Introduction
Cholangiocarcinoma (CCA) is a malignancy that
originates in bile duct epithelial cells. The disease may be
classified into three categories which are defined by the site of
the carcinoma: intrahepatic, perihilar and distal extrahepatic. The
location of the tumors, as well as the growth pattern of the
disease and a lack of diagnostic criteria, mean that CCA is often
diagnosed at an advanced stage. The only effective cure for CCA is
the resection of early stage tumors, following which there is a
5-year recurrence rate of 60–90%. However, due to late diagnosis
this treatment is only appropriate for <40% of patients. This,
coupled with an inadequate response to chemotherapy, results in a
poor prognosis for patients with CCA: 75% of patients with CCA
succumb to the disease within 1 year of diagnosis. There is some
difference in the prognoses of the different types of CCA, as the
median survival rates for patients with intrahepatic and those with
perihilar CCA are 18–30 and 12–24 months, respectively. With a
5-year survival rate of <5%, there is a clear need to study CCA
in order to improve the rate of early diagnosis and also to develop
new treatment options (1–9).
Similarly to most neoplasms, CCA does not result
from a single genetic change, but rather a series of mutations in
oncogenes and tumor-suppressor genes. These changes can cause
resistance to external stimuli and therefore lead to the
unregulated proliferation of cells and the transformation from
epithelium to carcinoma (10). One
family of proto-oncogenes whose dysregulation is known to be
associated with various types of cancer is the Myc family,
consisting of three main genes: C-Myc, N-Myc and L-Myc. These genes
are involved in apoptosis and the differentiation and proliferation
of cells and encode basic helix-loop-helix leucine zipper
transcription factors. However, the mechanisms by which the
transcription factors affect these phenomena and the role they play
in tumorigenesis are not fully understood and certain targets of
the Myc genes have yet to be identified (11–18).
One of the most widely studied proto-oncogenes is
C-Myc (17,18). The expression of C-Myc is correlated
with cell proliferation and is downregulated in cells which are
quiescent and fully differentiated. C-Myc has been shown to
directly affect the expression of Mina53 (Myc-induced nuclear
antigen with a molecular weight of 53 kDa) (19,20),
which is also associated with proliferation. The inhibition of
Mina53 expression results in the suppression of cell proliferation
in certain cell lines. Mina53 has also been found to have an
increased level of expression in human colon and esophageal
squamous cell carcinomas and it is therefore thought to be involved
in carcinogenesis (18–24).
Thus, we detected the levels of expression of
Mina53, p53 and Ki67 in CCA tissues and studied the correlation
between the level of expression of Mina53 and clinicopathological
characteristics, anti-oncogene expression and tumor proliferation
in CCA. We also explored the role of Mina53 in carcinogenesis and
tumor progression and its value in clinical application.
Materials and methods
Patients and tissue samples
Between 2006 and 2010, 69 CCA specimens and 21
adjacent non-cancerous tissues were obtained during routine
surgical procedures at the Jingzhou First People’s Hospital
(Jingzhou, China) and the Wuhan People’s Hospital (Wuhan, China).
Informed consent was obtained from all subjects. The
clinicopathological characteristics of the 69 CCA cases were as
follows: mean age, 59.2 years (range, 32–86); 41 males and 28
females; 20 stage I cases, 22 stage II cases, 16 stage III cases
and 11 stage IV cases according to the pathological TNM staging
criteria.
Immunohistochemistry
Immunohistochemistry was performed using the labeled
streptavidin-biotin immunoperoxidase technique to determine the
level of expression of Mina53. Sections (4 μm) of formalin-fixed
and paraffin-embedded samples were mounted on silane-coated glass
slides, deparaffinized in xylene and rehydrated through a graded
series of ethanols. The sections were microwaved in 10 mM
citrate-phosphate buffer (pH 6.0) for antigen retrieval for 15 min,
and incubated with 3% hydrogen peroxide for 10 min to block
endogenous peroxidase activity, followed by incubation with bovine
serum albumin (BSA) for 10 min to block non-specific binding. The
sections were then incubated for 2 h with mouse anti-human Mina53
monoclonal antibody (Zymed, San Francisco, CA, USA), diluted
100-fold in Tris-buffered saline (TBS, pH 7.6) with 1% BSA in a
humidified chamber at room temperature. The slides were incubated
in sequence with secondary biotinylated antibody for 10 min and
peroxidase-labeled streptavidin for 10 min using an LSAB kit
(Zhongshan Corp., Beijing, China). Finally, 3,3′-diaminobenzidine
(DAB, Zhongshan Corp.) was applied as the chromogen and the
sections were counterstained with Mayer’s hematoxylin and examined
under a light microscope. A negative control was performed with
serial sections, omitting the incubation with the primary antibody.
