Introduction
Prostate cancer is the most common type of cancer in
men in Western countries, accounting for one-third of all male
cancer diagnoses and 11% of all mortalities due to cancer (1). Epidemiological and screening studies
performed over the past several decades have raised important
questions regarding the pathogenesis of prostate cancer, but a
definitive cause for this disease has not been established.
Although family history and ethnicity are critical risk factors,
the diagnosis of prostate cancer is primarily associated with
increasing age (2,3). Several epidemiological studies support
the association between inflammation and prostate cancer risk
(4,5).
Previous studies have suggested that inflammation
may be important in the pathogenesis of prostate cancer by altering
the tumor environment (6,7). The production of cyclooxygenase (COX)
enzymes due to inflammation caused by precancerous tissues provides
significant evidence of the role played by inflammation in the
development of cancer (8). The
inflammatory process is driven by the interactions between various
types of cells, proteins and cytokines within the tumor environment
(4,7). Various inflammatory cytokines are
important mediators of inflammation and are produced by activated
macrophages and other immune cells. One of these cytokines,
macrophage inhibitory cytokine-1 (MIC-1) was first isolated based
on an increased mRNA expression in activated macrophages (9).
MIC-1, also known as growth differentiation
factor-15 (GDF-15) or prostate-derived factor (PDF), is a member of
the TGF-β superfamily and its increased expression has been
associated with a variety of cells including breast, gastric and
colorectal cancer cells (10,11).
However, MIC-1 is significant due to its increased association with
high-grade prostate tumors. Protein profiling on micro-dissected
samples of matched normal prostate tissue, high-grade prostatic
intraepithelial neoplasia (hPIN) and prostate cancer revealed MIC-1
expression in hPIN and cancer cells but not in normal cells,
suggesting a potential role of MIC-1 in the pathogenesis of
prostate cancer (12,13). Elevated serum MIC-1 levels are
associated with a number of disease conditions including chronic
inflammatory pathways, and as a predictor of miscarriage in
pregnant women (14–16). Specifically in the prostate,
increasing serum MIC-1 levels are associated with the progression
of metastatic prostate cancer (13,17–19).
Despite its association with multiple disease conditions, the basic
concept of MIC-1 gene regulation in prostate cancer
development and progression remains largely unknown. To understand
the functional regulation of MIC-1, we previously proposed that the
MIC-1 gene provides a potential link between inflammation
and prostate cancer (20). In this
study, using prostate cancer as a model, we studied the impact of
inflammation-associated cytokines on MIC-1 expression.
Materials and methods
Mouse prostate tissue collection and
tumor cell lines
Prostate tissue samples from different age groups of
male prostate- specific antigen transgenic (PSA-Tg) mice were
harvested. These tissue samples were fixed in formalin and/or
RNAlater (Ambion, Austin, TX, USA), and stored at −80°C. The
PSA-Tg mice were a gift from Dr David Lubaroff at the University of
Iowa, USA. Prior to prostate tissue harvesting, mice were
sacrificed in accordance with guidelines and regulations approved
by the Institutional Animal Care and Use Committee. A human
prostate cancer cell line (LNCaP cells) was purchased from ATCC
(Maryland, MD, USA). The LNCaP cell line was maintained in regular
culture medium RPMI-1640 supplemented with 10% fetal bovine serum
(FBS), 1% glutamine, 1% sodium pyruvate (Invitrogen, Carlsbad, CA,
USA) and 0.05 mg/ml of gentamicin (Mediatech, Manassas, VA,
USA).
Histopathology
Tissue blocks containing the most representative and
well-preserved tissue areas were selected for histopathology.
Sections (5 μm) were cut and stained for hematoxylin and eosin
(H&E) by the core facility at the University of Kansas Medical
Center. The H&E-stained slides from at least three mice per age
group were analyzed by the pathologist. Histopathological
evaluation included the examination of glandular architecture,
nuclear and cellular degenerative changes and the intensity of
inflammation along with the presence or absence of premalignant and
malignant changes.
RNA isolation and reverse transcription
PCR (RT-PCR)
Total RNA was extracted from the prostate tissue
samples recovered from the RNAlater as well as LNCaP cells
using a Qiagen kit as per the manufacturer's instructions.
