113 cells was evaluated using an MTT assay. The growth
inhibitory rates of human tongue carcinoma TCA8113 cells treated
with the different concentrations of Cassia tora L. are
demonstrated in Table II. When
Cassia tora L. solution was administered to TCA8113 cells,
the growth inhibitory rates observed with concentrations of 0.25,
0.5 and 1.0 mg/ml were 16, 43 and 72%, respectively (P<0.05).
These results demonstrated that Cassia tora L. has a
significant anti-proliferative effect on TCA8113 cells. In
addition, the higher the concentration of Cassia tora L.,
the stronger the anticancer effect.
 | Table IIGrowth inhibition of human tongue
carcinoma TCA8113 cells by different concentrations of Cassia
tora L., as evaluated by a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. |
Table II
Growth inhibition of human tongue
carcinoma TCA8113 cells by different concentrations of Cassia
tora L., as evaluated by a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay.
| OD540
(concentration of sample, mg/ml)
|
|---|
| Treatment | 0.25 | 0.5 | 1.0 |
|---|
| Control
(untreated) | |
0.497±0.005a | |
| Cassia tora
L. |
0.417±0.008b(16) |
0.283±0.010c(43) |
0.139±0.007d(72) |
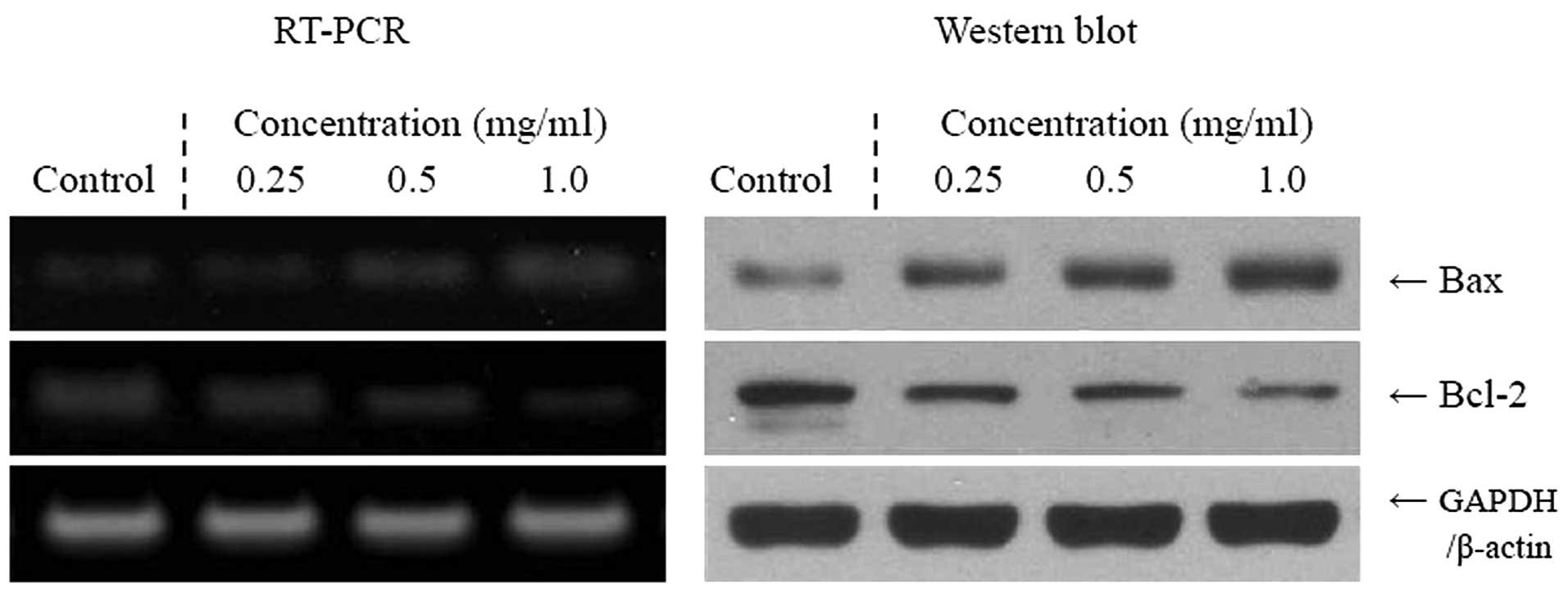
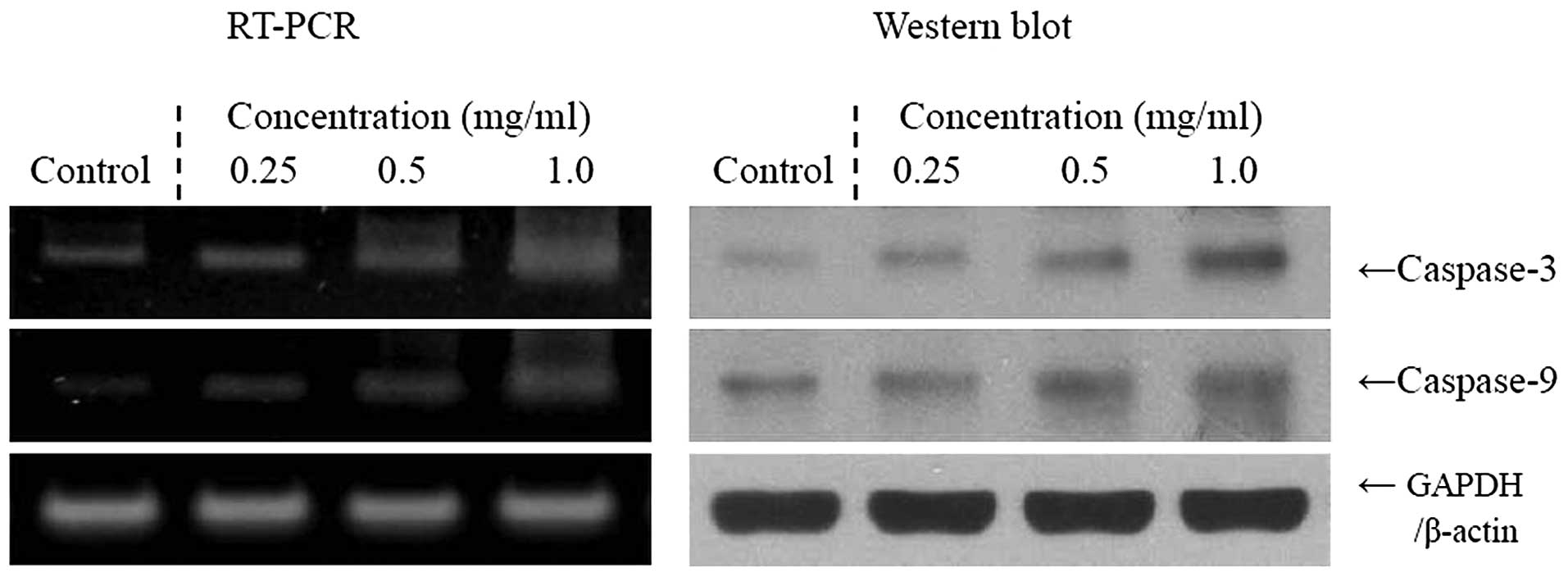
Apoptosis-related gene expression of Bax,
Bcl-2 and caspases
To elucidate the mechanisms underlying the
inhibition of cancer cell growth by the Cassia tora L., the
expression of Bax, Bcl-2, and caspase-3 and -9 was measured in
human tongue carcinoma TCA8113 cells by RT-PCR and western blot
analyses after a 48-h incubation with different concentrations of
Cassia tora L. solution. As demonstrated in Fig. 1, the expression of pro-apoptotic Bax
and anti-apoptotic Bcl-2 demonstrated significant changes in the
presence of 1.0 mg/ml Cassia tora L. These results suggest
that Cassia tora L. induced apoptosis in the TCA8113 cells
via a Bax- and Bcl-2-dependent pathway. The mRNA and protein
expression levels of caspase-3 and -9 were very low in untreated
control TCA8113 cells, but significantly increased following
treatment of the cells with 1.0 mg/ml Cassia tora L.
