Introduction
Head and neck squamous cell carcinoma (HNSCC) is a
common malignant disease with more than 600,000 new cases
registered worldwide every year (1). Despite improved treatment options,
including surgery, radiation and chemotherapy, HNSCC is associated
with a high mortality rate. The overall 5-year survival rate of
approximately 50% has not changed over the last decades. As such,
new therapeutic agents in the treatment of head and neck cancer are
required. One objective in cancer research is to find novel drugs
that induce apoptosis in tumor cells. Another approach is to
identify new combinations of agents that improve therapeutic
results and reduce adverse effects.
Arsenic is an odorless and tasteless semimetal and
has been used in medical history for more than 2,400 years
(2). Arsenic preparations were
prescribed for the treatment of various diseases. In 1910, Paul
Ehrlich identified arsphenamine, an organic arsenical, also known
as salvarsan or compound 606 (3).
It was applied for the treatment of syphilis prior to the
introduction of penicillin. Currently, melarsoprol, a trivalent
organic melaminophenyl arsenical, is used for the treatment of
late-stage human African trypanosomiasis infections (4). Arsenic trioxide (ATO,
As2O3), a trivalent form of arsenic, has been
established for the treatment of acute promyelocytic leukaemia
(APL). In this disease, ATO is capable of inducing complete
remission even in relapsed patients resistant to conventional
chemotherapeutic agents (5,6). Of note, the common side effects of ATO
treatment are limited to gastrointestinal disorders, cough, fatigue
and skin rash, and myelosuppression is minimal (7).
Besides APL, ATO was found to be an effective
treatment option in other hematological malignancies and solid
tumors. Promising in vitro effects of ATO on gastric cancer
(8), HNSCC (9), neuroblastoma (10), esophageal (11), prostate and ovarian carcinomas
(12) have been reported.
Furthermore, Phase II studies of ATO in patients with relapsed or
refractory multiple myeloma (13),
metastatic melanoma (14),
hepatocellular carcinoma (15) and
myelodysplatic syndromes (16) have
been carried out.
As yet, the combined effect of ATO with standard
chemotherapeuticals has not been described in HNSCC. Therefore, the
aim of this study was to evaluate the potential of ATO in enhancing
the effect of cisplatin in the four HNSCC cell lines SCC9, SCC25,
CAL27 and FADU as well as to confirm previously published data. For
a better understanding of the apoptosis-inducing mechanisms of ATO,
we investigated the expression of the anti-apoptotic protein Mcl-1
(myeloid cell leukemia protein) in the four cell lines. Mcl-1 is a
member of the Bcl-2 family and is involved in inhibiting cell death
in various cell types. Mcl-1 is highly expressed in HNSCC (17).
Materials and methods
Cells and reagents
The HNSCC SCC9, SCC25 and FADU cell lines were
obtained from the American Type Culture Collection (Manassas, VA,
USA). CAL27 was obtained from the German Collection of
Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany).
Tumor cells were cultured in RPMI medium (Cambrex, Walkersville,
MD, USA) supplemented with 10% fetal bovine serum (PAA
Laboratories, Linz, Austria) and 1% penicillin-streptomycin (Gibco
BRL, Gaithersburg, MD, USA) at 37°C in a humidified atmosphere of
5% CO2. ATO was purchased from Sigma-Aldrich (St. Louis,
MO, USA). Cisplatin was obtained from a ready-to-use infusion.
Cytotoxicity assay
A CCK-8 cell proliferation assay (Dojindo Molecular
Technologies, Gaithersburg, MD, USA) was used to determine the
cytotoxic effects of ATO on tumor cells in vitro. Cells
(3×103/well) were seeded into 96-well plates and
incubated for 24 h. The cells were then treated with increasing
concentrations of ATO and cisplatin either alone or in combination
(range, 0.25–16 μM). Untreated cells served as a control. Following
72 h of incubation, cell proliferation was measured by CCK-8
according to the manufacturer’s protocol. Experiments were carried
out in triplicate at least three times independently.
Analysis of combination effects
To determine the concentrations of the drugs to be
investigated in the combination study, dose response curves were
generated with Prism® 5.0 software (GraphPad Software
Inc., San Diego, CA, USA) for ATO and cisplatin alone. Experiments
with ATO and cisplatin in a fixed ratio combination were carried
out. Possible drug interactions were calculated with CalcuSyn
software (Version 2.0, Biosoft, UK).