Quantization of RNA was analyzed using a spectrophotometer. Total
RNA (~2 μg) from the tissue samples and the LNCaP cells was
reverse-transcribed (RT) using Superscript II (Invitrogen). RT
samples were subjected to PCR amplification in a total volume of 50
μl containing a mixture of PCR buffer, MgCl2, dNTPs,
each set of primers, and Taq DNA polymerase as per standard
protocol (Invitrogen). Oligonucleotide primers for the MIC-1
gene were synthesized from the previously published sequences
(Accession number AF019770 for the human sequence and NM_011819 for
the mouse sequence). GAPDH primers were designed to amplify the
product with both mouse and human compatibility. The reaction
mixture was denatured at 94°C for 2 min followed by 30 cycles at
94°C for 30 sec, 57°C for 45 sec, and 72°C for 1 min with a final
elongation at 72°C for 10 min using an MJ Mini Thermal Cycler
(Bio-Rad, Hercules, CA, USA). The amplified PCR products were
resolved electrophoretically on an agarose gel stained with
ethidium bromide to verify the size of the amplified product.
Western blot for MIC-1 protein
analysis
LNCaP cells were plated in a six-well plate at a
density of 2×105 cells per well under normal culture
conditions. Two days later, the medium was replaced with fresh
medium, and the cells were cultured for an additional day.
Subsequently, the cells were washed with RPMI-1640 phenol red-free
medium (without FBS) and starved for 4 h. The cells were then
incubated overnight (~16 h) with RPMI-1640 phenol red-free medium
(2 ml per well) containing various cytokines at a concentration of
100 ng/ml (PeproTech, Rocky Hill, NJ, USA). Following incubation,
the culture supernatant was collected for quantification of MIC-1
secretion and cells were lysed using RIPA buffer from Sigma (St.
Louis, MO, USA).
Approximately 20 μl of culture supernatant
containing MIC-1 protein was resolved on a ReadyGel and was
transferred onto a PVDF membrane (Bio-Rad). Following blocking with
3% non-fat dry milk in phosphate-buffered saline containing 0.1%
Tween-20 (PBST) for 2 h at room temperature, the blots were
incubated with anti-human MIC-1 primary antibody (1:500 dilution in
PBST) overnight at 4°C. Following repeated washing with PBST, the
blots were incubated with corresponding secondary antibody rabbit
polyclonal IgG (Abcam, Cambridge, MA, USA) in PBST with 3% milk for
2 h at room temperature. Following repeated washing with PBST, the
blots were developed using western blotting luminol reagent (Santa
Cruz Biotechnology Inc., Santa Cruz, CA, USA), and were exposed to
BioMax Film (Kodak), which was developed to analyze the specific
band size.
Results and Discussion
Prostate cancer is the second most common cause of
mortality from cancer for men of all ages. However, it is rare in
men aged younger than 40 years. Its incidence is known to increase
with age with higher percentages of men developing prostate cancer
as they become older. Studies (4,5) have
suggested that prostate cancer associated with increasing age is
also associated with increased susceptibility of the prostate
tissue to injury as well as increased predisposition to infections
and other insults leading to an increase in inflammatory response.
Therefore, to examine the role of MIC-1 in association with
inflammation, we analyzed the expression of MIC-1 gene in
prostate tissue collected from mice of different age groups. For
this purpose, we selected the PSA-Tg mouse based on the following
criteria: i) that PSA production is confined to the prostate
through the use of the probasin promoter, thus simulating the same
conditions described in studies on the human prostate; and ii) to
investigate the biological process of MIC-1 gene regulation
in the presence of self-antigen.
MIC-1 association with increasing age in
the mouse prostate
Different prostate lobes (ventral, dorsolateral and
anterior prostate lobes) from the naïve PSA-Tg mice of different
age groups were harvested to prepare total RNA, and were subjected
to RT-PCR using primer sets for MIC-1 gene expression. The
anticipated PCR product of 238 bp was resolved by agarose gel
electrophoresis (Fig. 1). In
agreement with published human studies (13,26,27),
MIC-1 gene expression was extremely low to non-detectable in
the normal (healthy) prostate lobes of young (4 weeks) and
middle-aged (13 months) mice (samples 3–8). However, higher MIC-1
levels were detectable in the prostate tissues of adult mice aged
15 months as well as those of 24-month-old elderly mice (samples
9–14).
