Introduction
Breast cancer is one of the most common cancers in
the world. There are 2.5 million women diagnosed with breast cancer
in the United States, and in Europe. Additionally, 350,000 new
cases are diagnosed each year, with a mortality rate of 130,000
patients, accounting for 17.5% of all cancer-related mortality in
Europe (1,2). In Korea, the incidence rate for breast
cancer has increased by 2.6% each year (3). In advanced adenocarcinoma, progressed
or stage IV cancer, malignant peritoneal and pleural fluid may
develop as the tumor progresses, and this occurs in 10% of all
cases (4).
Clinically, cancer antigen (CA) 15-3 is widely used
as a tumor marker for breast cancer, but it is mostly used with
plasma samples. Among the diagnostic methods using body fluid,
cytology is thought to be the most reliable, but it is limited by
low sensitivity (5). To compensate
for the low sensitivity, other diagnostic markers, such as
carcinoembryonic antigen (CEA), which has been reported to have a
diagnostic value for determining malignancy in pleural fluid, are
being used clinically (6). Ascitic
CEA has recently been reported to have an increased specificity in
peritoneal fluid for diagnosing gastric malignancy (7). However, the markers that are being
used to diagnose malignancy still pose problems of low sensitivity
with a wide variability, which is a limitation in routine clinical
use, particularly for predicting prognosis (8,9).
Matrix metalloproteinases (MMPs) are known to
promote cancer progression through extracellular matrix (ECM) and
basement membrane degradation, resulting in the exposure of cryptic
locations linked to invasion, metastasis and angiogenesis (10–12).
It has been reported that active MMPs are indicators for metastasis
in breast cancer (13).
Additionally, the overexpression of MMP-2 and -9 is reportedly
correlated with poor overall survival, suggesting that MMP-2 and -9
are possible prognostic markers (11,14).
Therefore, the improved ability to detect malignancy in body fluids
of breast cancer patients using biomarkers such as MMPs may be
helpful for determining the proper treatment and predicting
prognosis. In this study, we evaluated the possibility of using
MMP-2 and -9 expressed in body fluids as diagnostic markers for
metastatic breast cancer.
Materials and methods
Patients
We collected the body fluids of 36 patients, who
were clinically diagnosed with metastatic stage IV breast carcinoma
with malignant ascites or pleural effusion (10 ascites, 27 pleural
fluids; one patient had malignant ascites and pleural effusion) at
Yonsei Cancer Center, Yonsei University College of Medicine, Yonsei
University Health System between October 2000 and September 2009.
Medical records were retrospectively reviewed for patient
demographic and clinical information including serum CEA and CA
15-3. The patients had systemic metastasis with more than 2 sites
of metastasis, including at least one site of visceral metastasis.
The patients were heavily pretreated with systemic chemotherapy,
with the median chemotherapy regimen consisting of 3
chemotherapeutic agents (range, 1–7). Clinical and radiological
results confirmed that the body fluids originated from the
carcinomatosis of breast cancer, with no evidence of other
malignancies (15). When the body
fluid was detected for the first time in each patient, it was
collected through paracentesis or thoracentesis. Body fluid
cytology based on cell block and routine body fluid examinations
were performed and samples were kept at −70˚C until they were used
for experimentation. Body fluid cytology results were available in
7 peritoneal and 20 pleural fluids. In addition, CEA expression
from body fluids (aCEA for peritoneal and pCEA for pleural fluids)
was evaluated with a chemiluminescent enzyme immunoassay (Beckman
Coulter Inc., Minnesota, MN, USA). Following the previous result, a
positive cut-off level of 5 ng/ml CEA for body fluid was used
(7). Patient survival was
calculated from the date body fluid was collected until the date of
mortality due to any cause. Signed consent was obtained from all
patients.
