Introduction
Ovarian cancer is the fifth most common cause of
cancer-related mortality among women (1). Over the past two decades, despite
recent advances in surgery and chemotherapy, the overall cure rate
of ovarian cancer has remained approximately 30% (2). Taxol has been widely used in the
treatment of cancers, including ovarian cancer (3,4).
However, the multidrug resistance of tumor cells has been the main
cause for the failure of chemotherapy. Combination chemotherapy is
one of the strategies being used to overcome drug resistance. The
current standard of initial chemotherapy for ovarian cancer is
carboplatin and paclitaxel (5).
Previous studies have reported the inhibitory
effects of COX-2 inhibitors in combination with taxol on tumor
growth (6,7). The results demonstrated clear
antagonistic effects when COX-2 inhibitors were administered
concurrently with taxol. Cyclooxygenase (COX) is a key enzyme that
catalyzes the committed step in prostaglandin synthesis (8). Two enzyme isoforms of COX are known,
referred to as COX-1 and COX-2 (9).
COX-1 is constitutively expressed in most tissues and plays a role
in various physiological functions, whereas COX-2 is transiently
inducible by stimuli such as cytokines, growth factors, mitogens,
tumor promoters and hormones, and also regulates inflammation,
differentiation, mitogenesis and angiogenesis (8,9).
Previous findings showed that COX-1 is the predominant COX isoform
expressed in ovarian cancer (10–14).
These results suggest that COX-1 is a target for the prevention
and/or treatment of epithelial ovarian cancer. In addition,
numerous studies have shown that COX-1 selective inhibitors may
have potent antitumor activity in combating ovarian tumors
(10,12,13,15).
These results indicate that COX-1 is involved in the progression of
ovarian carcinoma and that COX-1 selective inhibitors may inhibit
tumor growth by inhibiting tumor angiogenesis, reducing cell
proliferation and/or accelerating apoptosis. However, studies of
the combined effects and antitumor mechanisms of COX-1 inhibitors
and taxol on ovarian cancer have not yet been reported.
In this study, we investigated the effects of
SC-560, a COX-1 inhibitor, combined with taxol, on the growth of
tumors, cell proliferation, apoptosis and angiogenesis using nude
mice transplanted with a human ovarian cancer SKOV-3 cell line as
an experimental model system.
Materials and methods
Human ovarian tumors in nude mice
In the present study, we used SKOV-3 cells to study
tumor growth in vivo. The SKOV-3 cells were implanted
subcutaneously into the dorsal skin (2×106 cells) of
female athymic nude mice (nu/nu, 7–8 weeks old). When the tumors
were visible (7 days after inoculation), the mice were randomly
divided into four groups, (n=6 mice per group): the SC-560 group,
the taxol group, the combination group (SC-560 plus taxol), and the
control group. SC-560 (Sigma Chemical Co., St. Louis, MO, USA) was
administered by oral gavage twice every other day at a dose of 6
mg/kg/day. Taxol (Bristol Myers Squibb SRL, Italy) was administered
by intraperitoneal injection once a week at a dose of 20 mg/kg. The
drugs were suspended in a 0.5 ml solution of 0.5% methycellulose
and 0.025% Tween-20 (both obtained from Sigma Chemical Co.). The
dose of SC-560 was selected for its specificity in inhibiting COX
isotypes (16). The control group
was administered 0.9% normal saline by gavage twice every other day
at a dose of 0.5 ml a time as well as 0.9% normal saline
administered intraperitoneally once a week at a dose of 0.5 ml. The
drugs or vehicle were administered over a period of 21 days,
commencing 7 days after the tumors became palpable.