Immunocytochemistry
To visualize apoptosis we used the monoclonal mouse
antibody M30 directed against a neo-epitope of cytokeratin 18 that
is formed by caspase cleavage. Cells (1×106) were grown
on glass slides and then treated with ATO for 48 h. Untreated cells
served as the control. Slides were fixed with ice-cold methanol
(−20°C) for 30 min, washed twice in PBS and blocked with 1% BSA/TBS
for 30 min. Slides were then incubated overnight with M30 CytoDeath
antibody (1:200, Roche, Vienna, Austria). As a control, slides were
exposed to an IgG1 (Ancell, Bayport, MN, USA) antibody.
After washing with TBS, slides were incubated with a multilink
antibody (Dako, Glostrup, Denmark) for 1 h at room temperature
(RT), washed, and again exposed to alkaline phosphatase-conjugated
Streptavidin-AP/10% human serum (Dako) for 1 h at RT. Visualization
was performed with Fast Red TR,
4-chloro-2-methylbenzenediazonium-salt (Sigma-Aldrich, St. Louis,
MO, USA). Slides were then counterstained with hemalaun, dehydrated
and mounted.
Western blot analysis
Cell monolayers were washed twice with cold PBS,
frozen with liquid nitrogen and lysed with lysis buffer comprising
1% NP40, 0.1% SDS, 150 mM NaCl, 50 mM TRIS, pH 7.4, 10 mM EDTA, 10
mM p-nitrophenolphosphate, 250 U/l aprotinin, 40 μg/ml leupeptin, 1
mM PMSF, 1 mM sodium orthovanadate, 10 mM sodium fluoride and 40 mM
β-glycerolphosphate. The lysates were centrifuged at 14,000 rpm at
4°C for 20 min and the supernatants were collected. Protein
concentrations were determined using a Micro BCA protein counting
kit from Pierce (Rockford, IL, USA). Protein (20 μg) was separated
by SDS-PAGE (10%) and electroblotted onto nitrocellulose membranes
(Schleicher & Schuell, Dassel, Germany). Subsequent to blocking
with 5% BSA in TBS-Tween overnight, membranes were incubated with
the appropriate diluted primary antibody. Bound antigen was
visualized using the ECL Western blotting detection system
(Amersham Life Sciences, Buckinghamshire, UK) and deteced using the
ChemiDoc-It Imaging System (UVP, Upland, CA, USA).
Flow cytometry
The SCC9, SCC25, CAL27 and FADU cell lines were
seeded in 6-well plates at a density of 100,000 cells/well. After
24 h, cells were treated with 10 μM cisplatin (as a positive
control), medium (as a negative control), 2 μM ATO or 2 μM
cisplatin, or a combination of 2 μM ATO and 2 μM cisplatin.
Apoptosis was measured after 48 and 72 h using the Annexin-V
Apoptosis detection kit (Bender MedSystems, Vienna, Austria).
Apoptosis was defined as Ann+/PI-.
Ann-/PI+ and Ann+/PI+
were defined as necrotic. Late apoptosis and necrosis cannot be
differentiated with this assay.
Statistical analysis
Statistical analysis was performed using
Prism® 5.0 software (GraphPad Software Inc., San Diego,
CA, USA).
Results
Effects of ATO or cisplatin as single
agents on cell growth
The HNCC SCC9, SCC25, CAL27 and FADU cell lines were
exposed to ATO or cisplatin at doses of between 0.25 μM and 16 μM
for 72 h. Growth inhibition was measured with the CCK-8 assay. ATO
and cisplatin administration induced a dose-dependent inhibition of
cell proliferation (Fig. 1).
IC50 ranged from 1.66 μM (SEM ± 1.06%) to 3.20 μM (SEM ±
1.10%) for ATO and from 2.32 μM (SEM ± 1.11%) to 4.79 μM (SEM ±
1.07%) for cisplatin (Table I).
 | Table IEffects of cisplatin and ATO on cell
growth. |
Table I
Effects of cisplatin and ATO on cell
growth.
