Introduction
An epidermal growth factor receptor (EGFR) tyrosine
kinase inhibitor (TKI) may be used as first-line therapy in
patients with advanced non-small cell lung cancer (NSCLC) with EGFR
mutations (1–5). The results of the INTEREST trial
(6) suggest that gefitinib is able
to provide similar overall survival to docetaxel in patients across
a broad range of clinical subgroups and that EGFR biomarkers,
including mutation status, may additionally identify which patients
are likely to gain greatest progression-free survival (PFS) and
overall response rate (ORR) benefit from treatment with gefitinib.
Two hot spots of EGFR mutations are in-frame deletion at codons
747–749 (DEL) in exon 19 and a missense mutation at codon 858
(L858R) in exon 21. A case study concerning a Japanese patient with
gefitinib-responsive small cell lung cancer (SCLC) reported that
the patient had a deletion in exon 19 of EGFR (7). Another case study has reported that an
American SCLC patient who had never smoked and who had an EGFR
mutation responded to gefitinib (8). In China, there has also been a case
report of a patient with SCLC who responded to gefitinib, but the
status of the mutation is unknown (9). Therefore, the EGFR mutation status of
SCLC is significant.
We detected EGFR exon 19 and exon 21 mutations in 40
SCLC patients, three of whom had combined SCLC, from the Zhejiang
Cancer Hospital (Hangzhou, China) by xTAG technology. Only two of
the combined SCLC patients showed an EGFR mutation in exon 19
(10). To identify the clinical
features and incidence of EGFR mutations in cases of combined SCLC
in China, we retrospectively investigated seven cases of combined
SCLC which were treated surgically and detected mutations in EGFR
exons 19 and 21 using a pyrosequencing assay. The combined
components of these patients were not only adenocarcinoma but also
squamous cell carcinoma. Specimens obtained during surgery more
accurately reflect the pathological status of the tumor and all our
specimens were from surgically resected tumors.
Materials and methods
Patient characteristics
Seven cases of combined SCLC from the Zhejiang
Cancer Hospital (Hangzhou, China) between 2007 and 2010 were
investigated. Of the patients with combined SCLC, 71.4% were male,
71.4% were heavy smokers, most were >60 years old and 71.4% of
the cases were combined adenocarcinoma. None of the patients were