Positivity of body fluid MMP-2 and
-9
The cut-off level for positivity of body fluid MMPs
determined from a comparison of zymography and ELISA was used based
on previous results (16). Briefly,
conditioned media (CM) of HT-1080 and human fibrosarcoma cells,
were used as a positive control for MMP-2 and -9. To overcome the
difficulties of zymography, which shows the qualitative biological
activity of MMPs, and also to quantify their activities, an ELISA
assay was utilized. Enzymatic activity and the quantitative
expression level of MMP were compared with the protein
concentration of HT-1080 CM. After confirming the positive
correlation between zymography and ELISA results (p<0.05), we
determined the diagnostic cut-off for MMP-9 as 0.14 ng/ml and 8.6
ng/ml for MMP-2 (16). Patient
samples were then quantified with ELISA assay.
Statistical analysis
To compare the expression levels of body fluid MMPs
with body fluid CEA and cytology, the Mann-Whitney U-Test was used.
In analyzing the overall survival, we performed a log-rank test
using the Kaplan-Meier method. SPSS 13.0 was used to perform all
statistical analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
Biomarker expression in the body fluid of
breast cancer patients
Our patient sample included only females (n=36), and
the total sample number was 37, as one patient had both malignant
ascitic and pleural effusion. The median age of the patients was 54
years (range, 36–77). Body fluid cytology had a 40.7% (11/27)
positivity, serum CA15-3 had a mean value of 159.3±298.4 μg/ml,
serum CEA 22.9±67.9 ng/ml, and body fluid CEA had a mean value of
60.9±124.1 ng/ml in all patients (Table
I). Median overall survival of all patients was 37 days (range,
5–1463), suggesting that the patients had far advanced disease, and
patients were heavily pretreated when they developed the malignant
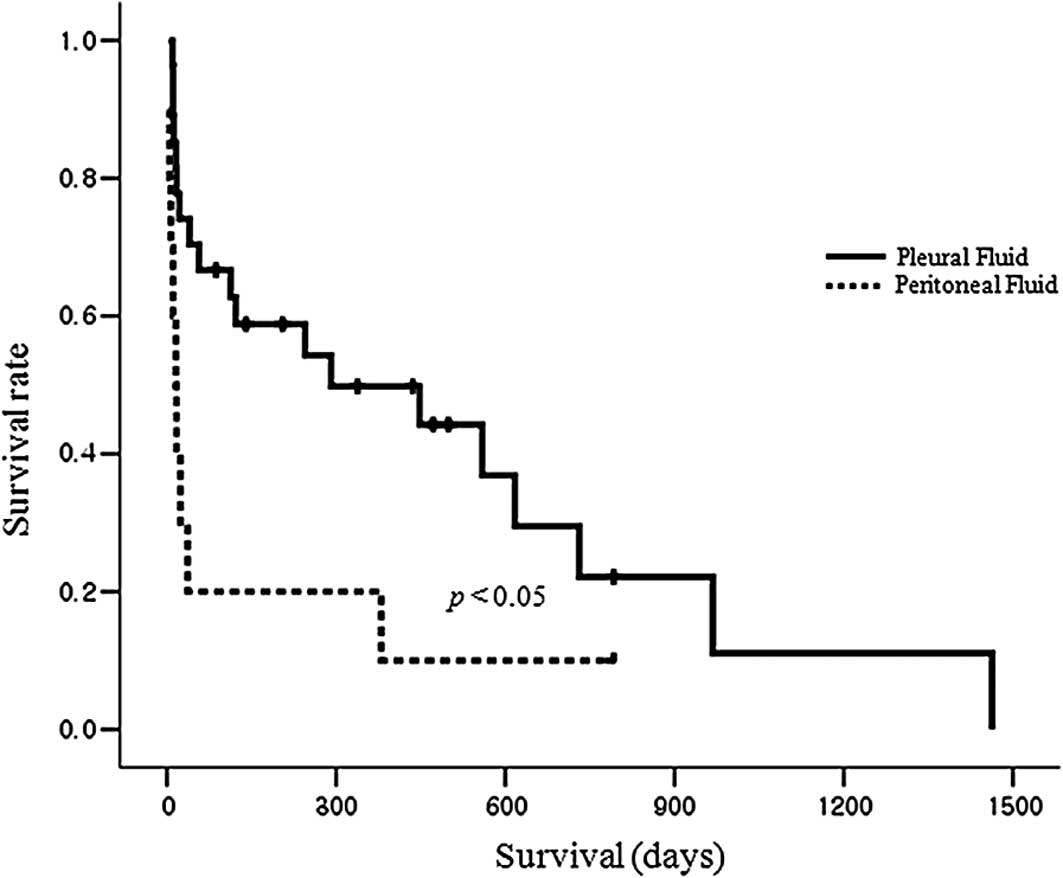
ascites of pleural effusion. Notably, our results showed that
patients with peritoneal fluid had a significantly shorter
survival, with a median of 16 days (range, 5–792), compared to
patients with pleural fluid, who had a median survival of 291 days
(range, 10–1463), p<0.05 (Fig.