The proliferative activity of the cholangiocarcinoma was determined
by assessing the Ki67 labelling index (Ki67-LI). Briefly, sections
were immunostained with the anti-Ki67 monoclonal antibody
(Biolegend, San Diego, CA, USA) in the same manner as the
immunohistochemical staining of Mina53 described above. Sections
were also immunostained with the anti-p53 monoclonal antibody
(Biolegend), but stained using the DAB kit (Zhongshan Corp.).
Evaluation of tissue staining
Tissue slides were evaluated independently by two
pathologists who were blinded for the patient characteristics and
outcome. Whole-tissue slides, each comprising a representative
cross section of the tumor, were evaluated. To account for regional
differences in staining, a semi-quantitative immunoreactivity
scoring system (IRS) was applied. To obtain the IRS for each
individual case, the staining intensity (0, no staining; 1, weak
staining; 2, moderate staining; 3, strong staining) as well as the
percentage of cells stained (0, no cells; 1, <10% of cells; 2,
11–50% of cells; 3, 51–80% of cells; 4, >80% of cells) were
evaluated and the respective scores were multiplied, resulting in
an IRS range from 0 to 12. For statistical analysis, cases were
grouped as either Mina53-negative (IRS 0–6) or Mina53-positive (IRS
7–12). Cases with discordant IRS values were discussed at a
multiheaded microscope until consensus was achieved. The Ki67-LI
was defined as the percentage of stained cells in a minimum of
1,000 counted tumor cells. Five randomly selected microscopic
fields were examined at high magnification (x200) under a light
microscope for this purpose, in the same manner as for Mina53. The
expression of p53 was scored as positive when staining was visible
in >10% of the nuclei within a specimen.
Statistical analyses
The software package SPSS version 13.0 (SPSS Inc,
Chicago, IL, USA) was used for data compilation and statistical
analysis. The χ2 and Fisher’s exact probability tests
were used to examine the association between the level of Mina53
expression and various other parameters, including
clinicopathological characteristics. The Student’s t-test was
performed to examine the correlation between the Ki67-LI and the
level of protein expression. The χ2 test was used to
examine the correlation between the expression of Mina53 and p53.
P<0.05 was considered to indicate a statistically significant
result.
Results
Mina53 expression in CCA tissues and
adjacent non-cancerous tissues
Mouse anti-human Mina53 monoclonal antibody was used
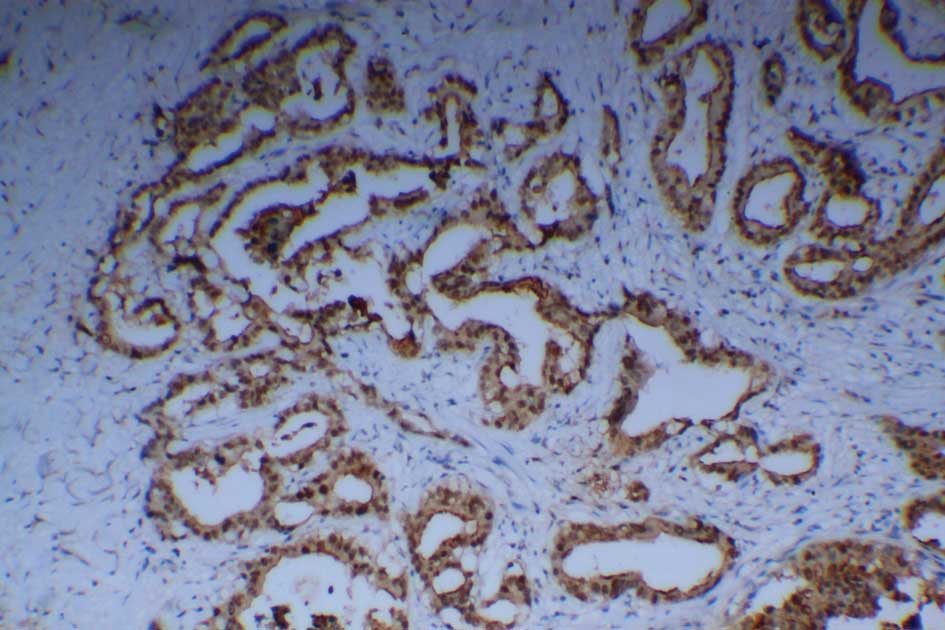
to detect the Mina53 protein immunohistochemically. Positive
staining of Mina53 (yellow or brown) was mainly located in the
nuclei but was also observed in the cytoplasm (Fig. 1). Adjacent non-cancerous tissues
showed positive staining for Mina53 in only 1 of 21 cases (4.8%).