A linear caliper was used to measure tumor
dimensions twice a week, and the tumor volume was calculated using
the equation V (mm3) = a ×
b2/2, where a is the largest diameter and
b is the smallest diameter (17). Tumor growth was evaluated by the
inhibition rate and calculated using the formula: IR = C -
T/C ×100%, where IR is the mean inhibition rate,
T is the mean tumor volume in the treatment group, and
C is the mean tumor volume in the control group. The animals
were weighed weekly throughout the experiment. On day 28, the mice
were sacrificed, and tumor tissue samples were collected and then
fixed in 10% phosphate-buffered formalin solution for
immunohistology or stored at −80°C until analysis. The tumor tissue
samples were snap-frozen in liquid nitrogen prior to storage at
−80°C.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from tissues using TRIzol
reagent from Invitrogen Life Technologies (Carlsbad, CA, USA),
according to the manufacturer’s instructions. Total RNA (5 μg) was
reverse-transcribed using SuperScript II from Invitrogen Life
Technologies according to the manufacturer’s instructions.
Transcribed products were subjected to PCR for VEGF (sense primer,
5′-atggacgtctaccagcgaag-3′; antisense primer,
5′-aatgctttctccgctctgaa-3′) and β-actin (sense primer,
5′-ttgctgacaggatgcagaag-3′; antisense primer, 5′-acatctgctgg
aaggtggac-3′). The oligodeoxynucleotides were synthesized on a
Model DNA synthesizer (Shenggong, Shanghai, China). Amplification
for VEGF cDNA started with a 3-min denaturation cycle at 94°C
followed by a 30-sec denaturation at 94°C, 30-sec annealing at
52°C, 30-sec extension at 72°C and a final extension at 72°C for 15
min. The PCR profile for β-actin consisted of a 3-min initial
denaturation cycle at 94°C followed by a 1-min denaturation at
94°C, 1-min annealing at 55°C and 1-min extension at 72°C. The PCR
mixture was then maintained at 72°C for 15 min for final extension.
The composition of the PCR mixture has been described previously
(18). Final PCR products were then
electrophoresed on 2% agarose gel and stained with ethidium
bromide. Ultraviolet (UV)-illuminated gels were captured using
Polaroid Type 667 films. Photographs were quantitated using a
Bio-Rad 2000 image scanner. The intensity of β-actin amplification
was used as an internal standard. The results of real-time PCR were
analyzed by the DCT method: ΔCT = CT selected gene - CT
β-actin, RQ (relative quantitation) = 2−ΔCT
×100%. The results of real-time PCR were presented as the ratio
between the selected genes and β-actin transcripts.
Immunohistochemistry
Immunohistochemistry was performed as previously
described (19). Sections were
deparaffinized and hydrated by sequential immersion in xylene and
graded alcohol solutions. Immunohistochemical staining was
performed using the streptavidin-biotin method. For the staining
for CD34, the sections were immersed in normal goat
serum for 34 min. Microvessel density (MVD) was evaluated according
to the method first described by Weidner et al (20). The entire tumor section was first
carefully scanned using low magnification light microscopy (x40) to
locate the area demonstrating the most intense neovascularization.
As the immunohistochemistry of CD34 revealed slight
heterogeneity within the same tumor, the five most highly
vascularized areas (hot spots) were selected in ×200 magnification
fields. The mean of five counts was calculated and used in the
statistical analysis. Sections of the tumor samples were also fixed
in 10% neutral-buffered formalin and processed for
immunohistochemistry (H&E staining). The proliferation index
was evaluated by staining for Ki-67. Following deparaffinization,
the tissue sections were heated at 121°C for 15 min in 10 mM Tris
HCl with 1 mM EDTA (pH 9.0). Endogenous peroxidase was blocked with
3% hydrogen peroxide in methanol for 10 min at room temperature.
The samples were incubated with Ki-67 antibody [clone MIB-5
(M7248)] for 90 min at room temperature. The sections were then
incubated in EnVision reagent for 40 min and
DAB/H2O2 for 8–12 min at room temperature.
Proliferation was assessed by counting the number of nuclei stained
positive for Ki-67 and the total number of cancer cells at a
magnification of ×400 in five representative regions of the tumor.