1).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Ascites | Pleural effusion | Total |
|---|
| Patienta |
| Female | 10 | 27 | 36 |
| Sample |
| Body fluid | 10 | 27 | 37 |
| Age, median
(range) | 53 (40–64) | 54 (36–77) | 54 (36–77) |
| Fluid CEA mean ±
SD | 124.5±213 | 37.3±58.8 | |
| Serum CEA mean ±
SD | 14±16.6 | 31.9±76.5 | |
| Serum CA15-3
mean±SD | 380.6±542.4 | 88.4±106.5 | |
| Cytology |
| Positive | 2 (28.6%) | 9 (45%) | |
| Negative | 5 (71.4%) | 11 (55%) | |
| Survivalb |
| Median (range) | 16 (5–792) | 291 (10–1463) | |
Since body fluid CEA has been reported to have a
role as a biomarker (7), we
evaluated CEA in body fluids. CEA expression differed in body
fluids; ascites had a higher CEA expression of 124.5±213 ng/ml than
the CEA expression in pleural fluids, which was 37.3±58.8 ng/ml. We
then compared MMP-9 and -2 expression in body fluids. Peritoneal
fluid had a lower MMP-9 expression level of 0.09±0.26 ng/ml
compared to 0.25±0.64 ng/ml from the pleural fluids. However, MMP-2
expression in ascites (34.1±20 ng/ml) was higher than that in
pleural fluids (29.9±24.5 ng/ml) (Table II). When we compared the expression
levels of various biomarkers (CEA, MMP-2, MMP-9) in ascites and
pleural fluids, CEA and MMP-2 were not significantly different
(data not shown). In comparison, MMP-9 expression in pleural fluid
was higher than that in peritoneal fluid (p<0.05).
 | Table IIComparison of body fluid biomarker
expression between ascites and pleural effusion. |
Table II
Comparison of body fluid biomarker
expression between ascites and pleural effusion.
| N | MMP-9 | MMP-2 | CEA |
|---|
| Ascites | 10 | 0.09±0.26 | 34.1±20 | 124.5±213 |
| Pleural effusion | 27 | 0.25±0.64 | 29.9±24.5 | 37.3±58.8 |
| Total | 37 | 0.20±0.56 | 31.1±23.19 | 60.9±124.1 |
Improved malignancy detection using body
fluid MMP-2
Cytology information from 20 pleural and 7
peritoneal fluids showed positive rates of 45 (9/20) and 28.6%
(2/7), respectively, demonstrating that cytology has an overall low
sensitivity in our samples, as reported in previous studies
(5,9). Following evaluation of other
biomarkers in 27 pleural fluids, MMP-2 was found to have the
highest positivity with 85.2% (23/27), followed by CEA, with a
74.1% (20/27) positivity. MMP-9 showed the lowest positivity with
29.6% (8/27) (Table III).
