Introduction
Warburg first observed that even in the presence of
sufficient oxygen, cancer cells prefer to metabolize glucose and
produce lactic acid (1–4). The concomitant increase in glucose
uptake may be exploited clinically for the detection of most solid
malignant tumors by fluorodeoxyglucose positron emission tomography
(FDG-PET). One possible reason for cancers adopting this less
efficient pathway for producing adenosine triphosphate (ATP)
compared with oxidative phosphorylation is its advantage for
survival and proliferation in the unique hypoxic tumor environment
(5). This preference for anaerobic
respiration is also considered to be the reason for the resistance
cancer cells exhibit to anticancer drugs which induce apoptosis via
the mitochondrial pathway. Bonnet et al have reported that
treating cancer cells with dichloroacetate (DCA), an approved
treatment for congenital lactic acidosis, reverses the Warburg
effect and inhibits tumor growth (3,4,6–8).
DCA increases the flux of pyruvate into the mitochondria by
inhibiting the pyruvate dehydrogenase kinase and promotes glucose
oxidation over glycolysis. As a result, DCA decreases the
production of lactic acid by the tumor and increases the
intracellular pH. DCA induces apoptosis via two pathways, one in
the mitochondria, where depolarization and superoxide (SOD)
production activates mitochondria-dependent apoptosis, and the
other at the plasmalemmal level, where activation/upregulation of
Kv1.5 channels decreases the (K+)i, activating caspases
(6). DCA is a promising anticancer
drug due to the convenience of its oral administration, low cost,
few side effects and long experience of clinical use (7,8).
Although it appeared to be a promising treatment for malignant
tumors, its effect is limited in an ongoing report of clinical
trials. Therefore, we aimed to find clinically-used drugs that
enhance the effects of DCA.
Omeprazole (OMP) is a proton pump inhibitor (PPI)
and is also known to enhance the effects of anticancer drugs
(9). OMP inhibits the activity of
V-ATPase besides targeting the gastric acid pump. It was reported
to inhibit the proliferation of tumor cells, possibly by
alkalinization of lysosomes and permeabilization of lysosome
membranes, followed by the production of reactive oxygen species
(ROS) (10,11).
As described, DCA and OMP are thought to inhibit
tumor cell growth through the common pathway of ROS production by
influencing the intracellular pH levels. Since these two drugs are
used clinically with minor side effects, we believe that their
combination is a promising protocol for cancer treatment.
Materials and methods
Chemicals
DCA and vitamin E (tocopherol acetate) were
purchased from Wako Chemical Industries, Ltd. (Tokyo, Japan). OMP
was purchased from Astra Zeneca Japan (Osaka, Japan). Z-VAD-FMK was
purchased from the Peptide Institute, Inc. (Osaka, Japan).
Cell culture
HT1080 human fibrosarcoma cells, WI-38 human
fibroblast cells and RKO colon cancer cells were seeded and grown
in Dulbecco's modified Eagle's medium containing 10% fetal bovine
serum (FBS) and maintained in an incubator at 37˚C and 5%
CO2. For the cell proliferation experiments, cells were
seeded in 12-well plates and incubated under the same conditions.
After 7 days, the cells were treated with trypsin-EDTA and counted
under a microscope.
Animal experiments
HT1080 cells (5×106) were inoculated
subcutaneously into nude mice (8w female). The drugs were added to
the drinking water in order to achieve a daily dose similar to that
used clinically (DCA 50 mg/kg, OMP 2 mg/kg). The size of each tumor
was measured with a caliper and the tumor volume was calculated by
the multiplication of three diameters.
