Introduction
Renal cell cancer (RCC) is a cause of mortality
worldwide and its incidence rate continues to rise. Although
several therapeutic approaches have been applied to the treatment
of RCC, including surgery, immunological therapies and vaccine
treatment (1), further improvements
are needed. Notably, the modulation of the apoptotic response
provides new prospects for therapeutic strategies in cancer
(2,3). Thus, novel and promising anticancer
drugs may be identified by further investigations into the
induction of apoptosis.
Tanshinone IIA (Tan IIA,
14,16-epoxy-20-nor-5(10),6,8,13,15-abietapentaene-11,12-dione),
one of the phytochemical compounds isolated from the Chinese
medicinal herb Danshen (root of Salvia miltiorrhiza Bunge),
possesses anti-inflammatory (4,5) and
antioxidant properties (6,7). Tan IIA has also been shown to have
anticancer activity through the induction of apoptosis in a variety
of cancers (8–10). However, it remains unclear whether
Tan IIA is capable of inhibiting cell growth and inducing apoptosis
in human RCC cells. In the present study, we investigated the
effect of Tan IIA on human RCC 786-O cells in vitro and the
mechanisms by which it functions.
Materials and methods
Reagents
Tan IIA (>99% pure) and MTT were purchased from
Sigma Chemical Co. (St. Louis, MO, USA). The annexin V-FITC
apoptosis detection kit was purchased from BD Biosciences (San
Jose, CA, USA). The antibodies used in this study were: monoclonal
anti-p53 (sc-126, Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), polyclonal anti-p21 (71–1000, Zymed Laboratories, San
Francisco, CA, USA), polyclonal anti-bax (2772, Cell Signaling
Technology, Inc., MA, USA), polyclonal anti-caspase-3 (Upstate
Biotechnology, Lake Placid, NY, USA) and monoclonal anti-actin
(ms-1295-po, NeoMarkers, Fremont, CA, USA).
Cell culture
The human RCC cell line 786-O was provided by Dr Jun
Hu (Sun Yat-sen University, Guangzhou, China) and cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) and 100 U/ml penicillin and
streptomycin.
MTT assay
786-O cells were seeded in 96-well microtitre plates
with 1×103 cells/well and incubated for 24 h in 100 μl
culture medium. The cells in the experimental group were then
treated with 1, 2, 4 or 8 μg/ml of Tan II-A for 24 h. MTT [100 μl
(5 g/l)] was added to the cells which were then cultivated for a
further 4 h. Following the removal of the supernatant fluid, 100
μl/well DMSO was added to the cells which were agitated for 15 min.
The absorbance was measured at 570 nm by an ELISA reader. The
untreated 786-O cells served as controls. Each assay was repeated
three times.
Immunoblotting (IB)
The cell lysates were boiled with 3X SDS loading
buffer and then fractionated by SDS-PAGE. The proteins were
transferred to a PVDF membrane, which was then incubated with a
primary specific antibody in 5% milk, followed by horse radish
peroxidase (HRP)-conjugated anti-mouse or anti-rabbit antibodies
and ECL detection reagent (Amersham Life Science, Piscataway, NJ,
USA).
Cell cycle distribution analysis
Cells were plated in 6-well culture dishes at
concentrations determined to yield 60–70% confluence within 24 h.
The cells were then treated with Tan IIA (0, 2, 4 or 8 μg/ml).
After 24 h, the cells were washed twice with PBS and then
centrifuged. The pellet was fixed with 70% ethanol at 4°C. The
ethanol was washed away and the cells were treated with 40 mg/ml
propidium iodide (PI) and 0.1 mg/ml RNase (Boehringer, Germany) for
30 min at 37°C. The cells (2×104) were analyzed and the
DNA content was measured using a FACStar cytofluorometer (BD
Biosciences) equipped with an argon-ion laser at 488 nm.
Apoptosis assessed by flow cytometry
The extent of apoptosis was evaluated by annexin
V-FITC and flow cytometry. The cells were grown at a density of
1×106 cells in 6-well culture dishes and were treated
with Tan IIA (0, 2, 4 or 8 μg/ml) for 24 h. Following treatment,
the cells were harvested, washed twice with pre-chilled PBS and
resuspended in 1X binding buffer at a concentration of
1×106 cells/ml. This solution (100 μl) was mixed with 5
μl annexin V-FITC and 5 μl PI for 15 min, then 400 μl 1X binding
buffer was added. The analysis was carried out using a FACStar
cytofluorometer with CXP software.