Notably, in five patients with ascites and negative cytology from
peritoneal fluid, four patients were positive for CEA expression
(80%), one patient was positive for MMP-9 expression (20%), and all
five patients were positive for MMP-2 expression (100%). By
contrast, in 11 pleural fluids with negative cytology, the
positivity for CEA, MMP-2 and MMP-9 were 63.6 (7/11), 72.7 (8/11)
and 27.3% (3/11), respectively. These results suggest that body
fluid MMPs, especially MMP-2, could be used as diagnostic
biomarkers in metastatic breast cancer.
 | Table IIIPositivity of single and multiple
biomarkers in body fluids. |
Table III
Positivity of single and multiple
biomarkers in body fluids.
| Ascites (Total
n=10) | Pleural fluids (Total
n=27) |
|---|
|
|
|
|---|
| n | % | n | % |
|---|
| Single marker |
| CEA | 8 | 80 | 20 | 74.1 |
| MMP-9 | 1 | 10 | 8 | 29.6 |
| MMP-2 | 10 | 100 | 23 | 85.2 |
| Multiple markers |
| CEA+MMP-9 | 8 | 80 | 23 | 85.2 |
| CEA+MMP-2 | 10 | 100 | 26 | 96.3 |
| MMP-9+MMP-2 | 10 | 100 | 24 | 88.9 |
| CEA+MMP-9+MMP-2 | 10 | 100 | 26 | 96.3 |
When the biomarkers were combined, an increase in
sensitivity was observed. In pleural fluids, combining CEA and
MMP-2 increased the positivity to 96.3% (26/27). Combining MMP-9
and MMP-2 showed a positive rate of 88.9% (24/27), combining CEA
and MMP-9 improved the positivity to 85.2% (23/27), and combining
all three markers had the same positive rate as combining just CEA
and MMP-2, 96.3% (26/27) (Table
III). In the 10 peritoneal fluid samples, MMP-2 had the highest
positive rate with 100% (10/10), followed by CEA which had a
positivity of 80% (8/10), and MMP-9 had the lowest positive rate
with 10% (1/10). The combination of body fluid CEA and body fluid
MMP-2 had a positive rate of 100% (10/10), which was equal to
combining body fluid MMP-9 and body fluid MMP-2 or combining all
three biomarkers. The combination of body fluid CEA and body fluid
MMP-9, however, increased positivity to 80% (8/10) (Table III). As a result, MMP-2 was more
sensitive to detecting malignancy in body fluids and had an
additional diagnostic role when combined with body fluid CEA.
Previous reports have suggested that body fluid CEA
is a marker with a relatively high sensitivity of approximately 80%
in the body fluid of various types of cancer, confirming results of
this study, obtained from the body fluid of metastatic breast
cancer patients (6,7,16).
However, our results showed that body fluid MMP-2 had an even
higher sensitivity than body fluid CEA for detecting malignancy in
breast carcinoma. In pleural fluids, the combination of body fluid
MMP-2 and body fluid CEA improved sensitivity to almost 100%,
indicating that MMP-2 alone, or in addition to CEA, may be used as
a diagnostic biomarker. In peritoneal fluid, MMP-2 had a positivity
of 100%, suggesting that body fluid MMP-2 is a useful diagnostic
biomarker in metastatic breast cancer patients, in addition to
cytology and body fluid CEA.
Discussion
We evaluated the expression of MMP-2 and -9 in the
body fluid of metastatic breast cancer patients to determine the
possibility of using MMPs as biomarkers. MMPs are reportedly
involved in prognosis and are used as prognostic markers in tissue
and plasma samples (17,18). Our study focused on body fluid
samples, which have an advantage over tissue and plasma samples in
terms of their availability and representation of the direct effect
from cancer. Body fluids may be obtained from patients as soon as
the fluids accumulate, but tissue samples are limited in their
availability. Moreover, plasma samples are not directly in contact
with the cancer, and thus may contain numerous non-specific target
molecules, whereas body fluids form directly at the site of cancer
and are capable of reflecting cancer status as well as tumor
burden. Therefore, body fluids provide advantages over tissue and
plasma samples for understanding pathogenesis and diagnosis, and
for predicting clinical outcomes in breast cancer.