Results
Combined treatment of DCA and OMP
inhibited cell proliferation
HT1080 human fibrosarcoma cells were cultured with
increasing concentrations of DCA and OMP for 7 days, which resulted
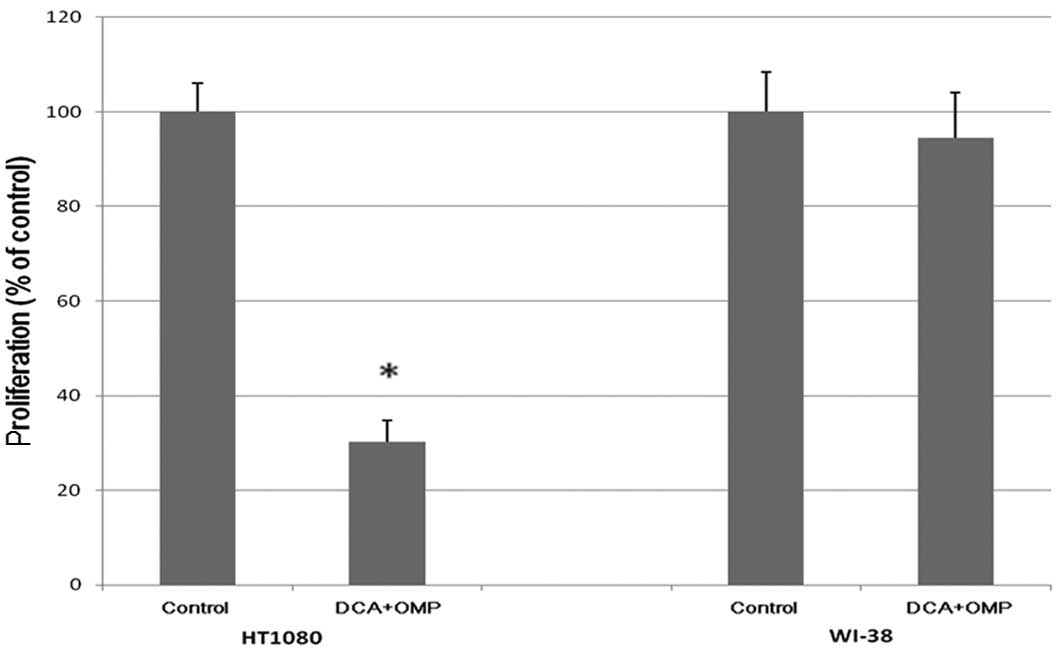
in a dose-dependent inhibition of proliferation (Fig. 1). As shown in Fig. 2A, the combination of DCA and OMP
markedly blocked the proliferation of HT1080 fibrosarcoma cells,
while the same combination did not affect the proliferation of
WI-38 normal human fibroblast cells.
Vitamin E inhibited the effects of DCA
and OMP
Although there has been controversy over the
mechanism of the caspase-dependent cell growth inhibition by OMP
(10,11), DCA and OMP have been reported to
inhibit tumor cell growth through the production of SODs (6,10). To
establish whether the same mechanism applied in the HT1080
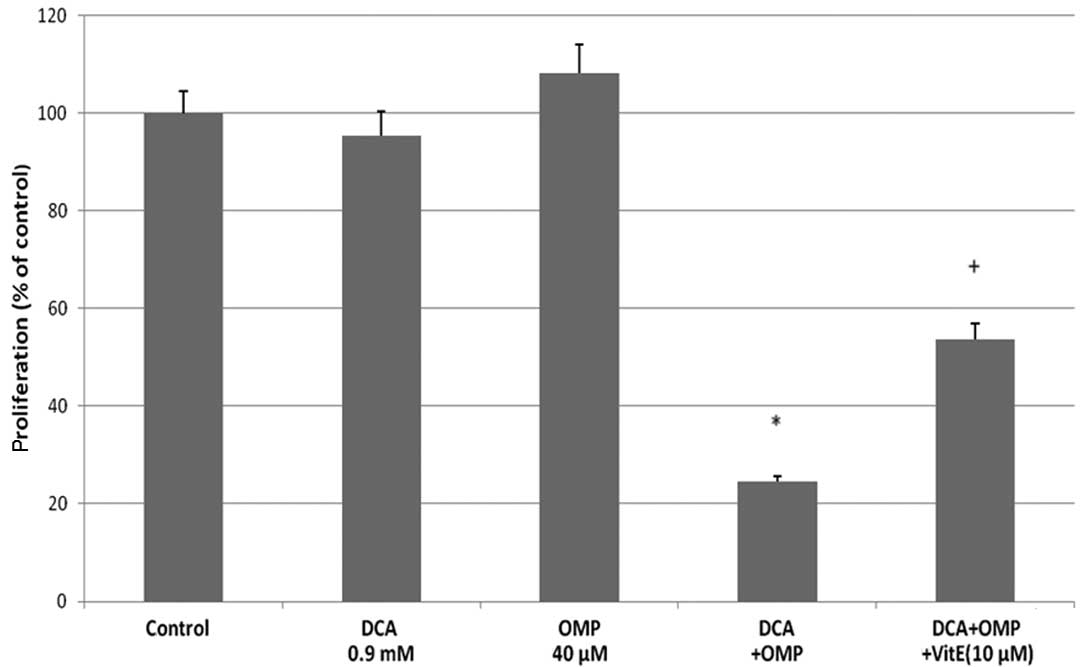
fibrosarcoma cells, we used vitamin E as an inhibitor of SOD.