clearly pathologically diagnosed prior to surgery. The specimens
were obtained from surgically resected tumors. The histological
diagnosis of combined SCLC was based on the standard criteria
defined by the WHO classification. The patients were aged 47–74
years (median, 62), two were female and five were male. Two cases
were stage IB, one was IIB and four were IIIA according to the
seventh edition of the TNM classification for lung cancer. Two of
the patients were non-smokers and five were heavy smokers. There
were five cases of SCLC combined adenocarcinoma and two cases of
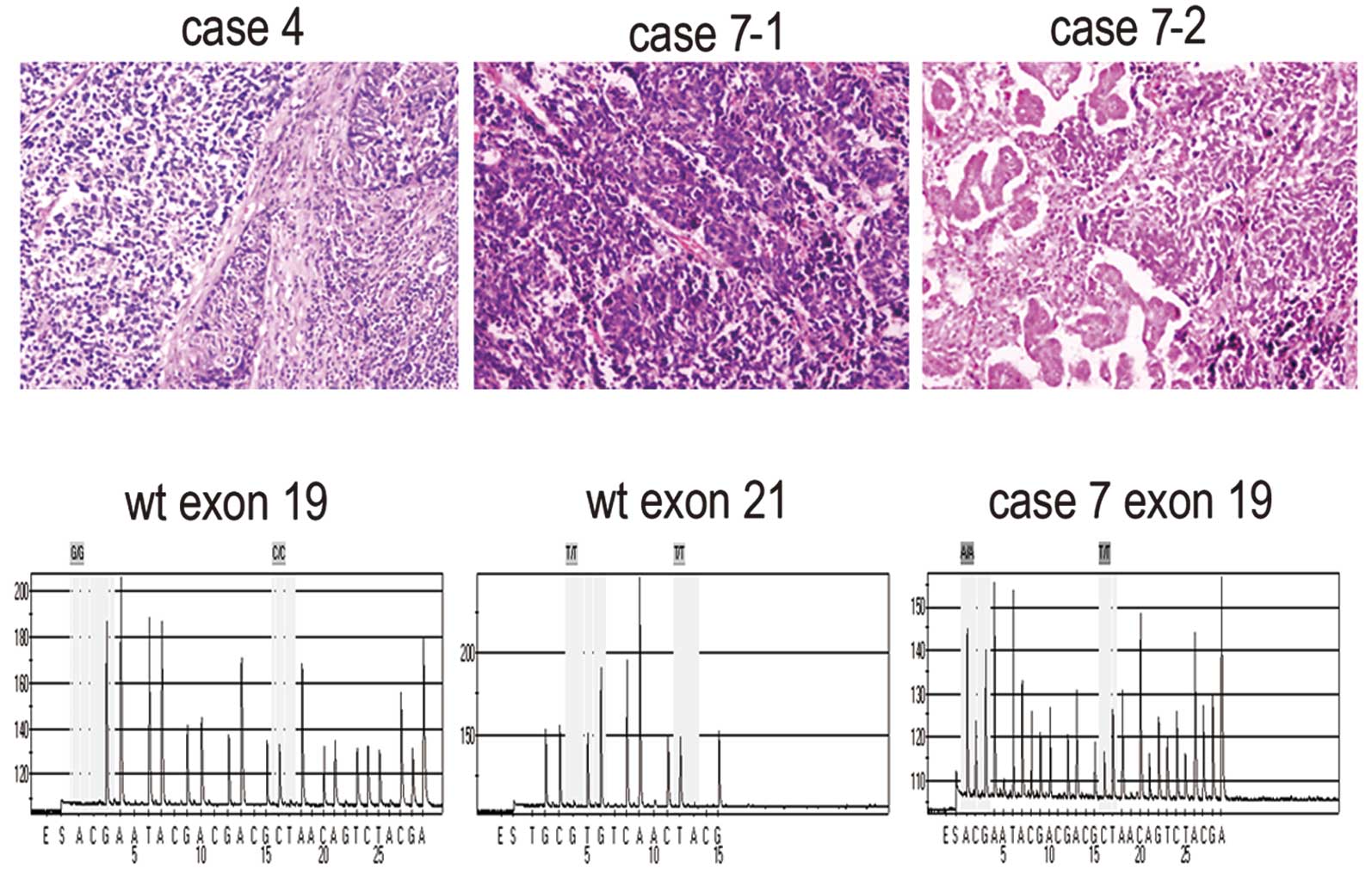
SCLC combined squamous cell carcinoma (Fig. 1A). Most of the patients underwent
lobectomy and lymph node dissection. Five patients received
chemotherapy and two patients received no chemotherapy. Only one
patient received thoracic radiotherapy (Table I). This study was approved by the
ethics committee of the Zhejiang Cancer Hospital.
 | Table IClinical characteristics of combined
SCLC patients. |
Table I
Clinical characteristics of combined
SCLC patients.
| Patient no. | Surgery | Pathology | Age/gender | Smoking history | First-line
chemotherapy | Thoracic
radiotherapy | Stage |
|---|
| 1 | LUL+LND | SCLC combined
adenocarcinoma | 58/male | Heavy smoker | TP (1 cycle) | Yes | IIIA pT2bN2M0 |
| 2 | LP+LND | SCLC combined
adenocarcinoma | 47/female | Never smoker | NP (4 cycles) | No | IIIA pT2aN2M0 |
| 3 | LLL+LND | SCLC combined
squamous cell carcinoma | 74/male | Heavy smoker | No | No | IIIA pT3N1M0 |
| 4 | LUL+LND | SCLC combined
squamous cell carcinoma | 66/male | Heavy smoker | EP (4 cycles) | No | IIB pT3N0M0 |
| 5 | LUL+LND | SCLC combined
adenocarcinoma | 53/male | Heavy smoker | EP (4 cycles) | No | IB pT2aN0M0 |
| 6 | RLRR | SCLC combined
adenocarcinoma | 71/male | Heavy smoker | No | No | IB pT2aN0M0 |
| 7 | RUML+LND | SCLC combined
adenocarcinoma | 62/female | Never smoker | IP (5 cycles) | No | IIIA pT1N2M0 |
Pyrosequencing assay for gene
mutation
We detected two examples of adenocarcinoma and SCLC
combined adenocarcinoma components in case 6. We also detected two
examples of conventional SCLC and SCLC combined adenocarcinoma
components in case 7. In the other five patients, only one sample
for each patient was detected. Genomic DNA was isolated and
purified from formalin-fixed paraffin-embedded tissues using a
GTpure FFPE Tissue DNA Extraction kit (GeneTech, Shanghai, China).