Cytology from body fluid is known to be the most
reliable marker for diagnosis, but has an extremely low sensitivity
(5). Due to the low sensitivity,
other cancer-related biomarkers such as CEA and telomerase activity
are being used in patient samples, but still yield unsatisfactory
sensitivities and specificities, and are not applicable due to
difficulties in detection methods. We aimed to identify diagnostic
biomarkers with increased sensitivity by evaluating MMP-2 and -9
expression in body fluids to be used alone or in addition to body
fluid CEA, for detecting malignancy within body fluids from
metastatic breast cancer.
Since our study focused on assessing body fluid
MMP-2 and -9 as diagnostic markers in breast cancer, the choice of
method for evaluation was important, as the assay directly affects
accuracy and practicality. While zymography allows visualization of
the enzymatic activity of MMPs qualitatively, ELISA provides a
quantitative amount of MMP protein expression. The combination of
methods allows us to create an accurate assessment of MMP
expression (19). Using the two
assays with HT-1080 CM, we determined a cut-off level for MMP-9 of
0.14 and 8.6 ng/ml for MMP-2 based on the minimal level of
expression that could be sufficient for diagnosis.
In our experiment, body fluid MMP-2 had a positivity
of 85.2% in pleural fluid and 100% in peritoneal fluid. When body
fluid MMP-2 was combined with body fluid CEA, positivity was
increased to 96.3% in pleural fluid. Compared to body fluid MMP-9
with limited diagnostic features, body fluid MMP-2 alone or in
combination with body fluid CEA was useful as an additive
diagnostic marker in the body fluid of breast cancer patients.
Although body fluid MMPs did not seem to have any prognostic role
in our study (data not shown), the type of body fluid had a
prognostic role. Breast cancer patients with peritoneal fluid had a
significantly shorter survival compared to patients with pleural
fluid. This observation may be correlated with disease burden
considering the site of metastasis from the original breast tumor.
Previously, it was reported that MMP-2 and MMP-9 are involved in
breast cancer invasion (20,21).
Our results have shown that MMP-2 has a higher expression and
positivity than MMP-9. Findings of a previous report that evaluated
23 malignant body fluids showed that MMP-2 (87%) had a higher
positivity than MMP-9 (78.3%) in different cancer origins,
corresponding with our result (22). In addition, MMP-9 expression was
significantly higher in pleural than in peritoneal fluid. Breast
cancer patients initially form pleural fluid, which may invade to
cause the formation of peritoneal fluid in the abdomen. Since MMP-2
and -9 are involved in cancer invasion, MMP expression may be
higher in pleural fluid in preparation for cancer invasion, whereas
MMP expression may be lower in peritoneal fluid, suggesting that
invasion has already occurred. Studies have shown that MMP-9 is
capable of being downregulated after invasion and body fluid
formation has occurred, suggesting that MMP-9 expression is tightly
controlled, which may contribute to the low level of MMP-9 in
peritoneal fluid (23,24). In one patient who had both ascitic
fluid and pleural effusion, MMP-9 expression in the pleural fluid
(0.55 ng/ml) was much greater than that in the ascitic fluid (0.02
ng/ml).
Previous reports determined that cancer invasion is
correlated with poor survival (25). We observed that the patients with
malignant ascites showed a shorter survival compared to the
patients with pleural effusion. As pleural and peritoneal effusions
occur in various parts of the body with different biology, we
consider that the body fluid itself may differ in the expression
and biological role of each molecule, which may also predict
prognosis. Previous reports have suggested that MMP-9 and MMP-2
overexpression correlates with poor overall survival in tissue and
plasma samples of various cancers (17,18).
However, results from our experiment did not concur with these
reports, except for the prognostic potential of the site of
malignant effusion. This may be related to the small sample size in
our study. Moreover, since our study used body fluid, rather than
tissue and plasma, the expression of MMPs may differ, since MMPs
are under tight control in the body and in cells (23,24).