| Cisplatin | ATO | ATO+Cisplatin |
|---|
| SCC9 |
| IC50 | 4.79 | 2.83 | 2.26 |
| SEM of
IC50 | 1.07 | 1.10 | 1.45 |
| SCC25 |
| IC50 | 2.41 | 1.66 | 1.09 |
| SEM of
IC50 | 1.12 | 1.06 | 1.09 |
| CAL27 |
| IC50 | 3.11 | 3.2 | 1.54 |
| SEM of
IC50 | 1.07 | 1.10 | 1.09 |
| FADU |
| IC50 | 2.32 | 2.38 | 1.69 |
| SEM of
IC50 | 1.11 | 1.10 | 1.11 |
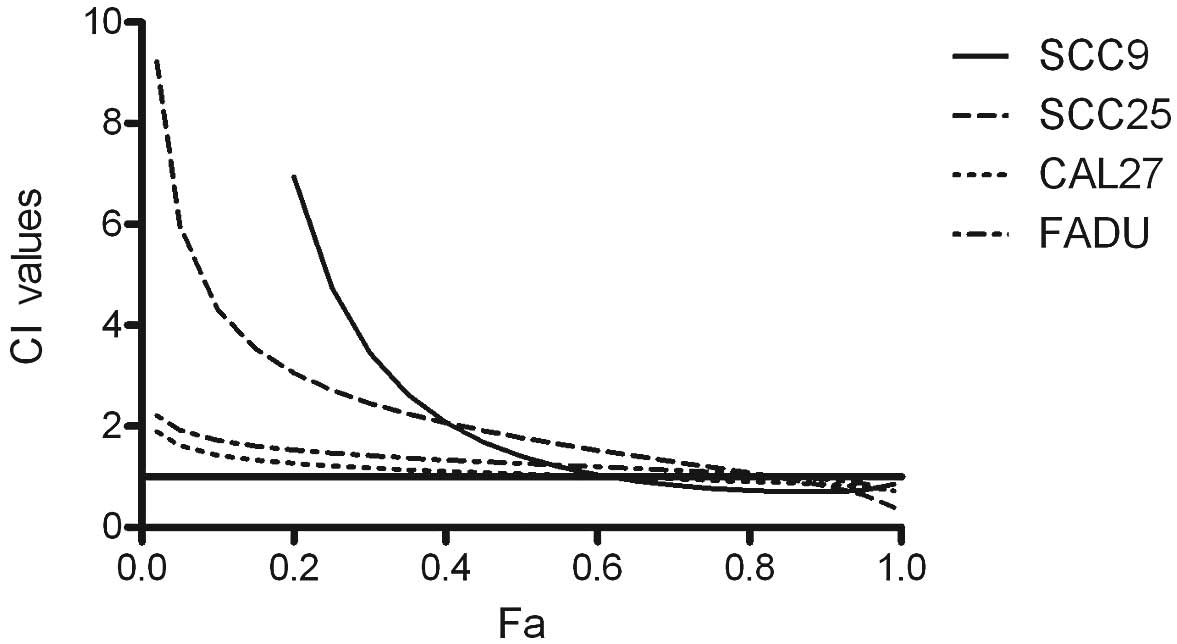
Effects of combined treatment with ATO
and cisplatin on cell growth
For the combination studies, cells were treated
simultaneously with 0.25–16 μM ATO and 0.25–16 μM cisplatin at a
fixed ratio. The combined regimen showed higher cytotoxic effects
in all four cell lines than each drug alone (Fig. 1). Combined drug effect was
quantified by combination index (CI) analysis with the CalcuSyn
software (Version 2.0, Biosoft) and expressed as CI versus Fa
(fraction affected). CI<1 indicated synergy; CI=1 an additive
effect; and CI>1 indicated antagonism. Our experiments showed
that ATO in combination with cisplatin acts synergistically at high
doses in all four tested cell lines (Fig. 2). An additive effect was observed in
CAL27 and FADU over a wide dose range.