For the amplification of fragments of exons 19 and 21 of the EGFR
gene from isolated genomic DNA, we designed PCR amplification
primers for pyrosequencing: EGFR-19, forward:
5′-GGATCCCAGAAGGTGAGAAAGTT-3′; EGFR-19, reverse biotinylated
primer: 5′-GAGAAAAGGTGGGCCTG AGGT-3′; and EGFR-21, forward:
5′-GGGCATGAAC TACTTGGAGG-3′; EGFR-21, reverse biotinylated primer:
5′-TCCCTGGTGTCAGGAAAATG-3′. Each PCR assay contained forward and
reverse primers (each 4 pmol), 2 μl template DNA solution and 2
units hotstart Taq DNA Polymerase (Takara, Shiga, Japan) in a 40 ml
volume. The PCR conditions consisted of initial denaturation at
95°C for 3 min; 50 cycles of 95°C for 15 sec, annealing at 56°C for
30 sec and 72°C for 30 sec; and final extension at 72°C for 5 min.
The PCR products were sequenced using the Pyrosequencing PyroMark
ID system (Qiagen, Hilden, Germany) following the manufacturer's
instructions, using the two pyrosequencing primers (5′-3′
orientation): EGFR-19, TCCCGTCGCTATCAA; EGFR-21, AAGATCACAGATTTTGG.
Pyrosequencing was performed using PyroMark Gold Q96 Reagents
(Qiagen) containing enzyme and substrate mixture, dATP-S, dCTP,
dGTP and dTTP.
Statistical analysis
The statistical significance of the mean values was
determined using SPSS 13.0 (Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant result.
Results
The majority of the patients (71.4%) received
chemotherapy and most underwent more than 4 cycles. Cases 4 and 6
were alive at the end of their follow-up periods and cases 2 and 5
could not be followed up for longer than 31 and 18 months,
respectively. Cases 3 and 6 did not receive chemotherapy and the
survival time of case 6 (stage IB) was longer than that of case 3
(stage IIIA). The survival time of case 3 was shorter than that of
the other stage IIIA patients who received chemotherapy (Table II). No mutations of exons 19 and 21
were observed, with the exception of case 7. Case 7 was found to
have a mutation in exon 19 (codon 746–754) of EGFR in both the
conventional SCLC and SCLC combined adenocarcinoma components
(Fig. 1B).
 | Table IISurvival and EGFR mutation of combined
SCLC patients. |
Table II
Survival and EGFR mutation of combined
SCLC patients.
| Patient no. | Survival
(months) | EGFR 19 mutation | EGFR 21 mutation |
|---|
| 1 | 26 | No | No |
| 2 | >31 | No | No |
| 3 | 10 | No | No |
| 4 | >18 | No | No |
| 5 | >18 | No | No |
| 6 | >47 | No | No |
| 7 | 22 | Yes | No |
Discussion
Combined SCLC is defined as SCLC combined with an
additional component that consists of any of the histological types
of NSCLC, usually adenocarcinoma, squamous cell carcinoma or large
cell carcinoma. SCLC accounts for approximately 15% of lung cancers
(11). Combined SCLC has been
reported to account for 1–3.2% of all SCLC cases (12,13).
However, 28% of SCLC patients who undergo surgical resection
exhibit combined SCLC, with SCLC with large cell carcinoma being
the most common, followed by adenocarcinoma and squamous cell
carcinoma (14). Specimens obtained
during surgery more accurately reflect the pathological features of
the tumor than biopsy and malignant pleural effusion. In the study
by Tatematsu et al, most of the specimens were obtained from
biopsy (15). The specimens
reported by Shiao et al include 10 computed
tomography-guided biopsy specimens, 17 echo-guided aspiration
specimens, 37 echo-guided biopsy specimens, one surgical lobectomy
specimen and 11 malignant pleural effusion specimens (16), whereas, in the present study, all
the specimens were obtained during surgery.