Statistical analysis
Values are presented as the mean ± SD of the
control. The Student's t-test was used to analyze the statistical
significance between the Tan IIA-treated and control groups.
P<0.05 was considered to indicate a statistically significant
result.
Results
The effects of Tan IIA on the viability
of 786-O cells
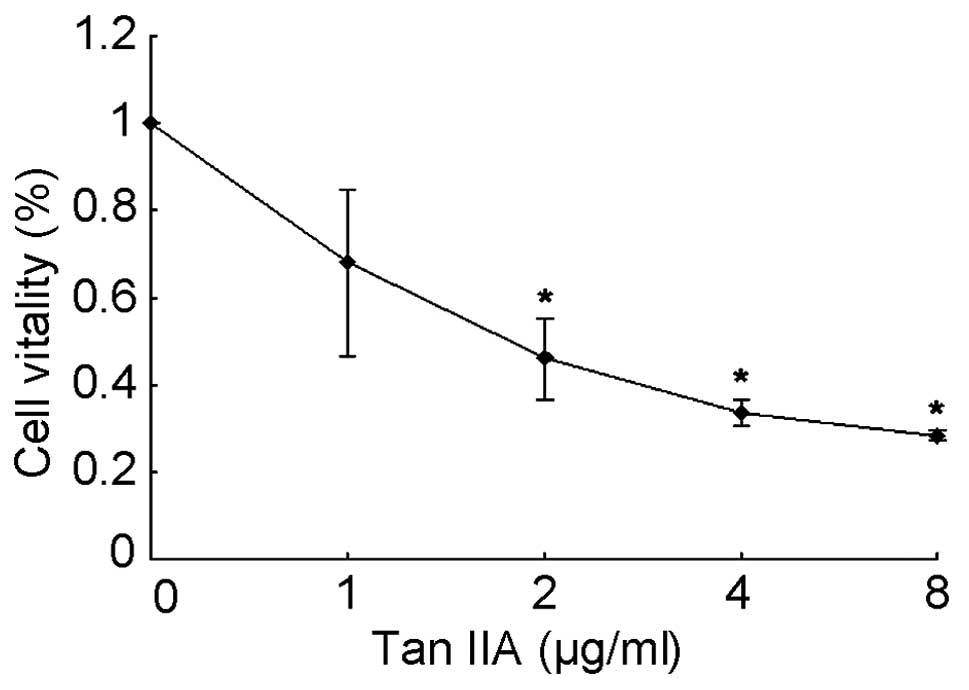
To examine the cytotoxicity of Tan IIA on the human
RCC cell line 786-O, 786-O cells were cultured and treated with Tan
IIA. The cytotoxicity of Tan IIA in 786-O cells was detected using
the MTT assay. The percentages of viable cells relative to the
control (DMSO)-treated cells were 68.2, 46.4, 33.4 and 28.3% when
cultured with Tan IIA (1, 2, 4 and 8 μg/ml, respectively) for 24 h.
The half maximal inhibitory concentration (IC50) value
for Tan IIA treatment for 24 h was estimated to be 2 μg/ml. The
results show that Tan IIA treatment induced a marked dose-dependent
inhibition of the growth of 786-O cells (Fig. 1).
Tan IIA induces 786-O cell cycle arrest
in the S phase
To determine the effect of Tan IIA on the growth of
786-O cells, the cell cycle distribution was analyzed by flow
cytometry. When 786-O cells were treated with Tan IIA (0, 2, 4 and
8 μg/ml) for 24 h, the percentage of cells in the S phase was 10.0,
11.5, 20.4 and 23.3%, respectively (Fig. 2). The results indicate the
inhibition of the growth of 786-O cells following treatment with
Tan IIA.