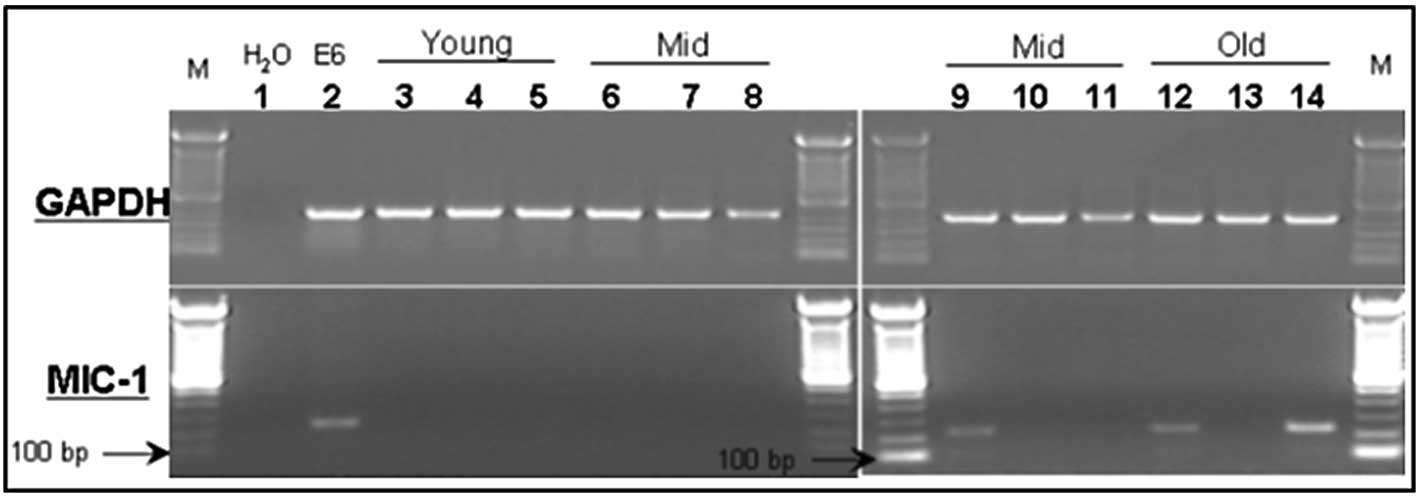 | Figure 1Expression of MIC-1 (lower panel) and
GAPDH (upper panel) mRNA in different prostate lobes from
prostate-specific antigen transgenic mice of different age groups.
Lanes 3–5, young age; lanes 6–11, middle age; lanes 12–14, elderly.
Lanes 3, 6, 9 and 12, dorsolateral prostate; lanes 4, 7, 10 and 13,
ventral prostate; and lanes 5, 8, 11 and 14, anterior prostate.
Lanes 1 and 2 are H2O and positive (RM11 mouse prostate
tumor cells) controls, respectively. |
Degree of inflammation (infiltrating
cells) in the prostates of PSA-Tg mice
Prostate tissue samples from different age groups of
PSA-Tg mice were further analyzed for the intensity of inflammation
(analyzed by quantifying infiltrating cells in the tissue).
Analysis of H&E-stained slides showed that there was no
infiltration of inflammatory cells in the prostate tissue samples
harvested from the youngest mice (Fig.
2A). However, we found an increase in the intensity of
inflammation in the samples harvested from 15-month-old mice
(Fig. 2B). Furthermore, we noted a
significant difference in the glandular architecture between
samples from the different age groups. The prostates of the younger
animals were healthy with normal-appearing glands that were
organized in an orderly manner with no evidence of inflammation
(Fig. 2A). By contrast, progressive
degenerative glandular changes as well as an increase in
inflammation were observed in the older mice, with the worst
changes being detected in the oldest mice. Degeneration of
epithelial lining and intraluminal sloughing of epithelial cells
was extremely high along with a significant depletion of the
prostatic secretion in the prostates of elderly PSA-Tg mice
(Fig. 2C). Overall, the prostate
tissue from 24-month-old elderly mice was severely damaged,
possibly due to chronic inflammation; however, the examined slides
did not reveal any sign of neoplasm. Thus, the presence of
inflammation corresponds with the mRNA expression of the
MIC-1 gene in the prostate tissues harvested from the PSA-Tg
mice, confirming the hypothesis that an increase in the expression
level of MIC-1 with increasing age may be due to inflammatory
response. It has been reported that inflammation and aging
influence the expression level of MIC-1 in correlation with
infiltration of macrophages in the rat prostate (21).
