Introduction
Neuroendocrine carcinoma (NEC) of the breast is a
rare distinct clinicopathological entity, comprising 0.5–2% of
breast carcinomas worldwide (1,2). Since
previous diagnostic criteria for NEC of the breast had been
contradictory, the World Health Organization Classification of
Tumours of the Breast and Genital Organs proposed the following
diagnostic criteria for breast NEC in 2003: i) Presence of
morphological neuroendocrine features resembling those of
neuroendocrine tumors of the gastrointestinal tract and lung, and
ii) expression of neuroendocrine markers in more than 50% of tumor
cells (1).
Mucinous carcinoma of the breast is also a rare
distinct clinicopathological entity, and accounts for approximately
2% of breast carcinomas worldwide (1). It is characterized by a proliferation
of clusters of generally small and uniform tumor cells floating in
large amounts of extracellular mucus. Pure mucinous carcinoma is
not recognized as a single homogenous entity. Capella et al
classified it based on structural and cytological features as type
A (paucicellular; a tumor showing a ribbon, annular, or cribriform
growth pattern with prominent extracellular mucin) and type B
(hypercellular; tumor showing clumps or sheet-like structures with
less extracellular mucin) (3). It
is well known that type B mucinous carcinoma frequently shows
neuroendocrine differentiation (3,4).
Solid NEC of the breast with a mucinous carcinoma
component is rarely reported (2).
In the present study, we report a case of solid NEC of the breast
with a mucinous carcinoma component and discuss the tumorigenesis
of this rare lesion.
The study was approved by the ethics committee of
the university and patient consent was obtained.
Patients and methods
Case report
A 37-year-old Japanese female presented with a right
breast tumor. A physical examination revealed a relatively
well-circumscribed tumor, measuring 3×2.5 cm in diameter, in the
right breast and swollen right axillary lymph nodes. No metastatic
lesions with the exception of the right axillary lymph nodes were
detected by computed tomography (CT). The biopsy specimen of the
right breast tumor revealed invasive carcinoma; thus, total
mastectomy and removal of the right axillary lymph nodes were
performed (cT2N1M0, stage IIB). Chemotherapy and hormonotherapy
were administered following the surgery. Three years later, local
recurrence was observed on the operation scar of her right thoracic
wall, and tumor resection was performed again. Seven years after
the first surgery, a liver tumor was detected during the follow-up
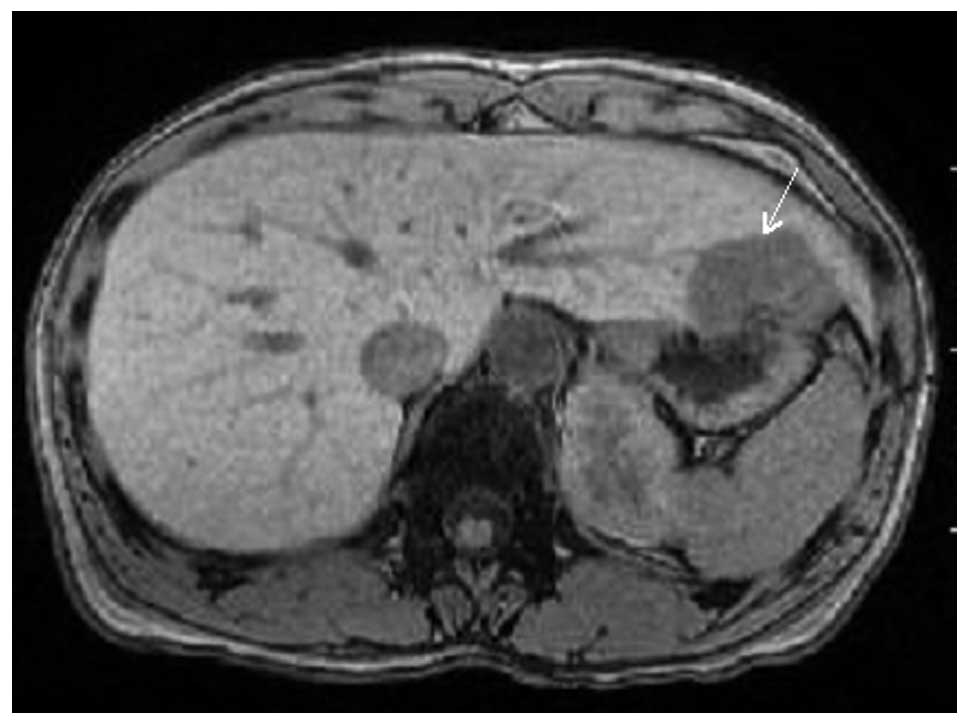
CT. CT and magnetic resonance imaging demonstrated a relatively
well-circumscribed tumor, measuring 42×37×30 mm, in S3 (Fig. 1). Metastatic breast cancer in the
liver was suspected clinically, resulting in the surgical resection
of the liver tumor. The post-operative course was uneventful, and
the patient has been free from recurrence for 18 months of medical
follow-up.