Overexpression of the Mina53 protein was found in 61 (88.4%) of the
69 CCA tissues. The expression of Mina53 in the tumor tissues was
compared with that in adjacent non-cancerous tissues. The level of
Mina53 expression in the carcinoma tissues was significantly higher
(P<0.01) than in the adjacent non-cancerous tissues (Table I).
 | Table IMina53 expression in human
cholangiocarcinoma tissues and adjacent non-cancer tissues. |
Table I
Mina53 expression in human
cholangiocarcinoma tissues and adjacent non-cancer tissues.
| Groups | Total | Negative | Positive | Cases with positive
staining |
|---|
| Adjacent non-cancer
tissues | 21 | 20 | 1 | 1 (4.8%) |
| Carcinoma
tissues | 69 | 8 | 61 | 61 (88.4%) |
Correlation between the expression of
Mina53 and the clinicopathological characteristics of CCA
Significant associations were not found between the
increased Mina53 expression and clinicopathological characteristics
including gender and age (P>0.05). There were statistically
significant associations between the increased Mina53 expression
and histological differentiation (χ2=4.934, P<0.05),
TNM stage (χ2=4.731, P<0.05) and lymph node
metastasis (χ2=4.525, P<0.05). However, significant
associations were not found between the level of Mina53 expression
and distant metastasis (P>0.05, Table II).
 | Table IICorrelation between the expression of
Mina53 and the clinicopathological characteristics of
cholangiocarcinoma cases. |
Table II
Correlation between the expression of
Mina53 and the clinicopathological characteristics of
cholangiocarcinoma cases.
| Clinical
characteristics | Total | Mina53
expression | P-value |
|---|
|
|---|
| Negative | Positive |
|---|
| All cases | 69 | 8 | 61 | |
| Gender |
| Male | 41 | 5 | 36 | 0.850 |
| Female | 28 | 3 | 25 | |
| Age |
| ≤60 years | 39 | 4 | 35 | 0.692 |
| >60 years | 30 | 4 | 26 | |
| Histological
differentiation |
| Well | 34 | 7 | 27 | 0.026 |
| Moderate | 22 | 1 | 21 | |
| Poor | 13 | 0 | 13 | |
| TNM stage |
| I | 20 | 4 | 16 | 0.030 |
| II | 22 | 4 | 18 | |
| III | 16 | 0 | 16 | |
| IV | 11 | 0 | 11 | |
| pN (lymph node
metastasis) |
| pN0 | 46 | 8 | 38 | 0.033 |
| pN1 | 23 | 0 | 23 | |
| Distant
metastasis |
| M0 | 59 | 8 | 51 | 0.216 |
| M1 | 10 | 0 | 10 | |
| p53 expression |
| Positive | 58 | 2 | 56 | 0.000 |
| Negative | 11 | 6 | 5 | |
Correlation between the expression of
Mina53 and p53 in CCA
Positive staining of p53 was mainly localized to the
nuclei (Fig. 2A). Accumulation of
p53 was detected in 58 of the 69 CCA tissues (84.1%). The
expression of Mina53 in CCA tissues was significantly associated
with the expression of p53 (χ2=23.553, P<0.01). Of
the 69 CCA tissue samples, 56 (81.2%) had simultaneous upregulation
of Mina53 and p53.