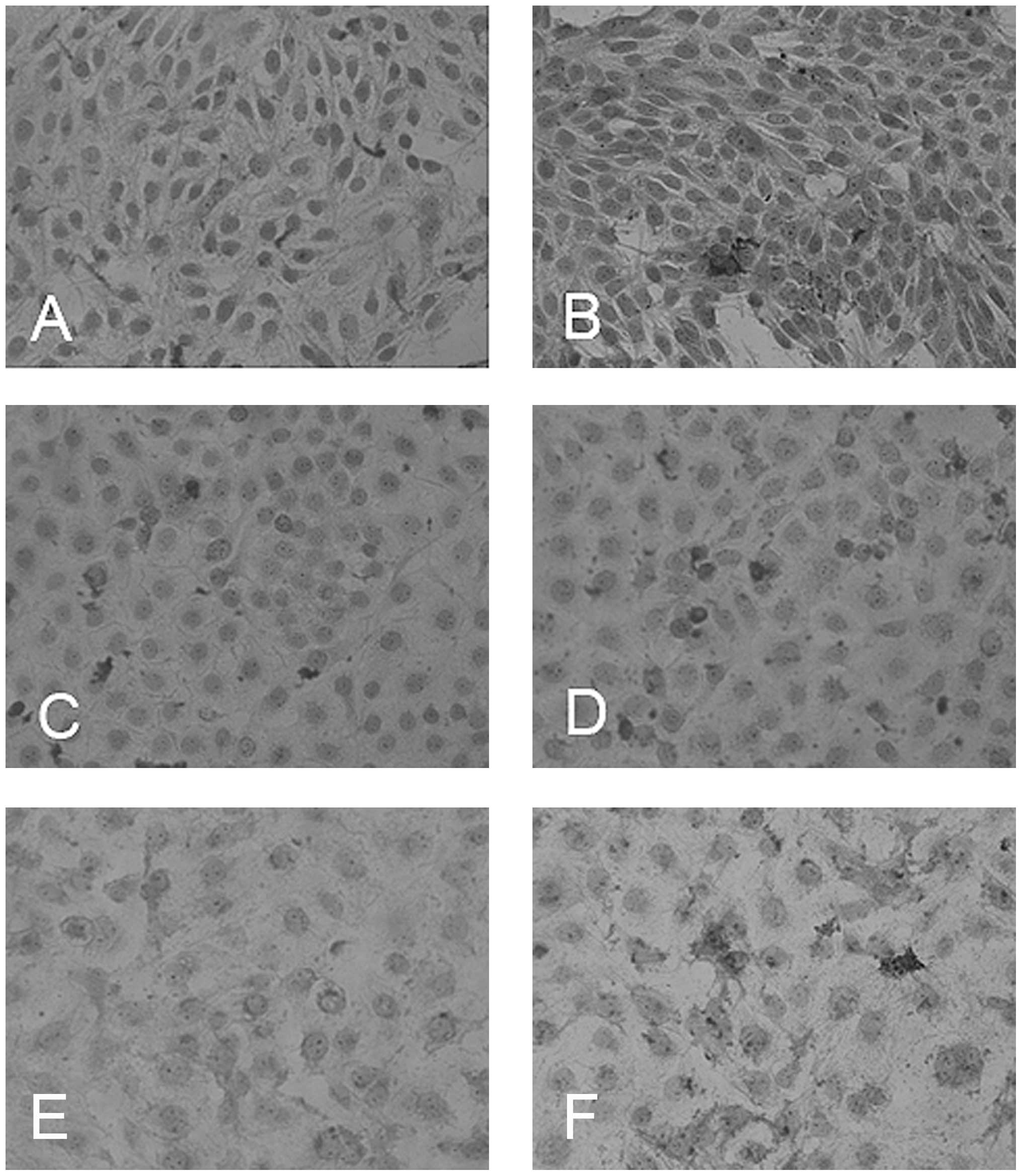
Visualization of apoptosis by
immunocytochemistry
To visualize apoptotic cells we used the M30
antibody, which detects caspase-cleaved cytokeratin-18 fragments.
ATO-treated cells showed a higher rate of apoptosis in the SCC9,
SCC25 and CAL27 cell lines than in the untreated control (Fig. 3). The cancer cell line FADU did not
adhere to the glass slides.
Regulation of the anti-apoptotic protein
Mcl-1
Western blot analysis of Mcl-1 (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) was performed to determine the
regulation of apoptosis-related proteins following treatment. Cells
were collected prior to and following 12, 24 and 48 h of treatment
with 2 μM ATO or 2 μM cisplatin alone, or 2 μM ATO and 2 μM
cisplatin in combination. Tubulin (Neomarkers, CA, USA) antibody
served as a control. The analysis showed no significant alteration
in the expression of the anti-apoptotic protein Mcl-1 following
treatment with ATO alone or with the combination (data not
shown).
Rate of apoptosis after treatment with
ATO and cisplatin alone or in combination
ATO induced apoptosis in the four cancer cell lines
alone and in combination with cisplatin. Apoptotic rates following
72 h of treatment were found to be higher than after 48 h. The
highest apoptotic rates following treatment with 2μM ATO were
observed in the SCC25 and FADU cell lines, whereas apoptosis in the
SCC9 cell line was minimal. Notably, the combination of ATO and
cisplatin showed no higher apoptosis rates than each drug alone
(Fig. 4).
Discussion
Head and neck squamous cell carcinoma is an
aggressive and often lethal malignancy. Treatment remains poor and
new therapeutic strategies are required to identify new drugs that
induce apoptosis in malignant tumors.