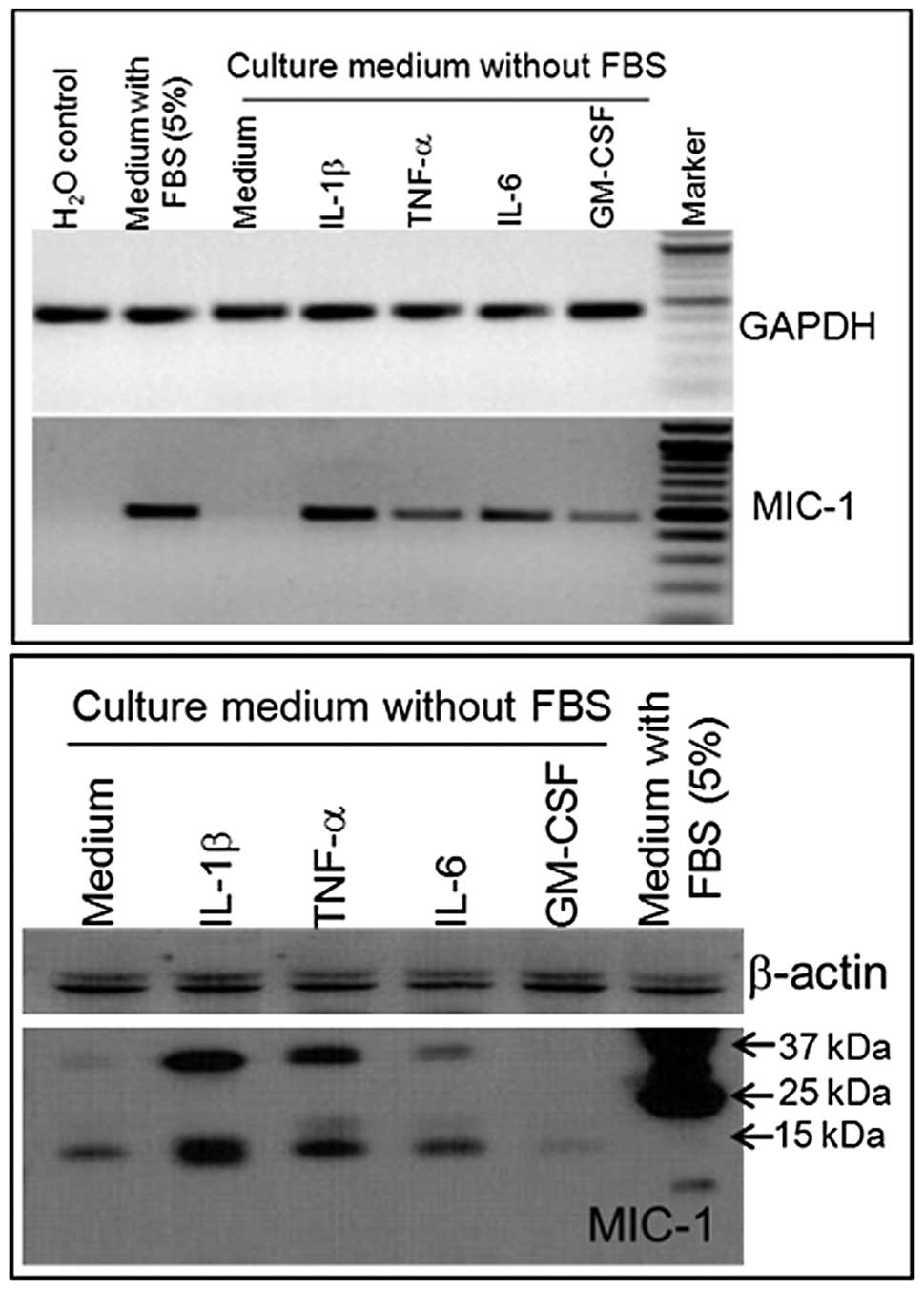
Modulation of MIC-1 expression in a human
prostate cancer cell line
To determine the possibility that MIC-1 gene
expression may be regulated in response to inflammation at the
early stage of cancer development, an androgen-responsive human
prostate cancer cell line (LNCaP) was treated with various
cytokines. Cytokine interleukin (IL)-1β, IL-6, tumor necrosis
factor-α (TNF-α), and granulocyte macrophage colony-stimulating
factor (GM-CSF) differentially regulate the expression of MIC-1 in
LNCaP cells at the mRNA (Fig. 3A)
and protein (Fig. 3B) levels. The
selection of these cytokines was based on their association with
inflammation. RT-PCR and western blot analysis showed consistency
in the MIC-1 expression level following cytokine treatment. Western
blot analysis revealed that two bands of approximately 40 and 15
kDa correspond to pro-MIC-1 and mature MIC-1 protein (Fig. 3B). These predicted sizes of
pro-MIC-1 and MIC-1 are consistent with previous studies (9,22–24).