Tan IIA-induced apoptosis of 786-O
cells
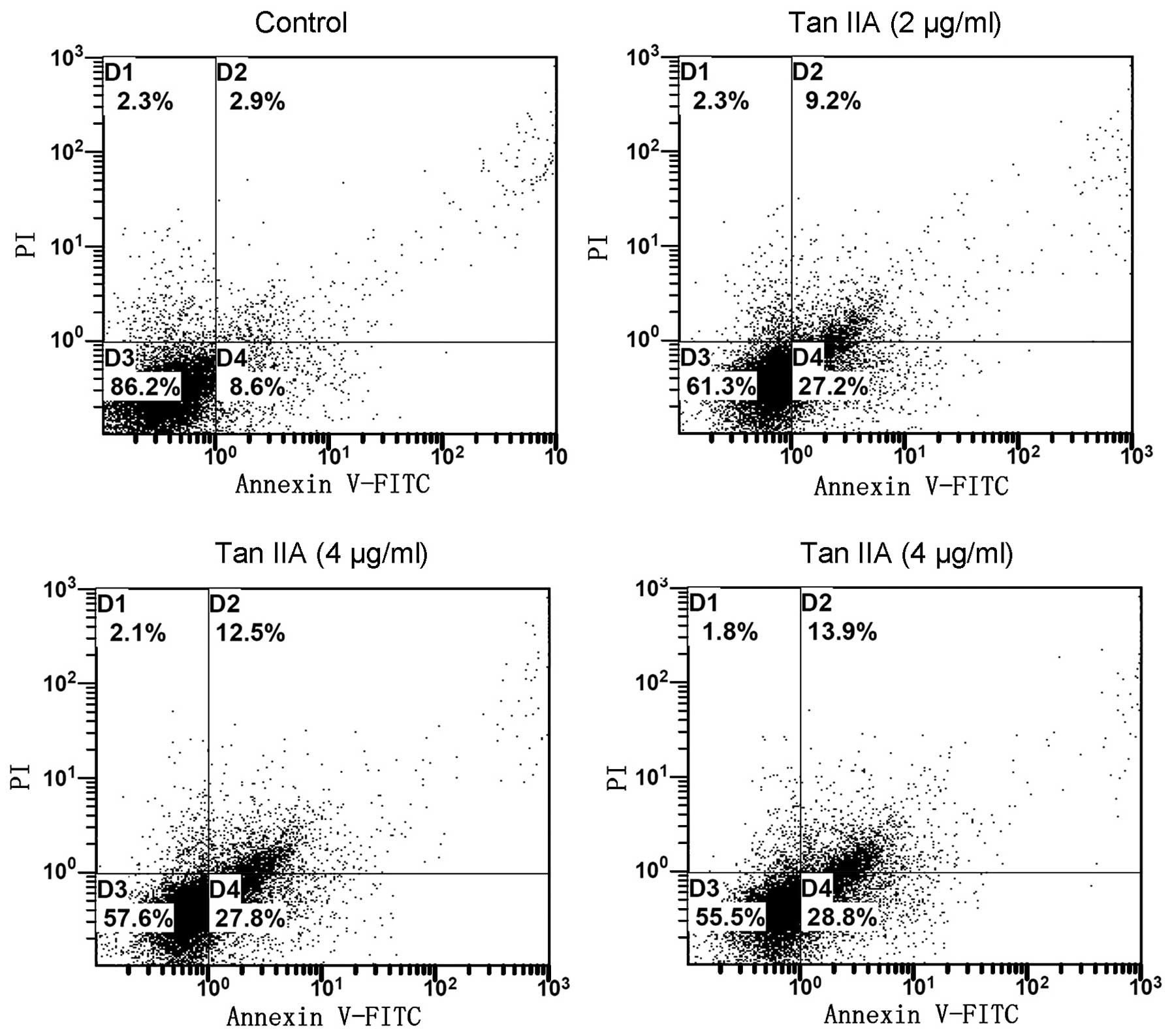
Since cell cycle perturbations may mediate the
induction of apoptosis, we examined the effect of Tan IIA on the
apoptosis of 786-O cells using the annexin V/PI approach. It was
observed that, following the treatment of 786-O cells for 24 h with
Tan IIA (0, 2, 4 and 8 μg/ml), the number of early apoptotic cells
increased to 8.6, 27.2, 27.8 and 28.8%, respectively. The number of
late apoptotic cells increased to 2.9, 9.2, 12.5 and 13.9%,
respectively. The total percentage of apoptotic cells was directly
related to the Tan IIA concentration, increasing from 11.5%
(control) to 36.4, 40.3 and 42.7% (2, 4 and 8 μg/ml Tan IIA,
respectively;Fig. 3), consistent
with the results of the cytotoxicity assay. The results revealed
that Tan IIA induced cell apoptosis of 786-O cells in a
concentration-dependent manner.