For many centuries, arsenic preparations have been
used through empirical observation for the treatment of numerous
diseases (18). ATO has been
successfully used in the treatment of APL and has been shown to be
effective even in patients resistant to conventional chemotherapy
(19). Studies have shown that ATO
induces apoptosis and loss of the PML/AR α fusion oncoprotein, a
protein that is specific for APL, leading to differentiation of
leukaemic cells (20,21). Since successful results have been
achieved, arsenic trioxide is regarded as a potential anticancer
agent, not only for hematological malignancies but also for
malignant diseases such as HNSCC.
Cisplatin is a standard agent of head and neck
cancer chemotherapy. Although concurrent chemoradiotherapy is well
established as an effective treatment for squamous cell carcinoma,
it is to the impairment of advanced toxicity in normal tissue.
Therefore, this study was conducted to identify a new combination
regimen with cisplatin.
In the present study, we investigated the effects of
ATO alone and in combination with cisplatin on the HNSCC cell lines
SCC9, SCC25, CAL27 and FADU. Our results showed that ATO is an
effective cytotoxic drug in vitro for head and neck cancer.
Treatment of the four cell lines led to an inhibited proliferation
in a dose-dependent manner with the IC50 ranging from
1.66 to 3.20 μM. Our results are in accordance with those of Seol
et al (22), who
demonstrated a dose-dependent cytotoxicity of ATO and revealed that
ATO is capable of inducing apoptosis in head and neck cancer
cells.
Few studies have reported the combined effect of ATO
and cisplatin. In their study, Wang et al (23) showed that in human hepatoma cells
the inhibition rates of ATO in combination with cisplatin are
higher than those of ATO or cisplatin alone. In this study, the
interaction between ATO and cisplatin was reported as synergistic.
Other studies in lung and ovarian cancer cells also demonstrated a
synergistic effect of ATO and cisplatin in vitro (24,25).
No studies are currently available regarding the combined effect of
ATO and cisplatin in HNSCC. One study has examined the effect of
tetra-arsenic oxide and cisplatin in head and neck cell lines
(26), however, tetra-arsenic oxide
has not yet been approved.
In the present study, we found a synergistic effect
in the combination of ATO and cisplatin in the four tested HNSCC
cell lines at high concentrations. A limiting factor, however, is
that this was a single-dose drug application in a cytotoxicity
assay. Comparison with clinical low-dose, long-term application
requires further investigation.
We also investigated whether the two substances
together would lead to increased rates of apoptosis. However, the
flow cytometry did not reveal higher apoptotic rates for the
combination compared to each drug alone.
Findings of the present study showed that the
combination of ATO and cisplatin has an enhanced cytotoxic effect
on the four HNSCC cell lines SCC9, SCC25, CAL27 and FADU after 72 h
of treatment when compared to the use of a single drug. ATO and
cisplatin had apoptotic properties in the four cell lines
tested.
In conclusion, ATO appears to be a noteworthy
substance in the treatment of head and neck cancer, due to its
apoptotic properties and its limited side effects in clinical
application. Adverse effects of established chemotherapeutics such
as cisplatin may be reduced if used in combination with ATO, since
lower doses of both agents may be possible.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Miller WH Jr, Schipper HM, Lee JS, Singer
J and Waxman S: Mechanisms of action of arsenic trioxide. Cancer
Res. 62:3893–3903. 2002.PubMed/NCBI
|
|
3
|
Gensini GF, Conti AA and Lippi D: The
contributions of Paul Ehrlich to infectious disease. J Infect.
54:221–224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Docampo R and Moreno SN: Current
chemotherapy of human African trypanosomiasis. Parasitol Res.
90(Supp 1): S10–S13. 2003.
|
|
5
|
Soignet SL, Maslak P, Wang ZG, et al:
Complete remission after treatment of acute promyelocytic leukemia
with arsenic trioxide. N Engl J Med. 339:1341–1348. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen ZX, Chen GQ, Ni JH, et al: Use of
arsenic trioxide (As2O3) in the treatment of
acute promyelocytic leukemia (APL): II. Clinical efficacy and
pharmacokinetics in relapsed patients. Blood. 89:3354–3360.
1997.PubMed/NCBI
|
|
7
|
Soignet SL, Frankel SR, Douer D, et al:
United States multicenter study of arsenic trioxide in relapsed
acute promyelocytic leukemia. J Clin Oncol. 19:3852–3860.