Correlation between the expression of
Mina53 and cell proliferation
Ki67 is a widely used biomarker of cell
proliferation. Positive staining of Ki67 was mainly localized to
the nuclei (Fig. 2B). The examined
carcinoma specimens showed definite positive nuclear staining for
Ki67 and some adjacent non-cancer tissues also showed positive
staining for Ki67. The values of the Ki67-LI for the carcinoma
samples and the adjacent non-cancerous tissues were 58.84±15.72%
and 29.63±14.52%, respectively (mean ± SD). The level of Ki67
expression in the CCA tissues was higher than that in the adjacent
non-cancerous tissues (P<0.05). An increased level of expression
of Mina53 was positively associated with the Ki67 level (r=0.801,
P<0.01, as calculated by association analysis).
Discussion
Tsuneoka et al previously identified Mina53
as a Myc target gene and revealed a clear correlation between
Mina53 expression and cell proliferation (18,19).
Certain studies have reported that Mina53 is overexpressed in a
number of tumor cells and tissues, including colon carcinoma,
esophageal squamous cell carcinoma and gingival squamous cell
carcinoma (18–24), which led us to suspect that Mina53
may be involved in the abnormal cell growth observed in neoplastic
diseases, including CCA. In this study, we used mouse anti-human
Mina53 monoclonal antibody to study the level of expression of
Mina53 in CCA tissues. Our results revealed that while almost all
the CCA tissues examined exhibited elevated levels of expression of
Mina53, only one adjacent non-cancerous tissue sample showed weak
positive staining. We also observed that significant associations
were not found between the level of Mina53 expression and
clinicopathological characteristics including gender, age and
distant metastasis. Statistically significant associations were
found between increased levels of Mina53 expression and lymph node
metastasis, histological differentiation and TNM stage. Therefore,
Mina53 may play a role in biliary tract carcinogenesis and may be
used as a marker for CCA.
The p53 gene, a tumor suppressor gene or
anti-oncogene, is associated with apoptosis. Mutations in the p53
gene are the most common genetic alterations in a number of human
carcinomas. The loss of p53 accelerates tumorigenesis associated
with Myc activation by preventing apoptosis (25–30).
In our study, the accumulation of mutated p53 was significantly
associated with Mina53 expression in CCA. As Mina53 is a Myc target
gene, Mina53 may suppress the activity of p53 via the Myc pathway.
Therefore, Mina53 may contribute to biliary tract carcinogenesis by
suppressing apoptosis.
Ki67 is a widely used biomarker of cell
proliferation (31). Using
association analysis, we found that the overexpression of Mina53 in
CCA tissues was positively associated with the level of Ki67. Cell
proliferation was promoted with the increasing expression of
Mina53. Therefore, the overexpression of Mina53 may be involved in
the proliferation of CCA cells and have certain functions in
carcinogenesis.
In conclusion, Mina53 was overexpressed in CCA and
was associated with tumor proliferation and anti-oncogene
expression. Mina53 was important in the carcinogenesis and
development of CCA. We suggest that Mina53 may be used as a marker
for CCA and could be exploited as a target for the treatment of
CCA.
References
|
1
|
Skipworth JR, Olde Damink SW, Imber C, et
al: Review article: surgical, neo-adjuvant and adjuvant management
strategies in biliary tract cancer. Aliment Pharmacol Ther.
34:1063–1078. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blechacz B, Komuta M, Roskams T and Gores
GJ: Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev
Gastroenterol Hepatol. 8:512–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamamoto M and Ariizumi S: Surgical
outcomes of intrahepatic cholangiocarcinoma. Surg Today.
41:896–902. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wadsworth CA, Dixon PH, Wong JH, et al:
Genetic factors in the pathogenesis of cholangiocarcinoma. Dig Dis.