Notably, our studies revealed that IL-1β induction increased the
expression of MIC-1 to the maximum level while the effect of GM-CSF
was minimal in the LNCaP cell line. These results are supported by
the observations that IL-1β is primarily secreted by macrophages
which are crucial in regulating the inflammatory network in the
host tissue. In macrophages, cytokines such as IL-1β and TNF-α
induce the expression of MIC-1 (9).
Such inflammation-induced MIC-1 production may function as an
autocrine-paracrine regulator of MIC-1 in the host tissue,
facilitating the process of enhanced cell proliferation (9,25).
Most studies report on an increased expression of
the MIC-1 gene in cancer and non-detectable levels in normal
tissue when comparing matched prostate cancer and normal prostate
specimens. Gene expression analysis between the normal peripheral
zone (PZ) and transition zone (TZ) of the specimens obtained from
prostate cancer patients revealed preferential expression of MIC-1
in the PZ (predominant site of tumor occurrence) compared to the TZ
(site of benign prostatic hyperplasia) (26). The expression of MIC-1 is also
reported in adult human prostate tissues with the existence of
infiltrating lymphocytes in normal-appearing prostate tissues
(27,28). An induction in MIC-1 gene
expression in kidney, lung and liver tissue due to injury following
surgical, chemical or heat shock methods has also been observed
(29,30).
In conclusion, this is the first study to show that
the MIC-1 gene may be directly regulated by
inflammation-associated cytokines in prostate cancer cells. Thus,
activation of the MIC-1 gene may be an early response due to
inflammation, infection or injury in the prostate for cell growth
advantage leading to an environment favoring prostate cancer
development. Since MIC-1 and macrophages are associated with
inflammation, further studies are under way to investigate the
mechanistic regulation of inflammation-induced MIC-1 gene in
prostate cancer.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Karan D, Thrasher JB and Lubaroff D:
Prostate cancer: genes, environment, immunity and the use of
immunotherapy. Prostate Cancer Prostatic Dis. 11:230–236. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pienta KJ and Esper PS: Risk factors for
prostate cancer. Ann Intern Med. 118:793–803. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Marzo AM, Platz EA, Sutcliffe S, et al:
Inflammation in prostate carcinogenesis. Nat Rev Cancer. 7:256–269.
2007.
|
|
5
|
Platz EA and De Marzo AM: Epidemiology of
inflammation and prostate cancer. J Urol. 171:S36–S40. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar
|
|
7
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang D and Dubois RN: Prostaglandins and
cancer. Gut. 55:115–122. 2006. View Article : Google Scholar
|
|
9
|
Bootcov MR, Bauskin AR, Valenzuela SM, et
al: MIC-1, a novel macrophage inhibitory cytokine, is a divergent
member of the TGF-beta superfamily. Proc Natl Acad Sci USA.
94:11514–11519. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Senapati S, Rachagani S, Chaudhary K,
Johansson SL, Singh RK and Batra SK: Overexpression of macrophage
inhibitory cytokine-1 induces metastasis of human prostate cancer
cells through the FAK-RhoA signaling pathway. Oncogene.
29:1293–1302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Breit SN, Johnen H, Cook AD, et al: The
TGF-beta superfamily cytokine, MIC-1/GDF15: A pleotrophic cytokine
with roles in inflammation, cancer and metabolism. Growth Factors.