2001.PubMed/NCBI
|
|
8
|
Jiang XH, Wong BC, Yuen ST, et al: Arsenic
trioxide induces apoptosis in human gastric cancer cells through
up-regulation of p53 and activation of caspase-3. Int J Cancer.
91:173–179. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seol JG, Park WH, Kim ES, et al: Effect of
arsenic trioxide on cell cycle arrest in head and neck cancer cell
line PCI-1. Biochem Biophys Res Commun. 265:400–404. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akao Y, Nakagawa Y and Akiyama K: Arsenic
trioxide induces apoptosis in neuroblastoma cell lines through the
activation of caspase 3 in vitro. FEBS Lett. 455:59–62. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen ZY, Shen J, Li QS, Chen CY, Chen JY
and Yi Z: Morphological and functional changes of mitochondria in
apoptotic esophageal carcinoma cells induced by arsenic trioxide.
World J Gastroenterol. 8:31–35. 2002.PubMed/NCBI
|
|
12
|
Uslu R, Sanli UA, Sezgin C, et al: Arsenic
trioxide-mediated cytotoxicity and apoptosis in prostate and
ovarian carcinoma cell lines. Clin Cancer Res. 6:4957–4964.
2000.PubMed/NCBI
|
|
13
|
Hussein MA, Saleh M, Ravandi F, Mason J,
Rifkin RM and Ellison R: Phase 2 study of arsenic trioxide in
patients with relapsed or refractory multiple myeloma. Br J
Haematol. 125:470–476. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim KB, Bedikian AY, Camacho LH,
Papadopoulos NE and McCullough C: A phase II trial of arsenic
trioxide in patients with metastatic melanoma. Cancer.
104:1687–1692. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin CC, Hsu C, Hsu CH, Hsu WL, Cheng AL
and Yang CH: Arsenic trioxide in patients with hepatocellular
carcinoma: a phase II trial. Invest New Drugs. 25:77–84. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schiller GJ, Slack J, Hainsworth JD, et
al: Phase II multicenter study of arsenic trioxide in patients with
myelodysplastic syndromes. J Clin Oncol. 24:2456–2464. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Whisler LC, Wood NB, Caldarelli DD, et al:
Regulators of proliferation and apoptosis in carcinoma of the
larynx. Laryngoscope. 108:630–638. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Evens AM, Tallman MS and Gartenhaus RB:
The potential of arsenic trioxide in the treatment of malignant
disease: past, present, and future. Leuk Res. 28:891–900. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soignet SL: Clinical experience of arsenic
trioxide in relapsed acute promyelocytic leukemia. Oncologist.
6(Suppl 2): 11–16. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen GQ, Shi XG, Tang W, et al: Use of
arsenic trioxide (As2O3) in the treatment of
acute promyelocytic leukemia (APL): I. As2O3
exerts dose-dependent dual effects on APL cells. Blood.
89:3345–3353. 1997.PubMed/NCBI
|
|
21
|
Shao W, Fanelli M, Ferrara FF, et al:
Arsenic trioxide as an inducer of apoptosis and loss of PML/RAR
alpha protein in acute promyelocytic leukemia cells. J Natl Cancer
Inst. 90:124–133. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seol JG, Park WH, Kim ES, et al: Potential
role of caspase-3 and -9 in arsenic trioxide-mediated apoptosis in
PCI-1 head and neck cancer cells. Int J Oncol. 18:249–255.
2001.PubMed/NCBI
|
|
23
|
Wang W, Qin SK, Chen BA and Chen HY:
Experimental study on antitumor effect of arsenic trioxide in
combination with cisplatin or doxorubicin on hepatocellular
carcinoma. World J Gastroenterol. 7:702–705. 2001.PubMed/NCBI
|
|
24
|
Li H, Zhu X, Zhang Y, Xiang J and Chen H:
Arsenic trioxide exerts synergistic effects with cisplatin on
non-small cell lung cancer cells via apoptosis induction. J Exp
Clin Cancer Res. 28:1102009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang N, Wu ZM, McGowan E, et al: Arsenic
trioxide and cisplatin synergism increase cytotoxicity in human
ovarian cancer cells: therapeutic potential for ovarian cancer.
Cancer Sci. 100:2459–2464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chung WH, Sung BH, Kim SS, Rhim H and Kuh
HJ: Synergistic interaction between tetra-arsenic oxide and
paclitaxel in human cancer cells in vitro. Int J Oncol.
34:1669–1679. 2009.PubMed/NCBI
|