Gene expression profile correlated with
growth inhibition and apoptosis following Tan IIA treatment
Since Tan IIA treatment caused S phase cell cycle
arrest, we examined the effect of Tan IIA on cell cycle regulatory
molecules which are active in the S phase. IB indicated that Tan
IIA treatment induced the upregulation of the p21 protein, a key
regulator of S phase cell cycle transition (11), in a dose-dependent manner. In order
to establish the involvement of a mitochondrial apoptotic event,
the level of the bax and caspase-3 proteins was measured. As shown
in Fig. 4, Tan IIA treatment
resulted in a significant dose-dependent increase in the level of
bax and caspase-3.
p53, an upstream regulator of p21 and bax (12), is involved in cell cycle arrest and
apoptotic induction in response to cell stress. In the present
study, we also assessed the level of p53 expression by IB following
Tan IIA treatment. The data indicate that the level of the p53
protein increased markedly following treatment with Tan IIA.
Discussion
Tan IIA, traditionally administered to treat
cardiovascular disease (13), has
gained much attention for its anticancer properties in cell culture
and animal carcinogenesis models (8–10).
Although previous studies have shown the chemochemical/therapeutic
potential of Tan IIA against several cancer types, information
regarding the apoptotic effects of Tan IIA in human RCC cells is
unavailable. The present study provides evidence for the first time
that Tan IIA has an anticancer effect on human RCC.
A number of studies have demonstrated that Tan IIA
is able to reduce cell vitality due to its cytotoxic properties
(14). The results of the MTT assay
in our study also showed that treatment with Tan IIA resulted in a
marked and dose-dependent inhibition of the growth of 786-O cells.
We observed a slight enhancement of vitality of 786-O cells
following trace Tan IIA (<0.05 μg/ml) treatment compared with
untreated cells. Tan IIA is a well-established antioxidant present
in the Chinese medicinal herb Danshen (root of Salvia
miltiorrhiza Bunge) (15).
Therefore, trace amounts of Tan IIA may promote cell growth. By
contrast, treatment with a high dose of Tan IIA generally exerts
cytotoxicity, resulting in a reduction of cell vitality.
Furthermore, the analysis of the cell cycle distribution revealed
that Tan IIA treatment induced S phase arrest in 786-O cells. As
p21, a cyclin-dependent kinase (CDK) inhibitor, is crucial in
retarding S phase (11), we
analysed its expression and found that it was dose-dependently
upregulated following Tan IIA treatment. Moreover, Tan IIA
treatment led to a concentration-dependent increase in the levels
of p53, paralleling the accumulation of p21. These results suggest
that the observed p21 accumulation may be due to p53 activation,
since p53 (wild-type) upregulates p21 expression (16). It should be noted that it is
possible that the level of p21 was altered via a p53-independent
pathway.
In addition to promoting cell cycle arrest, p53
targets genes with apoptotic activity, among them the bax gene
(12). The expression level of bax
was assessed using IB. The results revealed that bax was
upregulated in parallel with p53 activation in Tan IIA-induced
apoptosis in RCC cells, suggesting that the mitochondrial apoptotic
pathway is involved in Tan IIA-induced cell death. The
mitochondrial apoptotic process eventually leads to the activation
of the caspases, which are both initiators and effectors of the
apoptotic programmed cell death. Similar to the observations of
bax, treatment with Tan IIA significantly increased the expression
level of caspase-3 in 786-O cells (Fig.
4). Notably, p53 targets the Fas gene, which is involved in the
mitochondria-independent pathway of apoptosis (17). In our study, we did not detect any
change in the level of expression of Fas, indicating that the
specific cell death signals used by Tan IIA appear to be dependent
on the cell type.
In conclusion, we report that human RCC 786-O cells
in culture respond to Tan IIA treatment by showing a reduction in
growth and an increase in apoptotic cell death. Furthermore, IB
results revealed that Tan IIA activates p53 expression and
subsequently induces the upregulation of p21 and bax. These
findings suggest that Tan IIA induced apoptosis in 786-O cells,
possibly through both the p21-mediated cell cycle perturbation and
the mitochondrial-mediated intrinsic cell-death pathways (Fig. 5). These findings suggest the
efficacy of Tan IIA as a chemopreventive candidate to exert
antigrowth and proapoptotic actions on human RCC.