Methods
The formalin-fixed, paraffin-embedded tissue blocks
of the tumors of the breast, local recurrence on the operation
scar, and liver were cut into 3-μm sections, deparaffinized and
rehydrated. Each section was stained with hematoxylin and eosin,
and then used for immunostaining. Immunohistochemical analyses were
performed using an autostainer (XT system Benchmark, Ventana
Medical System, Tucson, AZ, USA) according to the manufacturer’s
instructions. The primary antibodies used were: a mouse monoclonal
antibody against CD56 (clone CD564, Novocastra Laboratories, Ltd.,
Newcastle upon Tyne, UK), a mouse monoclonal antibody against
chromogranin A (clone DAK-A3, Dako Cytomation, Glostrup, Denmark),
a mouse monoclonal antibody against estrogen receptor (ER) (clone
6F11, Novocastra), a mouse monoclonal antibody against gross cystic
disease fluid protein-15 (GCDFP-15) (clone 23A3, Novocastra), a
mouse monoclonal antibody against progesterone receptor (PgR)
(clone PgR636, Dako), and a mouse monoclonal antibody against
synaptophysin (clone 27G12, Novocastra). In addition,
immunohistochemistry for the c-erbB-2 (HER2) oncoprotein was
performed using a Dako kit.
Results
Breast tumor
The tumor was relatively well-circumscribed,
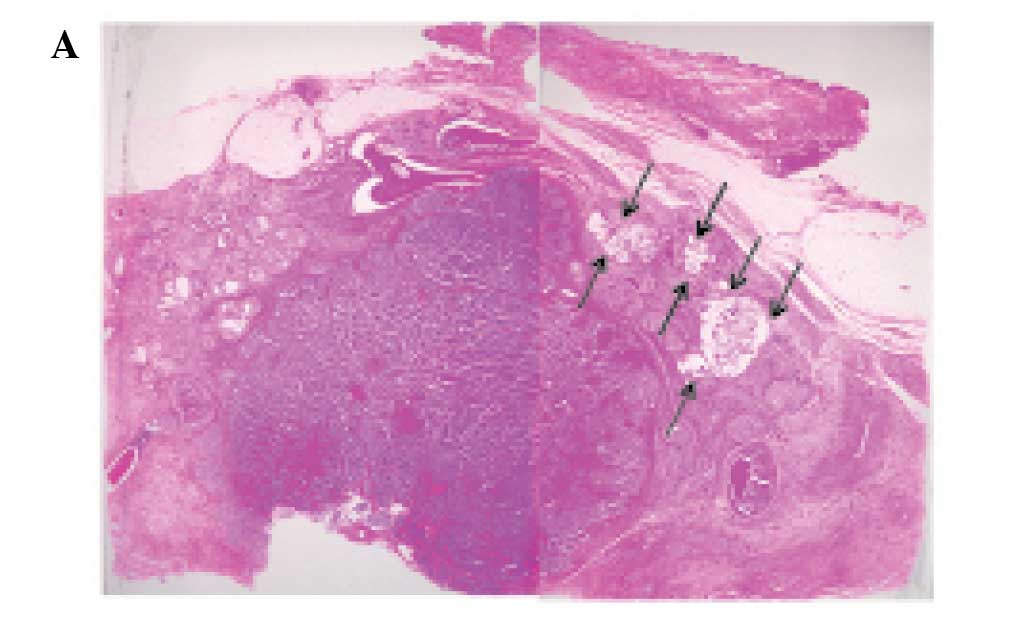
however, it had invaded into the surrounding fatty tissue. Most of
the tumor comprised variable-sized solid nests with or without
central necrosis separated by delicate fibrovascular stroma
(Fig. 2A and B). The solid nests
consisted of uniform tumor cells which had slightly enlarged oval
nuclei and inconspicuous nucleoli with slightly eosinophilic
cytoplasm (Fig. 2B, inset). Mitotic
figures were easily detected (45/10 high power fields).