Results are expressed as the proportion of positively stained cells
over the total number of cells. The proliferation index was
calculated as: (number of Ki-67-labeled cells/total number of
cells) ×100%.
TUNEL assay
Terminal deoxynucleotidyl transferase-mediated dUTP
nick end labeling (TUNEL) assay was used to measure apoptosis using
a TUNEL kit from Chemicon Co. Beijing Zhongshan Biotechnology Co.,
China). TUNEL assay is a method of demonstrating apoptotic cell
death. The tissue samples were fixed in 4% paraformaldehyde (PFA)
for 24 h then dehydrated and embedded in paraffin. The
paraffin-embedded tissues were cut into 4-μm sections. Following
deparaffinization in a graded alcohol series, the tissue sections
were covered with 20 μg/ml proteinase K in phosphate-buffered
saline (PBS) for 15 min at room temperature, and endogenous
peroxidase activity was blocked. The samples were then incubated
with TdT enzyme and biotin-16-dUTP in TdT buffer containing 0.01%
bovine serum albumin for 1.5 h at 37°C in a humidity chamber. The
avidin-biotin complex (ABC) method was used to detect
biotin-16-dUTP nucleotides that had been incorporated into DNA
fragments using DAB as the chromogen. In each tissue specimen, five
high-power fields at ×400 magnification were randomly selected and
the apoptotic index (AI) was calculated as the percentage of
positive cells, using the equation: AI = (number of positive
cells/total number of cells) ×100% (21).
Statistical analysis
Statistical analysis was performed using SPSS
software (SPSS version 17.0, SPSS). Statistical significance
between the control and treated groups was determined using the