Caspase-3 and -9 mRNA and protein expression was gradually elevated
by treatment with increased Cassia tora L. concentrations
(Fig. 2). More specifically, the
induction of apoptosis by Cassia tora L. was correlated with
the upregulation of Bax, caspase-3 and -9, and the downregulation
of Bcl-2, in terms of mRNA and protein expression. The effects of
1.0 mg/ml Cassia tora L. were greater compared with those of
0.25 and 0.5 mg/ml Cassia tora L.
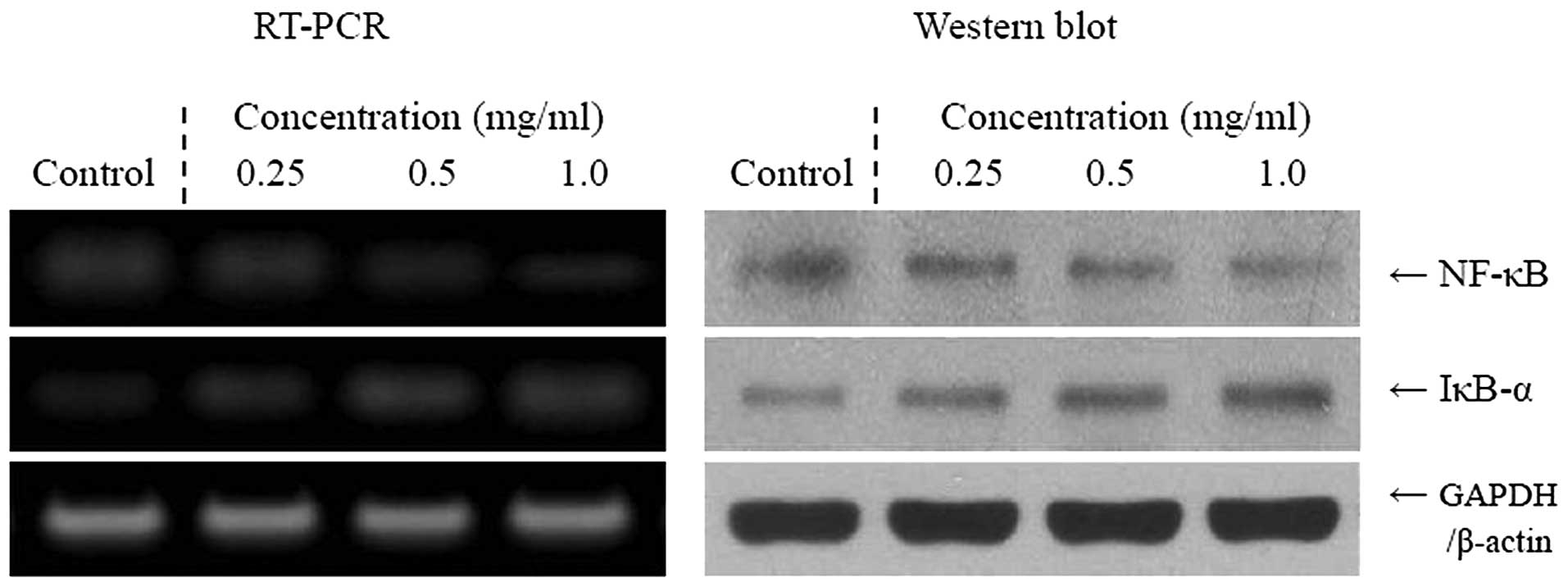
Inflammation-related gene expression of
NF-κB, IκB-α, iNOS and COX-2
We determined whether the anticancer effects of
Cassia tora L. were correlated with the inhibition of NF-κB,
IκB-α, iNOS and COX-2 gene expression. As demonstrated in Fig. 3, mRNA and protein expression of
NF-κB and IκB-α were reduced in TCA8113 cells treated with 1.0
mg/ml Cassia tora L. solution. Cassia tora L.
significantly modulated the expression of genes associated with
inflammation. The mRNA and protein expression of NF-κB decreased
while IκB-α mRNA levels increased. Additionally, the mRNA and
protein expression of COX-2 and iNOS gradually decreased in the
presence of the Cassia tora L., depending on the
concentration (Fig. 4). Our
findings indicate that Cassia tora L. may help to prevent
cancer in the early stages by increasing anti-inflammatory
activities. Overall, the results of this experiment demonstrate
that the higher concentration of Cassia tora L. had a
stronger anti-inflammatory effect on the tongue carcinoma cells
than the lower concentrations tested.