Although we focused on the role of body fluid
biomarkers, if we could use the serum biomarkers in addition to the
body fluid biomarkers, more reliable information would be obtained
in order to understand the status of the patients, including tumor
burdens and prognosis. Among the numerous tumor markers, serum CEA
and CA 15-3 are mostly used for breast cancer patients. However, in
our patient set, the level of those serum markers were not
correlated with each other or with body fluid biomarkers. In
addition, pleural CEA has been used for the detection of malignancy
from pleural fluid. However, ascitic CEA has recently been
suggested as a detection factor for malignancy by our previous
report in gastric cancer. Therefore, in this study we compared
ascitic and pleural CEA with body fluid MMPs. Our study was unique
in that it analyzed MMP expression in body fluids of metastatic
breast cancer patients, allowing us to compare the differences that
potentially exist between each patient. Moreover, the use of an
ELISA assay has several benefits for use in clinical practice; it
is easy, quantitative and a small amount of the body fluid is
required. Previous studies have suggested that MMP-2 and -9 be used
as diagnostic markers in tissue and plasma samples. However, this
is the first study to use malignant body fluids from breast cancer
to evaluate MMPs as possible diagnostic markers. In particular, our
results suggest that MMP-2 is a highly sensitive diagnostic marker
for metastatic breast cancer patients. The limitations of the
current study are: i) the patient heterogeneity, ii) the study is
retrospective with a small sample size, and iii) the determination
of the assay cut-off level is arbitrary. Patient heterogeneity with
tumor heterogeneity is the essential problem of translational
research. In our study, patients with relatively homogeneous
clinical features were selected. The patients were required to have
clinically evident malignant ascites or pleural effusion regardless
of cytology results, considering the false negativity of body fluid
cytology. Since systemic chemotherapy after body fluid formation
may affect prognosis, we selected patients who had received active
systemic chemotherapy prior to body fluid formation. Therefore,
patients who developed ascites or pleural effusion at the time of
breast cancer diagnosis were excluded. Multiple sites of metastasis
were also observed, including a minimum of 1 visceral metastasis
from the breast cancer. All the patients were previously heavily
pretreated with active systemic treatment. In addition, this study
is the first study to evaluate the proof-of-concept of whether the
biomarker in the body fluid may work in clinical practice and also
the feasibility of ELISA for stratifying the patients. In almost
all the patients who develop body fluids, the fluid examination is
easily performed in clinical practice, and the collection of body
fluid is feasible. Gathering the fluid provides biological
information, which may be of clinical use, and therefore this
practice is worthwhile. However, validation with large numbers of
prospectively collected samples is required for the further
clinical development of these novel body fluid biomarkers.
Acknowledgements
This manuscript was supported by the Public Welfare
and Safety research program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Education,
Science and Technology (2010-0020841).
References
|
1
|
Tyczynski JE, Plesko I, Aareleid T, et al:
Breast cancer mortality patterns and time trends in 10 new EU
member states: mortality declining in young women, but still
increasing in the elderly. Int J Cancer. 112:1056–1064. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holmes MD, Chen WY, Hankinson SE and
Willett WC: Physical activity’s impact on the association of fat
and fiber intake with survival after breast cancer. Am J Epidemiol.
170:1250–1256. 2009.
|
|
3
|
Jung KW, Won YJ, Park S, et al: Cancer
statistics in Korea: incidence, mortality and survival in 2005. J
Korean Med Sci. 24:995–1003. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Runyon BA, Hoefs JC and Morgan TR: Ascitic
fluid analysis in malignancy-related ascites. Hepatology.
8:1104–1109. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abe S, Yoshimura H, Tabara H, et al:
Curative resection of gastric cancer: limitation of peritoneal
lavage cytology in predicting the outcome. J Surg Oncol.