Student’s t-test. The experimental data were shown as the means ±
standard error (SE). P<0.05 was considered to indicate a
statistically significant result.
Results
Inhibition of ovarian cancer growth
Tumor growth increased throughout the study period
in the control group, whereas growth was gradually suppressed in
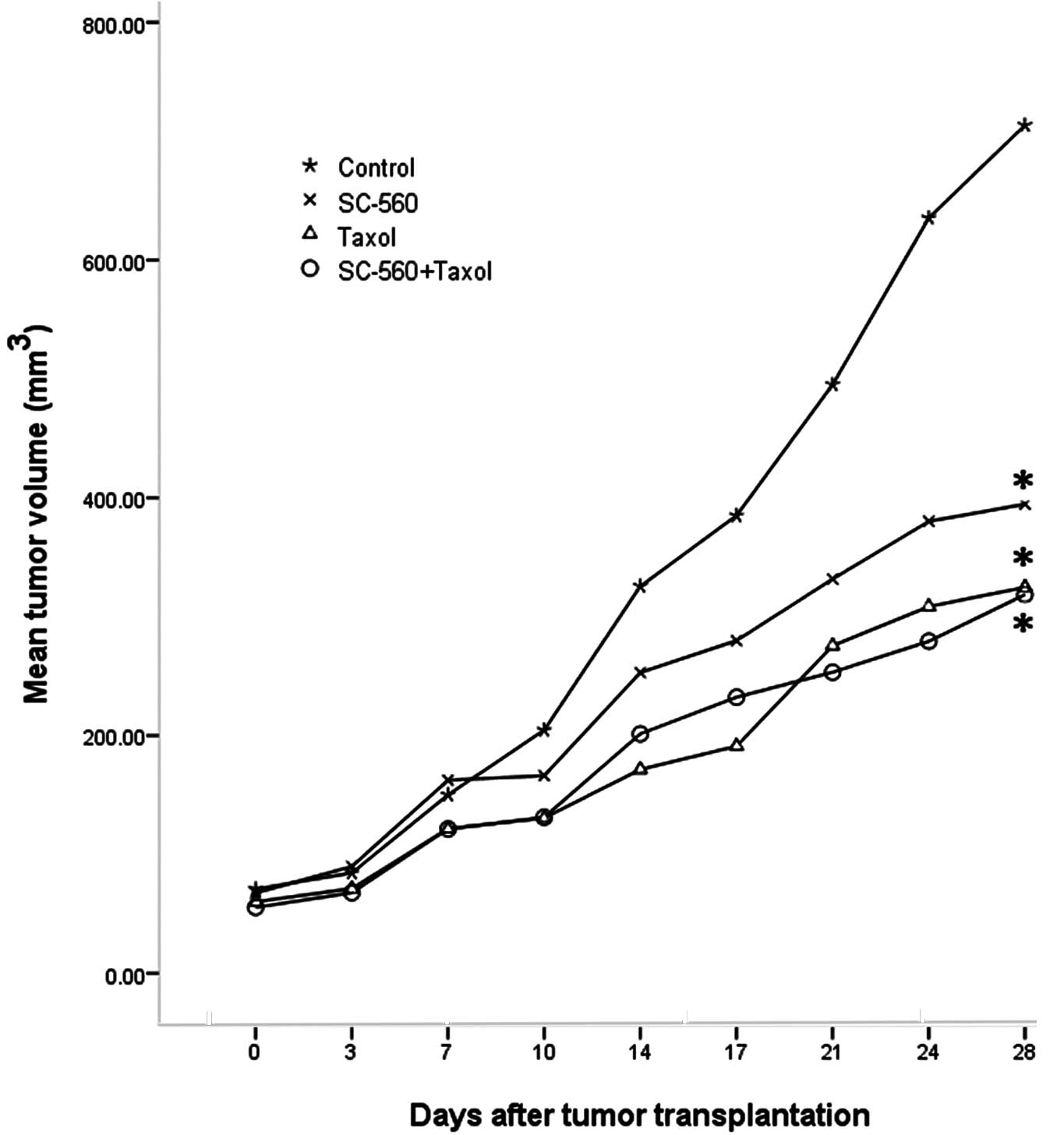
the treatment groups. Fig. 1 shows
the relative effect of SC-560 and/or taxol therapy. On day 28, the
tumor volume of mice in the SC-560, taxol and combination groups
was reduced by 44.67, 54.48 and 55.35%, respectively, compared with
the control mice. The inhibitory effect observed in the SC-560,
taxol and combination groups was statistically significant compared
with that of the control group (P<0.05 for all).
Effect on VEGF production
In this study, we measured VEGF levels in xenograft
tumors by real-time PCR analysis. Four molecular isoforms of VEGF
were generated by alternative splicing, rendering proteins
containing 206-, 189-, 165- and 121-amino acid residues (22). Although VEGF 206 transcripts were
not amplified, VEGF 189, 165 and 121 were routinely detected in
this series of ovarian cancer. Real-time PCR analysis indicated the
ΔCT (cycle threshold, = CT selected gene - CT
β-actin) of VEGF in the four groups (Table I). A comparison of the results of
the control and treatment groups revealed the expression levels of
VEGF mRNA to be significantly suppressed in the taxol (P<0.05)
and combination groups (P<0.01) (Fig. 2). The combination therapy
demonstrated a more synergistic effect than SC-560 on the
inhibition of mRNA expression (P<0.05).