Metastasis-related MMP and TIMP gene
expression
RT-PCR and western blot analyses were conducted to
determine whether the anti-metastatic effect of Cassia tora
L. was due to gene regulation of metastatic mediators, specifically
MMPs (MMP-2 and -9) and TIMPs (TIMP-1 and -2), in TCA8113 cells. As
demonstrated in Fig. 5, 1.0 mg/ml
Cassia tora L. significantly decreased the mRNA and protein
expressions of MMP-2 and -9, while it increased the expression of
TIMP-1 and -2. These changes in TIMP and MMP expression resulting
from Cassia tora L. treatment effectively led to metastatic
inhibition in vitro. Our results also demonstrated that the
higher concentration of Cassia tora L. had a stronger
anti-metastatic activity than the lower concentrations of Cassia
tora L. tested.
In vivo anti-metastatic effect of
Cassia tora L
Prophylactic inhibition of tumor metastasis by
Cassia tora L. was evaluated using an experimental mouse
metastasis model (Fig. 6). All mice
treated with Cassia tora L. had significantly fewer lung
metastatic colonies compared with the control mice (number of
metastatic tumors, 58±7; number in each group=10; P<0.05).
Cassia tora L. was most effective at inhibiting lung
metastasis at a concentration of 200 mg/kg. This concentration
(inhibitory rate, 64%; number of metastatic tumors, 21±4) inhibited
tumor formation and lung metastasis to a greater degree than the
100 mg/kg solution (inhibitory rate, 45%; number of meta-static
tumors, 32±4) or the 50 mg/kg solution (inhibitory rate, 19%;
number of metastatic tumors, 47±6) of Cassia tora L.
Discussion
Although Cassia tora L. has been used as a
medicine, scientific data concerning its effects is limited.
Cassia tora L. has previously been demonstrated to have
various therapeutic effects on numerous pathological conditions
such as inflammation, aging and cancer (3,4,6).
Apoptosis is a fundamental cellular event, and
understanding its mechanisms of action will aid in the exploitation
of this process in tumor diagnosis and therapy (22). In a healthy cell, the anti-apoptotic
protein Bcl-2 is expressed on the outer mitochondrial membrane
surface (23). As Bax and Bcl-2
genes are mainly expressed during apoptosis, we determined that
these genes regulate apoptotic activity. Apoptosis results from the
activation of caspase family members that act as aspartate-specific
proteases (24). Caspases form a
proteolytic network within the cell whereby upstream initiator
caspases (such as caspase-9) are activated early on in the
apoptotic process and in turn activate other downstream caspases
(such as caspase-3). Cytochrome c and procaspase-9
processing are highly dependent on caspase-3, placing this caspase
in a central position as a regulator of essential apoptotic
pathways in cancer cells (25).
Caspase-3 has also been demonstrated to be involved in the
amplification of apoptotic signals by cleaving Bcl-2 (26).
Additionally, anticancer mechanisms underlying the
effect of Cassia tora L. on human cancer cells involve the
induction of apoptosis by increasing the number of apoptotic
bodies, regulating the mRNA and protein expression of Bax and
Bcl-2, and promoting anti-inflammatory effects by down-regulating
iNOS and COX-2 gene expression. COX-2 has been suggested to be
important in colon carcinogenesis, while NOS, along with iNOS, may
be a good target for the chemoprevention of colon cancer (27). NF-κB is one of the most ubiquitous
transcription factors, and it regulates the expression of genes
required for cellular proliferation, inflammatory responses and
cell adhesion (28). NF-κB is
present in the cytosol where it is bound to the inhibitory protein,
IκB. Following its induction by a variety of agents, NF-κB is
released from IκB and trans-locates to the nucleus where it binds
to the κB binding sites in the promoter regions of target genes
(29). These mechanisms may be
involved in the anticancer effects of Cassia tora L. in
tongue carcinoma cells. Based on the results of the MTT assay and
the expression patterns of pro-apoptotic genes observed in the
present study, we conclude that cancer cells treated with Cassia
tora L. underwent apoptosis. The anticancer effects of
Cassia tora L. in JTC-26 human cervical cancer cells were
evaluated in a previous study by an MTT assay and RT-PCR or western
blot analysis, and were similar to our findings (6).