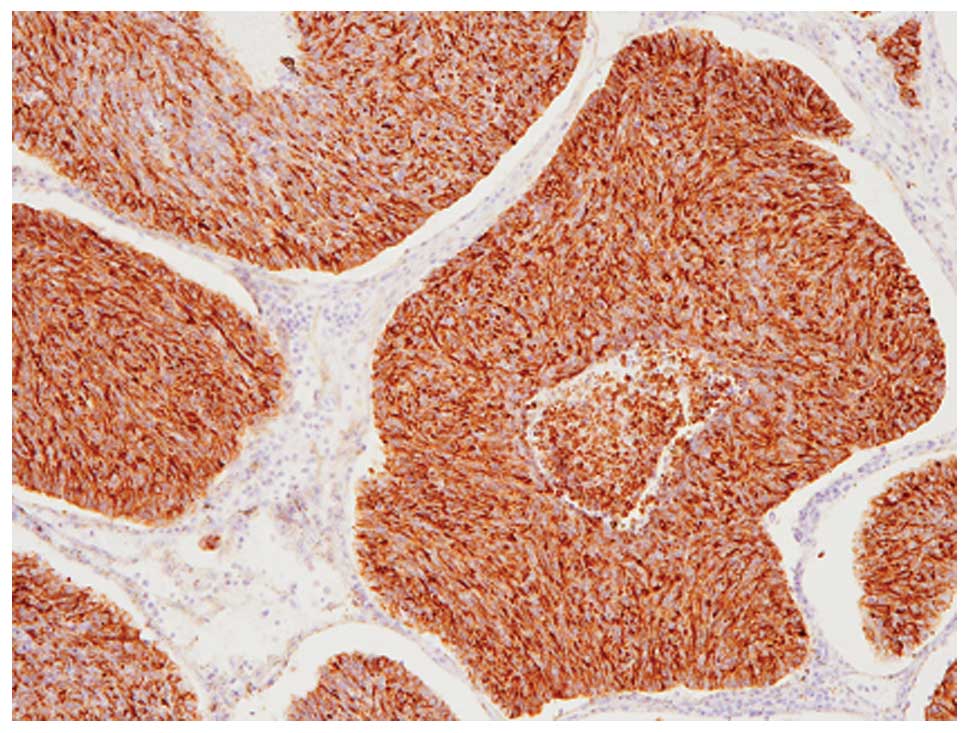
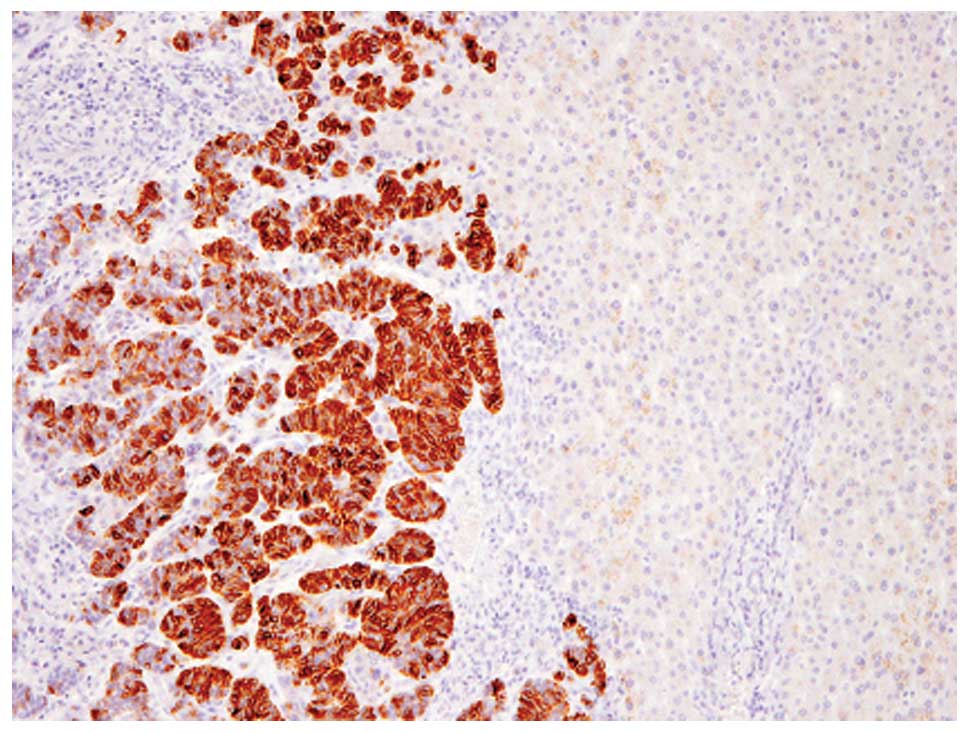
Immunohistochemical analyses revealed that these
tumor cells were diffusely (>95% of the tumor) positive for
chromogranin A (Fig. 3), although
CD56, synaptophysin and GCDFP-15 were not expressed. Therefore,
this component was considered to be solid NEC of the breast. In
addition, ER and PgR were diffusely expressed (100% of the tumor),
and the HER2 score was 1+.
Approximately 85% of the tumor belonged to the
above-mentioned solid NEC component. However, areas with
proliferation of clusters or sheets of uniform tumor cells with
mildly enlarged nuclei and slightly eosinophilic cytoplasm floating
in extracellular mucin, which are characteristic histopathological
features of mucinous carcinoma, were present at the periphery of
the tumor (Fig. 2A and C).
According to the classification by Capella et al (3), this mucinous carcinoma was classified
as type B. Results of the immunohistochemical analysis revealed
that certain tumor cells of the mucinous carcinoma component were
positive for chromogranin A, but negative for CD56, synaptophysin
and GCDFP-15. In addition, ER and PgR were diffusely expressed
(100% of the tumor) in this mucinous carcinoma component and the
HER2 score was 0.
According to these histopathological and
immunohistochemical findings, an ultimate diagnosis of solid NEC
with type B mucinous carcinoma component was made. In addition, the
right axillary lymph nodes had metastatic solid NEC (3/28).
Local recurrent tumor
The tumor was located in the dermis to superficial
subcutis and was composed of clusters of uniform tumor cells with
slightly enlarged nuclei floating in extracellular mucin (Fig. 4). These histopathological features
were consistent with local recurrence of mucinous carcinoma of the
breast. No NEC component was present.