 | Table IThe ΔCT of VEGF in the four groups
(control, SC-560, taxol and SC-560/taxol combination
group).a |
Table I
The ΔCT of VEGF in the four groups
(control, SC-560, taxol and SC-560/taxol combination
group).a
| VEGF | Control | SC-560 | Taxol | SC-560 + taxol |
|---|
| 121 | 6.94±0.23 | 7.13±0.30 | 7.87±0.32 | 9.14±0.33 |
| 165 | 4.58±0.26 | 5.34±0.30 | 6.28±0.49 | 7.00±0.48 |
| 189 | 6.34±0.23 | 6.54±0.21 | 7.79±0.29 | 8.99±0.23 |
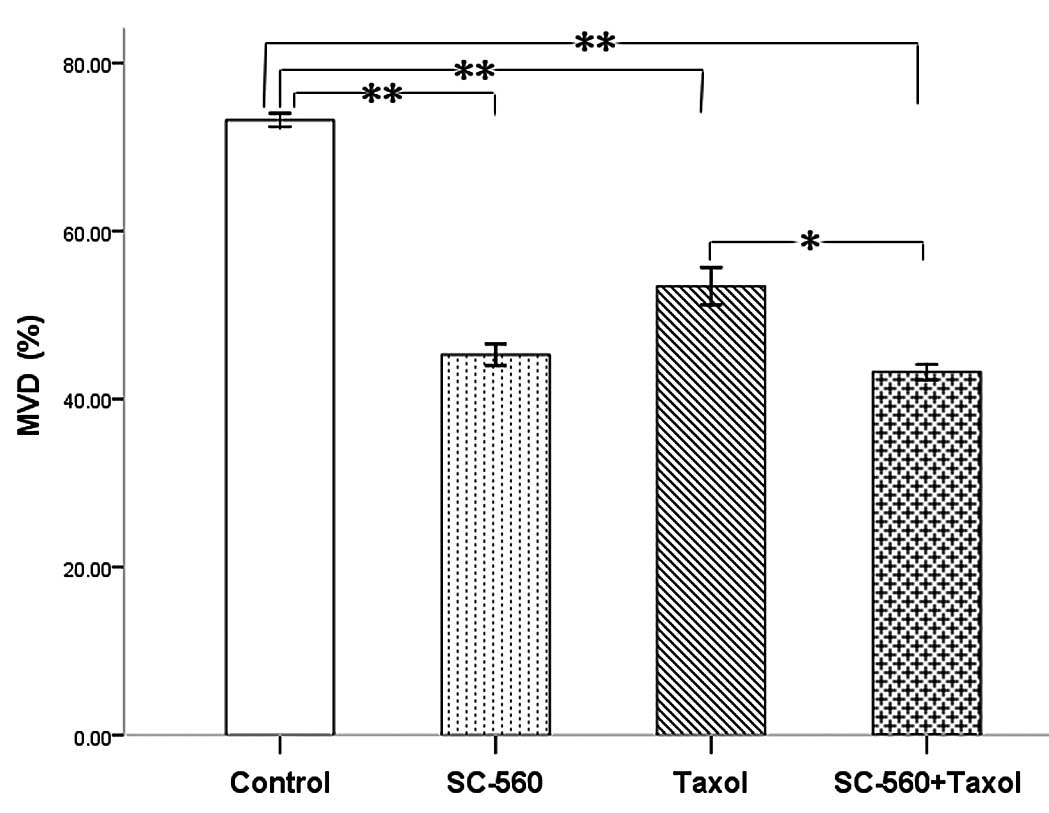
Effect on tumor blood vessels
To evaluate the consequence of antiangiogenic
therapy, we examined the residual tumors histologically.
Immunohistochemical analysis of frozen tumor sections revealed a
decrease in the number of CD34-positive microvessels in
mice treated with SC-560 and/or taxol. The MVD in the treatment
groups was 45.27±1.29% (SC-560), 53.43±2.22% (taxol) and
43.20±0.94% (SC-560/taxol), which was statistically significant
compared with that of the control group (73.20±0.80%) (P<0.01
for all; Fig. 3). In addition, the
SC-560/taxol combination therapy demonstrated a greater reduction
effect than taxol alone on MVD (P<0.05).