59:226–229. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Terracciano D, Di Carlo A, Papa P, et al:
New approaches in the diagnostic procedure of malignant pleural
effusions. Oncol Rep. 12:79–83. 2004.PubMed/NCBI
|
|
7
|
Jung M, Jeung HC, Lee SS, et al: The
clinical significance of ascitic fluid CEA in advanced gastric
cancer with ascites. J Cancer Res Clin Oncol. 136:517–526. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gaspar MJ, Arribas I, Coca MC and
Diez-Alonso M: Prognostic value of carcinoembryonic antigen, CA
19-9 and CA 72-4 in gastric carcinoma. Tumour Biol. 22:318–322.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Manzoni G, Verlato G, Di Leo A, et al:
Peritoneal cytology does not increase the prognostic information
provided by TNM in gastric cancer. World J Surg. 30:579–584.
2006.
|
|
10
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duffy MJ, Maguire TM, Hill A, McDermott E
and O’Higgins N: Metalloproteinases: role in breast carcinogenesis,
invasion and metastasis. Breast Cancer Res. 2:252–257. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Curran S and Murray GI: Matrix
metalloproteinases: molecular aspects of their roles in tumour
invasion and metastasis. Eur J Cancer. 36:1621–1630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jezierska A and Motyl T: Matrix
metalloproteinase-2 involvement in breast cancer progression: a
mini-review. Med Sci Monit. 15:RA32–40. 2009.PubMed/NCBI
|
|
14
|
Dragutinovic V, Izrael-Zivkovic L and
Radovanovic N: Relation of matrix metalloproteinase-9 to different
stages of tumors in the serum of gastric cancer. Dig Dis Sci.
54:1203–1207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yajima K, Kanda T, Ohashi M, et al:
Clinical and diagnostic significance of preoperative computed
tomography findings of ascites in patients with advanced gastric
cancer. Am J Surg. 192:185–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noh S, Jung JJ, Jung M, et al: MMP-2 as a
putative biomarker for carcinomatosis in gastric cancer.
Hepatogastroenterology. 58:2011.(Epub ahead of print).
|
|
17
|
Lengyel E, Schmalfeldt B, Konik E, et al:
Expression of latent matrix metalloproteinase 9 (MMP-9) predicts
survival in advanced ovarian cancer. Gynecol Oncol. 82:291–298.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kallakury BV, Karikehalli S, Haholu A,
Sheehan CE, Azumi N and Ross JS: Increased expression of matrix
metalloproteinases 2 and 9 and tissue inhibitors of
metalloproteinases 1 and 2 correlate with poor prognostic variables
in renal cell carcinoma. Clin Cancer Res. 7:3113–3119.
2001.PubMed/NCBI
|
|
19
|
Lombard C, Saulnier J and Wallach J:
Assays of matrix metalloproteinases (MMPs) activities: a review.
Biochimie. 87:265–272. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shah FD, Shukla SN, Shah PM, Shukla HK and
Patel PS: Clinical significance of matrix metalloproteinase 2 and 9
in breast cancer. Indian J Cancer. 46:194–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong MK, Cho KY, Oh SJ, Kim KM, Yu SJ and
Jung SS: Implications of the Activation of Matrix
Metalloproteinase-2 (MMP-2) on the Metastasis in Breast Cancer. J
Korean Breast Cancer Soc. 5:19–26. 2002. View Article : Google Scholar
|
|
22
|
Sun XM, Dong WG, Yu BP, Luo HS and Yu JP:
Detection of type IV collagenase activity in malignant ascites.
World J Gastroenterol. 9:2592–2595. 2003.PubMed/NCBI
|
|
23
|
McCawley LJ and Matrisian LM: Matrix
metalloproteinases: multifunctional contributors to tumor
progression. Mol Med Today. 6:149–156. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Belotti D, Paganoni P, Manenti L, et al:
Matrix metalloproteinases (MMP9 and MMP2) induce the release of
vascular endothelial growth factor (VEGF) by ovarian carcinoma
cells: implications for ascites formation. Cancer Res.
63:5224–5229. 2003.
|
|
25
|
Warren M: Metastatic breast cancer
recurrence: a literature review of themes and issues arising from
diagnosis. Int J Palliat Nurs. 15:222–225. 2009. View Article : Google Scholar : PubMed/NCBI
|