Vitamin E successfully inhibited the effects of DCA and OMP, thus
SOD production was thought to be involved in the inhibition of
tumor cell growth (Fig. 2B). The
pan-caspase inhibitor Z-VAD-FMK also inhibited the effects of these
drugs; therefore a caspase-dependent antitumor mechanism was
thought to be involved, at least in part, in these processes
(Fig. 2B). Taken together, DCA and
OMP inhibit the growth of HT1080 fibrosarcoma cells, possibly
through a caspase-dependent pathway by SOD production. As a result,
the drugs may exhibit a synergistic effect when combined. This
synergistic effect and the inhibitory effect with vitamin E was
also observed in RKO colon cancer cells (Fig. 3).
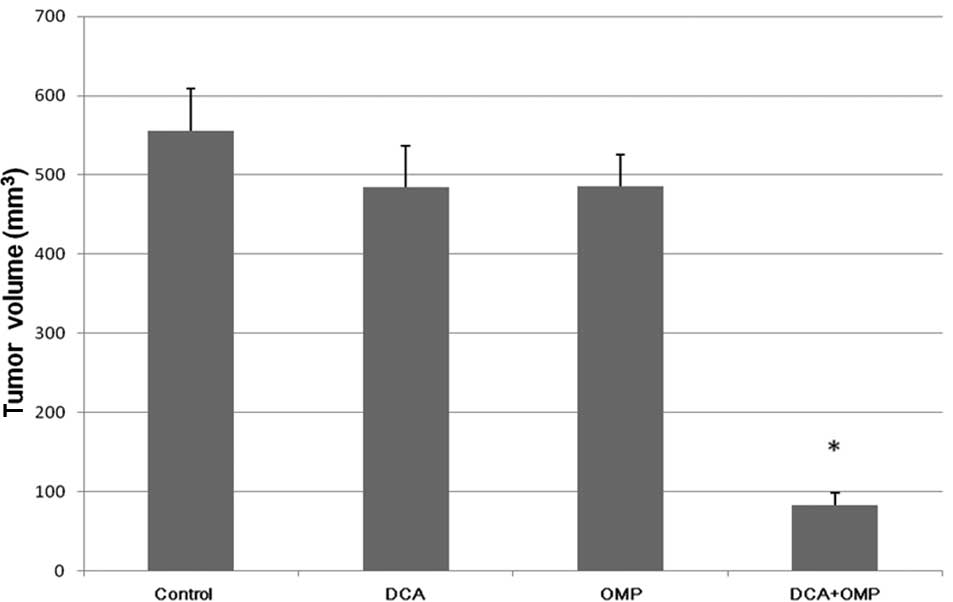
We performed animal experiments to
investigate the effects of DCA and OMP in vivo
As shown in Fig. 4,
the antitumor effect of the combination of DCA and OMP was also
greater than that of either drug alone.
Discussion
We have shown that a combined treatment of DCA and
OMP is more effective than treatment with either drug alone in
HT1080 human fibrosarcoma cells and RKO colon cancer cells. The
combined therapy was also effective in animal experiments.
DCA and OMP have been used safely for a number of
years but are not classified as anticancer drugs. DCA has been used
to treat congenital metabolic acidosis and OMP has been used to
treat acid-related diseases, both without major side effects
(7,8,12,
13). The combination of DCA and
OMP is expected to reduce the necessary dose of each drug and the
accompanying risks.
Oral doses of 20 mg OMP once daily have been
reported to lead to a maximum plasma concentration in patients of
~2.5 μg/ml (7 μM) 2 h after administering the dose (14). A dose of 120 mg OMP three times a
day has been used for the treatment of Zollinger-Ellison syndrome,
with only rare and mild side effects even following long-term use
(15,16). This dose corresponds with a
hypothetical in vivo peak concentration of ~15 μg/ml (42
μM). Overdoses of up to 2,400 mg [a hypothetical plasma peak
concentration of 300 μg/ml (840 μM)] have been tolerated with minor
side effects (17). DCA is already
established in clinical practice. The Cmax value of DCA in adults
following 6 months of continuous treatment (oral doses of 25
mg/kg/day) was 53±18 μg/ml (0.35±0.12 mM) (18). Patients were safely treated
chronically with DCA at the maximum dose of 50 mg/kg/day (19). A number of children with lactic
acidosis were administered >100 mg/kg DCA daily for a long
period (8). From these data we
consider that the necessary dose of the two drugs is tolerable.