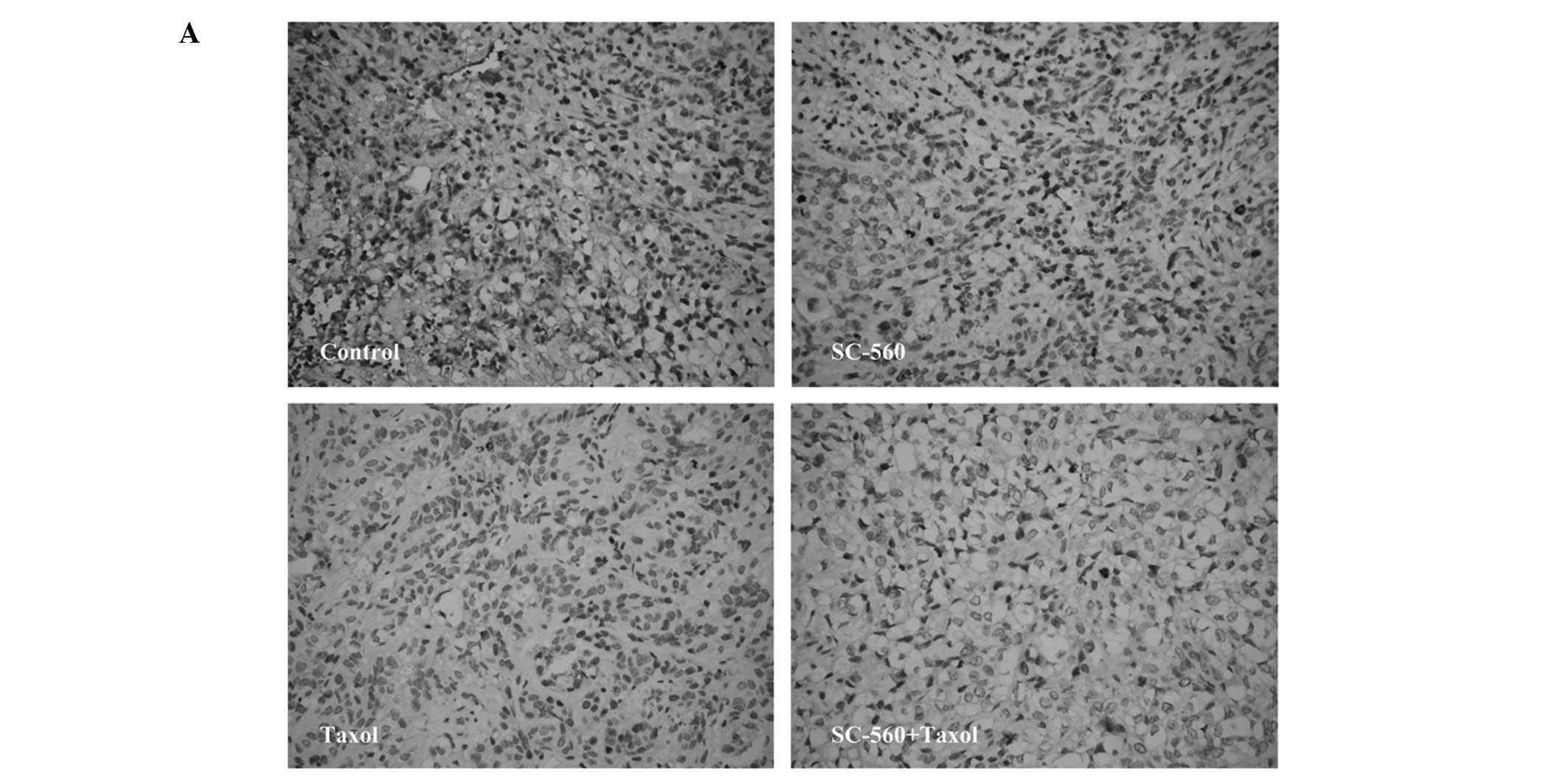
Effect on cell proliferation
We assessed cell growth in xenograft tumors in nude
mice treated with vehicle, SC-560, taxol and SC-560/taxol using
proliferation-associated nuclear antigen (Ki-67) staining. The
quantification of the Ki-67-positive cells in the tumors revealed
that SC-560 combined with taxol treatment in nude mice resulted in
a marked decrease in the proliferation index compared with the
control group (Fig. 4A). Data on
the proliferation index in the four groups are shown in Fig. 4B. In the SC-560 group, the
proliferation index was 12.00±2.13%, which is statistically
significant compared with that of the control group (24.67±4.61%,
P<0.05). The combination group demonstrated a notable reduction
in the proliferation index (8.67±1.15%) compared with the control
group (P<0.01). In addition, the combination group demonstrated
a greater reduction in the proliferation index compared with the
taxol group (P<0.05).