29:187–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheung PK, Woolcock B, Adomat H, et al:
Protein profiling of microdissected prostate tissue links growth
differentiation factor 15 to prostate carcinogenesis. Cancer Res.
64:5929–5933. 2004. View Article : Google Scholar
|
|
13
|
Karan D, Chen SJ, Johansson SL, et al:
Dysregulated expression of MIC-1/PDF in human prostate tumor cells.
Biochem Biophys Res Commun. 305:598–604. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brown DA, Bauskin AR, Fairlie WD, et al:
Antibody-based approach to high-volume genotyping for MIC-1
polymorphism. Biotechniques. 33:118–120. 122124
passim2002.PubMed/NCBI
|
|
15
|
Brown DA, Moore J, Johnen H, et al: Serum
macrophage inhibitory cytokine 1 in rheumatoid arthritis: a
potential marker of erosive joint destruction. Arthritis Rheum.
56:753–764. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tong S, Marjono B, Brown DA, et al: Serum
concentrations of macrophage inhibitory cytokine 1 (MIC 1) as a
predictor of miscarriage. Lancet. 363:129–130. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brown DA, Lindmark F, Stattin P, et al:
Macrophage inhibitory cytokine 1: a new prognostic marker in
prostate cancer. Clin Cancer Res. 2009.PubMed/NCBI
|
|
18
|
Sun J, Turner A, Xu J, Gronberg H and
Isaacs W: Genetic variability in inflammation pathways and prostate
cancer risk. Urol Oncol. 25:250–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Selander KS, Brown DA, Sequeiros GB, et
al: Serum macrophage inhibitory cytokine-1 concentrations correlate
with the presence of prostate cancer bone metastases. Cancer
Epidemiol Biomarkers Prev. 16:532–537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karan D, Holzbeierlein J and Thrasher JB:
Macrophage inhibitory cytokine-1: possible bridge molecule of
inflammation and prostate cancer. Cancer Res. 69:2–5. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taniguchi S, Taoka R, Inui M, Sugimoto M
and Kakehi Y: Influence of inflammation and aging on macrophage
inhibitory cytokine-1 gene expression in rat ventral prostate.
Urology. 73:410–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Albertoni M, Shaw PH, Nozaki M, et al:
Anoxia induces macrophage inhibitory cytokine-1 (MIC-1) in
glioblastoma cells independently of p53 and HIF-1. Oncogene.
21:4212–4219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bauskin AR, Jiang L, Luo XW, Wu L, Brown
DA and Breit SN: The TGF-beta superfamily cytokine MIC-1/GDF15:
secretory mechanisms facilitate creation of latent stromal stores.
J Interferon Cytokine Res. 30:389–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bauskin AR, Zhang HP, Fairlie WD, et al:
The propeptide of macrophage inhibitory cytokine (MIC-1), a
TGF-beta superfamily member, acts as a quality control determinant
for correctly folded MIC-1. Embo J. 19:2212–2220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen SJ, Karan D, Johansson SL, et al:
Prostate-derived factor as a paracrine and autocrine factor for the
proliferation of androgen receptor-positive human prostate cancer
cells. Prostate. 67:557–571. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van der Heul-Nieuwenhuijsen L, Hendriksen
PJ, van der Kwast TH and Jenster G: Gene expression profiling of
the human prostate zones. BJU Int. 98:886–897. 2006.PubMed/NCBI
|
|
27
|
Paralkar VM, Vail AL, Grasser WA, et al:
Cloning and characterization of a novel member of the transforming
growth factor-beta/bone morphogenetic protein family. J Biol Chem.
273:13760–13767. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bostwick DG, de la Roza G, Dundore P,
Corica FA and Iczkowski KA: Intraepithelial and stromal lymphocytes
in the normal human prostate. Prostate. 55:187–193. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zimmers TA, Jin X, Hsiao EC, McGrath SA,
Esquela AF and Koniaris LG: Growth differentiation
factor-15/macrophage inhibitory cytokine-1 induction after kidney
and lung injury. Shock. 23:543–548. 2005.PubMed/NCBI
|
|
30
|
Zimmers TA, Jin X, Hsiao EC, et al: Growth
differentiation factor-15: induction in liver injury through p53
and tumor necrosis factor-independent mechanisms. J Surg Res.
130:45–51. 2006. View Article : Google Scholar
|