Moreover, candidate analogs of DCA with less toxicity and better
binding affinity have been developed (20). Thus, the combination of DCA and OMP
is considered a potential therapeutic option for malignant tumors
and may lead to the development of a new therapeutic strategy.
Abbreviations:
|
DCA
|
dichloroacetate
|
|
PPI
|
proton pump inhibitor
|
|
OMP
|
omeprazole
|
|
SOD
|
superoxide
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Warburg O: Ueber den Stoffwechsel der
Tumoren. Constable; London: 1930
|
|
2
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hsu PP and Sabatini DM: Cancer cell
metabolism: Warburg and beyond. Cell. 134:703–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Michelakis ED, Webster L and Mackey LR:
Dichloroacetate (DCA) as a potential metabolic-targeting therapy
for cancer. Br J Cancer. 99:989–994. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Plas DR and Thompson CB: Cell metabolism
in the regulation of programmed cell death. Trends Endocrinol
Metab. 13:75–78. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonnet S, Archer SL, Allalunis-Turner J,
Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta
L, Bonnet S, et al: A mitochondria-K+ channel axis is suppressed in
cancer and its normalization promotes apoptosis and inhibits cancer
growth. Cancer Cell. 11:37–51. 2007.
|
|
7
|
Stacpoole PW: The pharmacology of
dichloroacetate. Metabolism. 38:1124–1144. 2006. View Article : Google Scholar
|
|
8
|
Stacpoole PW, Gilbert LR, Neiberger RE,
Carney PR, Valenstein E, Theriaque DW and Shuster JJ: Evaluation of
long-term treatment of children with congenital lactic acidosis
with dichloroacetate. Pediatrics. 121:1223–1228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luciani F, Spada M, Milito AD, Molinari A,
Rivoltini L, Montinaro A, Marra M, Lugini L, Logozzi M, Lozupone F,
et al: Effect of proton pump inhibitor pretreatment on resistance
of solid tumors to cytotoxic drugs. J Natl Cancer Inst.
96:1702–1713. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Milito AD, Iessi E, Logozzi M, Lozupone F,
Spada M, Marino ML, Federici C, Perdicchio M, Matarrese P, Lugini
L, et al: Proton pump inhibitors induce apoptosis of human B-cell
tumors through a caspase-independent mechanism involving reactive
oxygen species. Cancer Res. 67:5408–5417. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Milito A, Canese R, Marino ML, Borghi
M, Iero M, Villa A, Venturi G, Lozupone F, Iessi E, Logozzi M, et
al: pH-dependent antitumor activity of proton pump inhibitors
against human melanoma is mediated by inhibition of tumor acidity.
Int J Cancer. 27:207–219. 2010.PubMed/NCBI
|
|
12
|
Michelakis ED, Sutendra G, Dromparis P,
Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR,
Fulton D, et al: Metabolic modulation of glioblastoma with
dichloroacetate. Sci Trasl Med. 2:31–34. 2010.PubMed/NCBI
|
|
13
|
Shi S and Klotz U: Proton pump inhibitors:
an update of their clinical use and pharmacokinetics. Eur J Clin
Pharmacol. 64:935–951. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katagiri F, Inoue S, Itoh H and Takayema
M: Omeprazole raises somatostatin and motilin in human plasma. Biol
Pharm Bull. 28:370–372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Frucht H, Maton PN and Jensen RT: Use of
omeprazole in patients with Zollinger-Ellison syndrome. Dig Dis
Sci. 36:394–404. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thomson ABR, Sauve MD, Kassam N and
Kamitakahara H: Safety of the long-term use of proton pump
inhibitors. World J Gastroenterol. 16:2323–2330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Udelnow A, Kreyes A, Ellinger S,
Landfester K, Walther P, Klapperstueck T, Wohlrab J, Henne-Bruns D,
Knippschild U and Würl P: Omeprazole inhibits proliferation and
modulates autophagy in pancreatic cancer cells. PLoS One.
6:e201432011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shroads AL, Guo X, Dixit V, Liu HP, James
MO and Stacpoole PW: Age-dependent kinetics and metabolism of
dichloroacetate: possible relevance to toxicity. J Pharmacol Exp
Ther. 324:1163–1171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stacpoole PW, Henderson GN, Yan Z, Cornett
R and James MO: Pharmacokinetics, metabolism and toxicology of
dichloroacetate. Drug Metab Rev. 30:499–539. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Subramanian K and Ramaian AS: Development
of a less toxic dichloroacetate analogue by docking and descriptor
analysis. Bioinformation. 5:73–76. 2010. View Article : Google Scholar : PubMed/NCBI
|