Effect on cell apoptosis
To evaluate the extent of apoptosis in tumor tissue
in the cancer-bearing mouse model, apoptotic cells were stained
using the TUNEL method and the number of apoptotic-positive cells
was counted in a high-power field. A notable increase in
apoptotic-positive cells was observed in the combined therapy group
compared with the control group (Fig.
5A). Data on the apoptotic index of the four groups are shown
in Fig. 5B. The apoptotic index was
56.17±2.09, 51.33±1.26 and 69.50±2.87% in the SC-560, taxol and
combination groups, respectively, which is statistically
significant compared with that of the control group (33.00±3.22%;
P<0.01 for all). In addition, the combination group demonstrated
a synergistic effect on the induction of cell apoptosis in tumors
compared with the SC-560 (P<0.05) and taxol group
(P<0.01).
Discussion
Numerous studies have demonstrated that COX
molecules are involved in the onset and progression of a variety of
malignancies and are overexpressed in ovarian cancer (13,23,24).
Other studies have found that COX-1, but not COX-2, is the
predominant COX isoform expressed in human ovarian cancer (11,12,15,25).
Additional evidence that an elevated COX-1 expression contributes
to cancer development has emerged through studies of ovarian cancer
using animal models (13,26). Thus, COX-1 is overexpressed in
ovarian cancer, suggesting that COX-1 production plays a role in
ovarian cancer development. The present study has shown that COX-1
selective inhibitors inhibited the growth of tumor cells by
inhibiting COX-1 activity, thereby reducing prostaglandin
I2 (PGI2) and PGE2 levels,
inhibiting the production of angiogenic factors and ultimately
impeding tumor angiogenesis (10,12,27).
Angiogenesis refers to the recruitment of new blood
vessels and forms an essential component of the metastatic pathway.
Numerous studies have indicated that angiogenesis is considered
essential for tumor growth and the development of metastases
(28–30). As one of the most important
angiogenic factors, VEGF stimulates the proliferation of
endothelial cells and also increases vascular permeability and
protein extravasations, which then provides nutrition and gas
exchange for the growth of tumor cells (31). VEGF has been shown to play a key
role in the growth and progression of ovarian cancer (32,33).
Our results have shown that SC-560 and taxol therapy inhibited VEGF
mRNA expression, and a combination therapy of the two drugs
demonstrated a synergistic effect. Previous studies have found that
the expression of COX-1 leads to an increased expression of VEGF
and that the inhibition of COX-1 reverses this response (15,34,35).
The above studies indicate that COX-1 inhibitors may indirectly
inhibit VEGF expression by inhibiting COX-1 expression. Combined
with previous research results, the present study indicates that
the decrease in tumor-associated VEGF by SC-560 and taxol may be a
crucial mechanism in controlling angiogenesis and inducing the
inhibition of overall tumor growth. Studies showed that
VEGF-positive tissue was associated with a high MVD expression,
whereas VEGF-negative tissue demonstrated a low expression of MVD,
indicating a positive correlation between VEGF and MVD (36,37).
The increase of MVD, an indirect marker of intense tumor
vascularization, increases blood volume (38), and increased MVD is known to be
associated with both evolution of disease and survival (39–41).