The incidence of the EGFR mutation in NSCLC is
higher in China than in the United States and European countries
(17,18). Few large studies concerning the
mutations status of patients with SCLC have been performed. EGFR
mutations have been detected in five (4%) of 122 Japanese patients
with SCLCs, of which 15 cases were combined SCLCs. The patients
with EGFR mutations were mainly in the light smoker and
histological combined subtype. In three cases of the combined SCLC
subtype, both components of adenocarcinoma and SCLC harbored an
EGFR mutation (15). Fukui et
al retrospectively investigated six resected cases of combined
SCLC with an adenocarcinoma component in Japan to elucidate the
clinicopathological parameters and detect the EGFR mutation status
(19). With regard to EGFR, no
specific mutation was detected in five of the six patients, whereas
only one female patient who had never smoked had the same mutation
in exon 21 (L858R) in both the SCLC and adenocarcinoma components
(19). A prospective study on 76
specimens from patients with SCLC conducted between 2004 and 2009
at the National Taiwan University Hospital reported that two cases
(2.6%) tested positive for the EGFR mutation with reverse
transcription polymerase chain reaction (RT-PCR) and direct
sequencing and both cases were deletions in exon 19. Additionally,
three patients were diagnosed with combined SCLC but showed no EGFR
mutation in exon 19 or 21 (19). In
our previous study, we detected EGFR mutations in exons 19 and 21
of 40 cases and two combined SCLCs with EGFR mutation in exon 19
(10). However, more combined SCLC
cases are required to identify clinical features and detect EGFR
exon 19 and 21 mutations in China. EGFR mutations were predictive
of the response of single-agent TKIs, and EGFR gene copy number was
also associated with response to TKIs, albeit with lower
sensitivity and specificity (20).
Therefore, in this study, we detected EGFR mutations instead of
EGFR copy number. Compared with other genotyping and genetic
detection methods, pyrosequencing technology is unique. Unlike
hybridization-based assays, which may yield false-negative results,
pyrosequencing produces a correct sequence even in the presence of
a novel mutation. This is significant for microbiological
applications. Another benefit of pyrosequencing is that the data is
quantitative, thus it is possible to measure the relative amounts
of alleles.
The seventh edition of the TNM classification was
also cited in the National Comprehensive Cancer Network (NCCN)
guidelines for SCLC (2011 version 1). Surgery may be used to treat
NSCLC patients with stages IA, IB, IIA, IIB and IIIA disease, but
only T1-2N0M0 SCLC patients may be considered for surgical
treatment. Cases of T1-2N0M0 SCLC have been reported to account for
less than 5% of all SCLCs (21),
thus few SCLCs can be treated surgically. It is difficult to
diagnose combined SCLC by biopsy and the rarity of patients with
combined SCLC makes it difficult to determine the optimal
management and biological characteristics of this tumor. The
treatment of combined SCLC was managed according to NCCN guidelines
(Version 1.2011). There have been few studies concerning combined
SCLC and more studies should be conducted to identify the clinical
features of these patients. This study demonstrates that combined
SCLC frequently occurs in patients who are heavy smokers, male and
aged over 60 years and that most cases are combined adenocarcinoma.
Case 3 did not receive chemotherapy and the survival time of this
patient was shorter than that of the other stage IIIA patients who
received chemotherapy. We suggest that chemotherapy is significant
for combined SCLC and most of the patients in the present study
received chemotherapy. Cases 3 and 6 did not receive chemotherapy,
but the survival time of case 6 was longer than that of case 3. The
cause of the difference in survival time may be the different
stages of case 3 (IIIA) and case 6 (IB). Thus, stage may affect
survival time. Concurrent chemotherapy combined with mediastinal
radiotherapy should be used to treat SCLC patients with lymph node
metastasis following surgery and adjuvant chemotherapy and
radiotherapy may be considered for stage IIIA NSCLC patients.
Ideally, if the combined SCLC with lymph node metastasis is present
after surgery, radiotherapy should be used in conjunction with
chemotherapy. However, in our study, only one patient received
radiotherapy.
In conclusion, EGFR mutations may occur in combined
SCLCs, particularly in SCLC combined with adenocarcinoma in
China.
Acknowledgements
This study was supported by the funds: No. Y2110004,
Zhejiang Provincial Natural Science Foundation of China; No.
2010KYA035, Zhejiang Province Medical Science Fund Project of
China; No. 2010ZA006, Zhejiang Province Traditional Medical Science
Fund Project of China.