Liver tumor
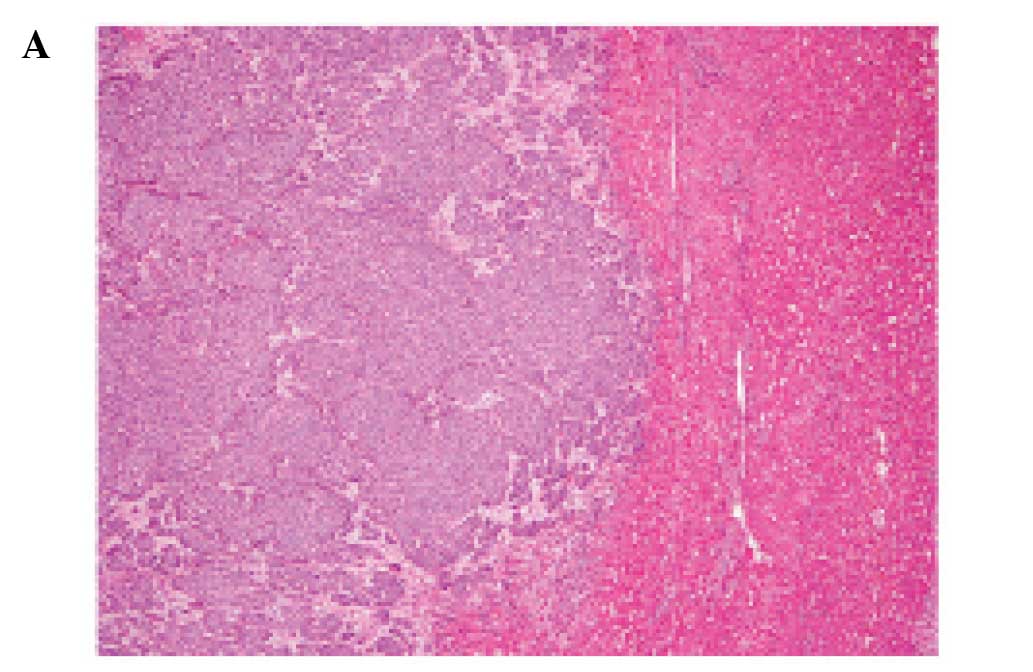
The tumor was relatively well-circumscribed from
surrounding liver tissue and separated by delicate fibrovascular
stroma (Fig. 5A). The tumor was
composed of solid nests of slightly enlarged oval nuclei with
inconspicuous nucleoli and slightly eosinophilic cytoplasm
(Fig. 5B). Mitotic figures were
easily detected (47/10 high power fields).
Immunohistochemical analyses revealed that these
tumor cells were diffusely (>95% of the tumor) positive for
chromogranin A (Fig. 6), although
CD56, synaptophysin and GCDFP-15 were not expressed. No mucinous
carcinoma component was present. Therefore, a diagnosis of
metastatic solid NEC of the breast in the liver was made. In
addition, ER and PgR were diffusely expressed (100% of the tumor)
and the HER2 score was 0.
Discussion
According to the WHO Classification, NEC of the
breast is classified into three subtypes: solid neuroendocrine
carcinoma, small cell/oat cell carcinoma, and large cell
neuroendocrine carcinoma (1). The
histopathological features of the present case corresponded to
solid NEC of the breast, which is the most common subtype (5), since the tumor was composed of solid
nests with or without central necrosis separated by delicate
fibrovascular stroma, which suggested neuroendocrine
differentiation, and no poorly differentiated component was
observed. López-Bonet et al summarized the
clinicopathological and immunohistochemical features of seven cases
of solid NEC of the breast (2).
Results of their study showed that solid NEC of the breast mainly
affects the elderly (the median age of the patients was 63), and
the frequency of axillary lymph node metastasis is relatively high
(3/6 cases underwent lymph node removal) (2). Immunohistochemically, ER and PgR were
positive in all cases, as in the present case (2), and overexpression of HER2 is uncommon
in NEC of the breast (2,5). Moreover, the expression pattern of
neuroendocrine markers is variable; all cases reported by
López-Bonet et al demonstrated positive immunoreactivity for
synaptophysin (>50% of tumor cells), but chromogranin A
expression was observed only focally in five of their seven cases
(the remaining two cases demonstrated no positive immunoreactivity
for chromogranin A) (2). By
contrast, Sapino et al reported that 53% of NEC expressed
chromogranin A (>50% of tumor cells) (6).