Metastasis is defined as the spread of cancer cells
from one organ or area to an adjacent organ or another location
(30,31). Malignant tumor cells are considered
to have the capacity to metastasize. Cancer occurs when the cells
in a tissue have been genetically damaged in a progressive manner,
resulting in cancer stem cells that possess a malignant phenotype.
When the tumor cells have come to rest in another site, they
penetrate the vessel walls, continue to multiply and eventually
form another tumor.
MMPs, a family of zinc-dependent endopeptidases, are
important in tumorigenesis and metastasis. MMPs are able to cleave
almost all extracellular matrix (ECM) substrates. Degradation of
the ECM is a key event in tumor progression, invasion and
metastasis (32). Among the MMP
family members, MMP-2 and -9 are important molecules for cancer
invasion (33,34), and are highly expressed in breast
and colon cancer cells (35–37).
Inhibition of MMP activity is useful for controlling tumorigenesis
and metastasis (38). TIMPs are
naturally occurring inhibitors of MMPs which prevent catalytic
activity by binding to activated MMPs, thereby blocking breakdown
of the ECM (39). Disturbances in
the ratio between MMPs and TIMPs have been observed during
tumorigenesis (40). Maintaining
the balance between MMPs and TIMPs, or increasing TIMP activity,
are useful ways to control tumor metastasis (41). Experimental evidence demonstrating
the role of MMPs in metastasis has been obtained by in vitro
invasion assays and in vivo xenograft metastasis
experiments.
MMP-2 and -9 are key factors in cancer cell invasion
and metastasis both in vivo and in vitro(42). Spontaneous and experimental
metastasis to the liver is decreased in mice over-expressing TIMP1,
and increased in mice expressing antisense TIMP-1 mRNA (43). Ectopic overexpression of TIMP-1 in
the brain of transgenic mice also reduces experimental metastasis
to the brain (44). In particular,
MMP-2 and -9 are important for tumor invasion and angiogenesis.
Thus, tumor metastasis may be inhibited by blocking MMP synthesis
and activity (45). Colon 26-M3.1
carcinoma cells have been used to evaluate anti-metastasis effects
in vivo(46).
In the current study, different concentrations of
Cassia tora L. were employed in our experiments. Cassia
tora L. exerted anticancer and anti-metastatic effects on
TCA8113 cells. All concentrations of Cassia tora L. were
found to have in vitro anti-metastasis effects based on the
RT-PCR and protein analysis of MMP and TIMP gene expression, and
also showed anti-metastasis effects in vivo. Further
research is required to explain the mechanisms associated with
these effects.
In summary, various in vitro experimental
methods, including MTT, RT-PCR and western blot analysis, were
employed to evaluate the anticancer effects of Cassia tora
L. A mouse model bearing tumors produced by 26-M3.1 colon carcinoma
cells was used to study the in vivo effects of Cassia
tora L. Overall, Cassia tora L. demonstrated potent
in vitro and in vivo anticancer activities,
particularly in combating in vivo tumor metastasis. The
functional contents of Cassia tora L. are important for
augmenting these anti-cancer effects. A high concentration of
Cassia tora L. solution increased the anticancer properties
in the present study. The active compounds resulting from Cassia
tora L. require identification and evaluation in future
studies.