29:93–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akamatsu N, Sugawara Y and Hashimoto D:
Surgical strategy for bile duct cancer: advances and current
limitations. World J Clin Oncol. 2:94–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Friman S: Cholangiocarcinoma - current
treatment options. Scand J Surg. 100:30–34. 2011.PubMed/NCBI
|
|
7
|
Tyson GL and El-Serag HB: Risk factors for
cholangiocarcinoma. Hepatology. 54:173–184. 2011. View Article : Google Scholar
|
|
8
|
Morise Z, Sugioka A, Tokoro T, et al:
Surgery and chemotherapy for intrahepatic cholangiocarcinoma. World
J Hepatol. 2:58–64. 2010.PubMed/NCBI
|
|
9
|
Ellis MC, Cassera MA, Vetto JT, et al:
Surgical treatment of intrahepatic cholangiocarcinoma: outcomes and
predictive factors. HPB (Oxford). 13:59–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fava G: Molecular mechanisms of
cholangiocarcinoma. World J Gastrointest Pathophysiol. 1:12–22.
2010.
|
|
11
|
Lutz W, Leon J and Eilers M: Contributions
of Myc to tumorigenesis. Biochim Biophys Acta. 16:61–71. 2002.
|
|
12
|
Dang CV: c-Myc target genes involved in
cell growth, apoptosis, and metabolism. Mol Cell. 19:1–11.
1999.PubMed/NCBI
|
|
13
|
Henriksson M and Luscher B: Proteins and
the Myc network: essential regulators of cell growth and
differentiation. Adv Cancer Res. 68:109–182. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cowling VH and Cole MD: HATs off to
capping: a new mechanism for Myc. Cell Cycle. 6:307–309. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prochownik EV and Li Y: The ever expanding
role for c-Myc in promoting genomic instability. Cell Cycle.
6:1024–1029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schick B, Wemmert S, Jung V, et al:
Genetic heterogeneity of the MYC oncogene in advanced juvenile
angiofibromas. Cancer Genet Cytogenet. 164:25–31. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jain M, Arvanitis C, Chu K, et al:
Sustained loss of a neoplastic phenotype by brief inactivation of
MYC. Science. 297:102–104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsuneoka M, Koda Y, Soejima M, et al: A
novel myc target gene, mina53, that is involved in cell
proliferation. J Biol Chem. 277:35450–35459. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsuneoka M, Nishimune Y, Ohta K, et al:
Expression of Mina53, a product of a Myc target gene in mouse
testis. Int J Androl. 29:323–330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Prendergast GC: Mechanisms of apoptosis by
c-Myc. Oncogene. 18:2967–2987. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Obaya AJ, Mateyak MK and Sedivy JM:
Mysterious liaisons: the relationship between c-Myc and the cell
cycle. Oncogene. 18:2934–2941. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuratomi K, Yano H, Tsuneoka M, et al:
Immunohistochemical expression of Mina53 and Ki67 proteins in human
primary gingival squamous cell carcinoma. Kurume Med J. 53:71–78.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsuneoka M, Fujita H, Arima N, et al:
Mina53 as a potential prognostic factor for esophageal squamous
cell carcinoma. Clin Cancer Res. 10:7347–7356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Teye K, Tsuneoka M, Arima N, et al:
Increased expression of a Myc target gene Mina53 in human colon
cancer. Am J Pathol. 164:205–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vousden KH: P53: death star. Cell.
103:691–694. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Levine AJ: P53, the cellular gatekeeper
for growth and division. Cell. 88:323–331. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang XH, Chun YWB, Yuen ST, et al:
Arsenic trioxide induces apoptosis in human gastric cancer cells
through up-regulation of p53 and activation of caspase-3. Int J
Cancer. 91:173–179. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin XY, Grove L, Datta NS, et al: C-myc
overexpression and p53 loss cooperate to promote genomic
instability. Oncogene. 18:1177–1184. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hong S, Pusapati RV, Powers JT and Johnson
DG: Oncogenes and the DNA damage response: Myc and E2F1 engage the
ATM signaling pathway to activate p53 and induce apoptosis. Cell
Cycle. 5:801–803. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schlüter C, Duchrow M, Wohlenberg C, et
al: The cell proliferation-associated antigen of antibody Ki67: a
very large, ubiquitous nuclear protein with numerous repeated
elements, representing a new kind of cell-cycle-maintaining
proteins. J Cell Biol. 123:513–522. 1993.
|