A noteworthy finding of the present case is the
coexistence of type B mucinous carcinoma and solid NEC within the
same breast tumor. Although mucinous carcinoma (particularly type
B) frequently shows neuroendocrine differentiation, the presence of
a dual (neuroendocrine and mucinous) divergent differentiation
within the same breast tumor is extremely rare (5). López-Bonet et al reported two
cases of solid NEC with a mucinous carcinoma component (the subtype
of the mucinous carcinoma is not available) (2). Thus, this is the third reported case
of solid NEC with mucinous carcinoma component of the breast.
Recently, Weigelt et al analyzed the
molecular characteristics of mucinous carcinoma and NEC of the
breast using genome-wide oligonucleotide microarrays (7). The study clearly revealed that no
differences in gene expression were present between type B mucinous
carcinoma and NEC, whether or not type A mucinous carcinoma
exhibited differences compared with type B mucinous carcinoma and
NEC (7). Taking these results into
consideration, the present case may represent NEC and type B
mucinous carcinoma as part of a spectrum with the same genetic
background.
The prognosis of solid NEC is thought to be better
than invasive ductal carcinoma, since in one report, none of the
investigated solid NEC (35 cases) had distant metastasis (5). In another study, only one of seven
cases of solid NEC demonstrated metastasis (soft tissue of the
cheek; the patient is alive with metastatic disease) (2). In addition, both patients with solid
NEC with mucinous carcinoma component reported by López-Bonet et
al are free from tumor recurrence (2). However, the present patient had liver
metastasis seven years after the surgery. Therefore, long-term
follow-up is necessary for patients with solid NEC of the breast
due to metastatic potential at a later stage.
References
|
1
|
Ellis IO, Schnitt SJ, Sastre-Garau X, et
al: Invasive breast carcinoma. World Health Organization of
Classification of Tumors. Pathology and Genetics of Tumours of the
Breast and Female Genital Organs. Tavassoli FA and Devilee P: IARC
Press; Lyon: pp. 30–34. 2003
|
|
2
|
López-Bonet E, Alonso-Ruano M, Barraza G,
Vazquez-Martin A, Bernadó L and Menendez JA: Solid neuroendocrine
breast carcinomas: Incidence, clinico-pathological features and
immunohistochemical profiling. Oncol Rep. 20:1369–1374.
2008.PubMed/NCBI
|
|
3
|
Capella C, Eusebi V, Mann B and Azzopardi
JG: Endocrine differentiation in mucoid carcinoma of the breast.
Histopathology. 4:613–630. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scopsi L, Andreola S, Pilotti S, et al:
Mucinous carcinoma of the breast: a clinicopathologic,
histochemical, and immunocytochemical study with special reference
to neuroendocrine differentiation. Am J Surg Pathol. 1994:702–711.
1994. View Article : Google Scholar
|
|
5
|
Righi L, Sapino A, Marchió C, Papotti M
and Bussolati G: Neuroendocrine differentiation in breast cancer:
established facts and unresolved problems. Semin Diagn Pathol.
27:69–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sapino A, Righi L, Cassoni P, Papotti M,
Gugliotta P and Bussolati G: Expression of apocrine differentiation
markers in neuroendocrine breast carcinomas of aged women. Mod
Pathol. 14:768–776. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weigelt B, Geyer FC, Horlings HM, Kreike
B, Halfwerk H and Reis-Filho JS: Mucinous and neuroendocrine breast
carcinomas are transcriptionally distinct from invasive ductal
carcinomas of no special type. Mod Pathol. 22:1401–1414. 2009.
View Article : Google Scholar : PubMed/NCBI
|