References
|
1
|
Yen GC and Chung DY: Antioxidant effects
of extracts from Cassia tora L. prepared under different
degrees of roasting on the oxidative damage to biomolecules. J
Agric Food Chem. 47:1326–1332. 1999.PubMed/NCBI
|
|
2
|
Liu JZ, Lin X, Li XE, et al: Effect of
protein and anthraquinone glucosides from Semen Cassia on learning
and memory capacity and related substances of senile mice induced
by D-galactose. China J Chinese Mater Med. 32:516–519. 2007.(In
Chinese).
|
|
3
|
Lin DJ and Jin Z: Experimental study on
protective effect of Semen Cassiae extract against acute liver
injury. LiShiZhen Med Mater Med Res. 17:214–215. 2006.
|
|
4
|
Yen GC, Chen HW and Duh PD: Extraction and
identification of an antioxidative component from Jue Ming Zi
(Cassia tora L.). J Agric Food Chem. 46:820–824. 1998.
View Article : Google Scholar
|
|
5
|
Kim SY, Kim JH, Kim SK, et al: Antioxidant
activities of selected oriental herb extracts. J Am Oil Chem Soc.
71:633–640. 1994. View Article : Google Scholar
|
|
6
|
Hao YJ, Sang YL and Zhao YQ: Research
progress of Jue-ming-zi. Chinese Tradit Herbal Drugs. 32:858–859.
2001.
|
|
7
|
Qi GF: Cassia analysis of lipid-lowering
active ingredients. Guang Ming Zhong Yi. 26:1569–1570. 2011.
|
|
8
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 1999. View Article : Google Scholar
|
|
9
|
Kwon JI, Kim GY, Park KY, et al: Induction
of apoptosis by linoleic acid is associated with the modulation of
Bcl-2 family and Fas/FasL system and activation of caspases in AGS
human gastric adenocarcinoma cells. J Med Food. 11:1–8. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guerrero AD, Guerrero M and Wang J:
Delineation of the caspase-9 signaling cascade. Apoptosis.
13:177–186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tak PP and Firestein GS: NF-κB: a key role
in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
|
|
12
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mantovani A, Allavena P, Sica A, et al:
Cancer-related inflammation. Nature. 454:436–444. 2008. View Article : Google Scholar
|
|
14
|
Itoh Y and Nagase H: Matrix
metalloproteinases in cancer. Essays Biochem. 38:21–36.
2002.PubMed/NCBI
|
|
15
|
Brew K, Dinakarpandian D and Nagase H:
Tissue inhibitors of metalloproteinases: evolution, structure and
function. Biochim Biophys Acta. 1477:267–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Woodhouse EC, Chuaqui RF and Liotta LA:
General mechanisms of metastasis. Cancer. 80:1529–1537. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Skehan P, Storeng R, Monks SA, et al: New
colorimetric cytotoxicity assay for anticancer-drug screening. J
Natl Cancer Inst. 82:1107–1112. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bak SS, Kong CS, Rhee SH, et al: Effect of
sulfur enriched young radish kimchi on the induction of apoptosis
in AGS human gastric adenocarcinoma cells. J Food Sci Nutr.
12:79–83. 2007. View Article : Google Scholar
|
|
20
|
Choi YH, Lee SJ, Nguyen P, et al:
Regulation of cyclin D1 by calpain protease. J Biol Chem.
272:28479–28484. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jung KO, Park SY and Park KY: Longer aging
time increases the anticancer and antimetastatic properties of
doenjang. Nutrition. 22:539–545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Milanezi F, Leitão D, Ricardo S, et al:
Evaluation of HER2 in breast cancer: reality and expectations.
Expert Opin Med Diagn. 3:607–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chao DT and Korsmeyer SJ: Bcl-2 family:
regulators of cell death. Annu Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar
|
|
24
|
Kidd VJ: Proteolytic activities that
mediate apoptosis. Annu Rev Physiol. 60:533–573. 1998. View Article : Google Scholar
|
|
25
|
Blanc C, Deveraux QL, Krajewski S, et al:
Caspase-3 is essential for procaspase-9 processing and
cisplatin-induced apoptosis of MCF-7 breast cancer cells. Cancer
Res. 60:4386–4390. 2000.PubMed/NCBI
|
|
26
|
Kirsch DG, Doseff A, Chau BN, et al:
Caspase-3-dependent cleavage of Bcl-2 promotes release of
cytochrome c. J Biol Chem. 274:21155–21161. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Delić R and Štefanović M: Optimal
laboratory panel for predicting preeclampsia. J Maternal-Fetal
Neonatal Med. 23:96–102. 2010.PubMed/NCBI
|
|
28
|
Baeuerle PA: IkappaB-NF-kappaB structures:
at the interface of inflammation control. Cell. 95:729–731.