Acknowledgements
We thank Dr Jun Hu (Sun Yat-sen University,
Guangzhou, China) for providing the human RCC cell line 786-O
cells. This study was supported by the National Natural Science
Foundation of China (no. 30772492).
References
|
1
|
Bird J and Hayter M: A review of the
literature on the impact of renal cancer therapy on quality of
life. J Clin Nurs. 18:2783–2800. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kyprianou N, Martikainen P, Davis L,
English HF and Isaacs JT: Programmed cell death as a new target for
prostatic cancer therapy. Cancer Surv. 11:265–277. 1991.PubMed/NCBI
|
|
3
|
Kyprianou N, Bains AK and Jacobs SC:
Induction of apoptosis in androgen-independent human prostate
cancer cells undergoing thymineless death. Prostate. 25:66–75.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jang SI, Kim HJ, Kim YJ, Jeong SI and You
YO: Tanshinone IIA inhibits LPS-induced NF-kappaB activation in RAW
264.7 cells: possible involvement of the NIK-IKK, ERK1/2, p38 and
JNK pathways. Eur J Pharmacol. 542:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li W, Li J, Ashok M, et al: A
cardiovascular drug rescues mice from lethal sepsis by selectively
attenuating a late-acting proinflammatory mediator, high mobility
group box 1. J Immunol. 178:3856–3864. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin R, Wang WR, Liu JT, Yang GD and Han
CJ: Protective effect of tanshinone IIA on human umbilical vein
endothelial cell injured by hydrogen peroxide and its mechanism. J
Ethnopharmacol. 108:217–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang AM, Sha SH, Lesniak W and Schacht J:
Tanshinone (Salviae miltiorrhizae extract) preparations
attenuate aminoglycoside-induced free radical formation in vitro
and ototoxicity in vivo. Antimicrob Agents Chemother. 47:1836–1841.
2003.
|
|
8
|
Wang X, Wei Y, Yuan S, et al: Potential
anticancer activity of tanshinone IIA against human breast cancer.
Int J Cancer. 116:799–807. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Wang X, Jiang S, et al: Growth
inhibition and induction of apoptosis and differentiation of
tanshinone IIA in human glioma cells. J Neurooncol. 82:11–21. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chiu TL and Su CC: Tanshinone IIA induces
apoptosis in human lung cancer A549 cells through the induction of
reactive oxygen species and decreasing the mitochondrial membrane
potential. Int J Mol Med. 25:231–236. 2010.PubMed/NCBI
|
|
11
|
Ogryzko VV, Wong P and Howard BH: WAF1
retards S-phase progression primarily by inhibition of
cyclin-dependent kinases. Mol Cell Biol. 17:4877–4882.
1997.PubMed/NCBI
|
|
12
|
el-Deiry WS: Regulation of p53 downstream
genes. Semin Cancer Biol. 8:345–357. 1998. View Article : Google Scholar
|
|
13
|
Sun J, Tan BK, Huang SH, Whiteman M and
Zhu YZ: Effects of natural products on ischemic heart diseases and
cardiovascular system. Acta Pharmacol Sin. 23:1142–1151.
2002.PubMed/NCBI
|
|
14
|
Lu Q, Zhang P, Zhang X and Chen J:
Experimental study of the anti-cancer mechanism of tanshinone IIA
against human breast cancer. Int J Mol Med. 24:773–780.
2009.PubMed/NCBI
|
|
15
|
Cao EH, Liu XQ, Wang JJ and Xu NF: Effect
of natural antioxidant tanshinone II-A on DNA damage by lipid
peroxidation in liver cells. Free Radic Biol Med. 20:801–806. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu C, Friday BB, Lai JP, et al: Cytotoxic
synergy between the multikinase inhibitor sorafenib and the
proteasome inhibitor bortezomib in vitro: induction of apoptosis
through Akt and c-Jun NH2-terminal kinase pathways. Mol Cancer
Ther. 5:2378–2387. 2006. View Article : Google Scholar
|
|
17
|
Müller M, Wilder S, Bannasch D, et al: p53
activates the CD95 (APO-1/Fas) gene in response to DNA damage by
anticancer drugs. J Exp Med. 188:2033–2045. 1998.PubMed/NCBI
|