Abbreviations:
|
EGFR
|
epidermal growth factor receptor
|
|
SCLC
|
small cell lung cancer
|
|
NSCLC
|
non-small cell lung cancer
|
|
RT-PCR
|
reverse transcription polymerase chain
reaction
|
|
NCCN
|
National Comprehensive Cancer
Network
|
References
|
1
|
Mok TS, Wu YL, Thongprasert S, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maemondo M, Inoue A, Kobayashi K, et al:
Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mitsudomi T, Morita S, Yatabe Y, et al:
Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): an open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar
|
|
4
|
Rosell R, Gervais R, Vergnenegre A, et al:
Erlotinib versus chemotherapy (CT) in advanced non-small cell lung
cancer (NSCLC) patients (p) with epidermal growth factor receptor
(EGFR) mutations: Interim results of the European Erlotinib Versus
Chemotherapy (EURTAC) phase III randomized trial. J Clin Oncol.
29(Suppl): abs.75032011.
|
|
5
|
Zhou C, Wu YL, Chen G, et al: Erlotinib
versus chemotherapy as first-line treatment for patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase
3 study. Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Douillard JY, Shepherd FA, Hirsh V, et al:
Molecular predictors of outcome with gefitinib and docetaxel in
previously treated non-small-cell lung cancer: data from the
randomized phase III INTEREST trial. J Clin Oncol. 28:744–752.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okamoto I, Araki J, Suto R, et al: EGFR
mutation in gefitinib responsive small-cell lung cancer. Ann Oncol.
17:1028–1029. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zakowski MF, Ladanyi M, Kris MG, et al:
EGFR mutations in small-cell lung cancers in patients who have
never smoked. N Engl J Med. 355:213–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qian J, Qin S, Tang Q, et al: Gefitinib in
patients with advanced refractory small cell lung cancer
contemporaneous with superior venacava syndrome. Chin Clin Oncol.
10:243–244. 2005.
|
|
10
|
Lu HY, Sun WY, Chen B, et al: Epidermal
growth factor receptor mutations in small cell lung cancer patients
who received surgical resection in China. Neoplasma. 59:100–104.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Govindan R, Page N, Morgensztern D, et al:
Changing epidemiology of small-cell lung cancer in the United
States over the last 30 years: analysis of the surveillance,
epidemiologic, and end results database. J Clin Oncol.
24:4539–4544. 2006.PubMed/NCBI
|
|
12
|
Mangum MD, Greco FA, Hainsworth JD, et al:
Combined small-cell and non-small-cell lung cancer. J Clin Oncol.
7:607–612. 1989.PubMed/NCBI
|
|
13
|
Fraire AE, Johnson EH, Yesner R, et al:
Prognostic significance of histopathologic subtype and stage in
small cell lung cancer. Hum Pathol. 23:520–528. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nicholson SA, Beasley MB, Brambilla E, et
al: Small cell lung carcinoma (SCLC): a clinicopathologic study of
100 cases with surgical specimens. Am J Surg Pathol. 26:1184–1197.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tatematsu A, Shimizu J, Murakami Y, et al:
Epidermal growth factor receptor mutations in small cell lung
cancer. Clin Cancer Res. 14:6093–6096. 2008. View Article : Google Scholar
|
|
16
|
Shiao TH, Chang YL, Yu CJ, et al:
Epidermal growth factor receptor mutations in small cell lung
cancer: a brief report. J Thorac Oncol. 5:195–198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shigematsu H, Lin L, Takahashi T, et al:
Clinical and biological features associated with epidermal growth
factor receptor gene mutations in lung cancers. J Natl Cancer Inst.
97:339–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu YL, Zhong WZ, Li LY, et al: Epidermal
growth factor receptor mutations and their correlation with
gefitinib therapy in patients with non-small cell lung cancer: a
meta-analysis based on updated individual patient data from six
medical centers in mainland China. J Thorac Oncol. 2:430–439. 2007.
View Article : Google Scholar
|
|
19
|
Fukui T, Tsuta K, Furuta K, et al:
Epidermal growth factor receptor mutation status and
clinicopathological features of combined small cell carcinoma with
adenocarcinoma of the lung. Cancer Sci. 98:1714–1719. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dahabreh IJ, Linardou H, Siannis F, et al:
Somatic EGFR mutation and gene copy gain as predictive biomarkers
for response to tyrosine kinase inhibitors in non-small cell lung
cancer. Clin Cancer Res. 16:291–303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rostad H, Naalsund A, Jacobsen R, et al:
Small cell lung cancer in Norway. Should more patients have offered
surgical therapy? Eur J Cardiothorac Surg. 26:782–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|