1998.PubMed/NCBI
|
|
29
|
Sánchez-Pérez I, Benitah SA,
Martinez-Gomariz M, et al: Cell stress and MEKK1-mediated c-Jun
activation modulate NFkappaB activity and cell viability. Mol Biol
Cell. 13:2933–2945. 2002.PubMed/NCBI
|
|
30
|
Klein CA: Cancer. The metastasis cascade
Science. 321:1785–1787. 2008.PubMed/NCBI
|
|
31
|
Chiang AC and Massagué J: Molecular basis
of metastasis. New Engl J Med. 359:2814–2823. 2008. View Article : Google Scholar
|
|
32
|
Vihinen P, Ala-aho R and Kähäri VM: Matrix
metalloproteinase as therapeurtic targets in cancer. Curr Cancer
Drug Targets. 5:203–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Davies B, Waxman J, Wasan H, et al: Levels
of matrix metalloproteinase in bladder cancer correlate with tumor
grade and invasion. Cancer Res. 53:5365–5369. 1993.PubMed/NCBI
|
|
34
|
Bogerieder T and Herlyn M: Axis of evil:
molecular mechanism of cancer metastasis. Oncogene. 22:6524–6536.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Canning MT, Postovit LM, Clarke SH, et al:
Oxygen-mediated regulation of gelatinase and tissue inhibitor of
metalloproteinase-1 expression by invasive cells. Exp Cell Res.
267:88–94. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bartsch JE, Staren ED and Appert HE:
Matrix metalloproteinase expression in breast cancer. J Surg Res.
110:383–932. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zucker S and Vacirca J: Role of matrix
metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis
Rev. 23:101–117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen PN, Chu SC, Chiou HL, et al: Mulberry
anthjocyanins, cyaniding 3-rutinoside and cyaniding 3-glucoside,
exhibited and inhibitory effect on the migration and invasion of a
human lung cancer cell line. Cancer Lett. 235:248–259. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Uzui H, Harpf A, Liu M, et al: Increased
expression of memebrane type 3-matrix metalloproteinase in human
athero-sclerotic plaque: role of activated macrophage and
inflammatory cytokines. Circulation. 106:3024–3030. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lambert E, Dasse E, Haye B, et al: TIMPs
as multifacial proteins. Crit Rev Oncol Hematol. 49:187–198. 2004.
View Article : Google Scholar
|
|
41
|
Mysliwiec AG and Ornstein DL: Matrix
metalloproteinases in colorectal cancer. Clin Colorectal Cancer.
1:208–219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kleiner DE and Stetler-Stevenson WG:
Matrix metalloproteinases and tumor metastasis. Cancer Metastasis
Rev. 25:9–34. 2006. View Article : Google Scholar
|
|
43
|
Krüger A, Fata JE and Khokha R: Altered
tumor growth and metastasis of a T-cell lymphoma in TIMP-1
transgenic mice. Blood. 90:1993–2000. 1997.PubMed/NCBI
|
|
44
|
Krüger A, Sanchez-Sweatman OH, Martin DC,
et al: Host TIMP-1 overexpression confers resistance to
experimental brain metastasis of a fibrosarcoma cell line.
Oncogene. 16:2419–2423. 1998.PubMed/NCBI
|
|
45
|
Shin DY, Kim GY, Kim JI, et al:
Anti-invasive activity of diallyl disulfide through tightening of
tight junctions and inhibition of matrix metalloproteinase
activities in LNCaP prostate cancer cells. Toxicol In Vitro.
24:1569–1576. 2010. View Article : Google Scholar
|
|
46
|
Ha ES, Hwang SH, Shin KS, et al:
Anti-metastatic activity of glycoprotein fractionated
fromacanthopanax senticosus, involvement of NK-cell and macrophage
activation. Arch Pharm Res. 27:217–224. 2004. View Article : Google Scholar : PubMed/NCBI
|