Our results indicated that SC-560 and/or taxol therapy caused a
marked reduction in MVD compared with vehicle-treated control, and
the combination therapy revealed a synergistic effect on the
inhibition of MVD. These results suggest that the potent
antiangiogenic activity of SC-560 combined with taxol is the
primary mechanism of inhibition of tumor growth in the animal model
of ovarian cancer.
The delicate balance between apoptosis and cell
proliferation is essential in controlling the cyclical growth of
the reproductive tissues and plays a significant role in the
prevention of neoplastic transformation (42,43).
Unrestricted cell proliferation and reduced apoptosis are hallmarks
of cancer cells (27). Our results
demonstrated that SC-560 combined with taxol therapy had a
synergistic effect on the inhibition of cell proliferation and
induction of apoptosis in tumors. Our previous study indicated that
SC-560 inhibited the PGE2 level by inhibiting the
expression of COX-1 in SKOV-3 ovarian carcinoma xenograft-bearing
mice (10). Munkarah et al
(44) found that PGE2
stimulated proliferation and inhibited cell apoptosis by promoting
tumor angiogenesis in epithelial ovarian cancer. The above studies
suggest that SC-560 reduces the PGE2 level by inhibiting
the production of COX-1, then inhibiting cell proliferation and
promoting apoptosis, and finally suppressing tumor growth. It is
understood that one of mechanisms by which taxol inhibits the
growth of ovarian cancer is by binding selectively and reversibly
to the B subunit of tubulin, promoting tubulin polymerization and
the formation of stable microtubules, causing cell cycle arrest at
the G2/M phase and eventually resulting in the inhibition of cell
proliferation by blocking cell division and cell death through an
apoptotic pathway (45). Therefore,
our results suggest that taxol supplemented by COX-1 inhibitors in
the treatment of ovarian cancer enhances the effect of taxol alone
on the inhibition of cell proliferation and induction of apoptosis,
in addition to inducing a synergistic inhibition effect on the
growth of ovarian cancer.
In conclusion, this study demonstrated that the
molecular mechanisms of the antitumor efficacy of SC-560 combined
with taxol therapy may act in part through the inhibition of tumor
angiogenesis, the reduction of cell proliferation and the induction
of cell apoptosis. However, whether COX-1 inhibitors combined with
taxol therapy can be adopted as a new chemotherapy regimen in the
treatment of ovarian cancer requires further investigation.
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: new opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jain A, Dubashi B, Reddy KS and Jain P:
Weekly paclitaxel in ovarian cancer - the latest success story.
Curr Oncol. 18:16–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kumar S, Mahdi H, Bryant C, Shah JP, Garg
G and Munkarah A: Clinical trials and progress with paclitaxel in
ovarian cancer. Int J Womens Health. 2:411–427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar
|
|
6
|
Bijman MN, Hermelink CA, van Berkel MP,
Laan AC, Janmaat ML, Peters GJ and Boven E: Interaction between
celecoxib and docetaxel or cisplatin in human cell lines of ovarian
cancer and colon cancer is independent of COX-2 expression levels.
Biochem Pharmacol. 75:427–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Munkarah AR, Ali-Fehmi R, Jiang JZ,
Elhammady E, Malone JM Jr and Saed GM: The effects of combining
docetaxel and cyclooxygenase-2 inhibitors on proliferation and
apoptosis in epithelial ovarian cancer. Anticancer Drugs.
18:889–896. 2007.PubMed/NCBI
|
|
8
|
Vane JR, Bakhle YS and Botting RM:
Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 38:97–120.
1998. View Article : Google Scholar
|
|
9
|
Smith WL, Garavito RM and DeWitt DL:
Prostaglandin endoperoxide H synthases (cyclooxygenases) -1 and -2.
J Biol Chem. 271:33157–33160. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Ji ZL, Zhuo GC, Xu RJ, Wang J and
Jiang HR: Effects of a selective cyclooxygenase-1 inhibitor in
SKOV-3 ovarian carcinoma xenograft-bearing mice. Med Oncol.
27:98–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dore M, Cote LC, Mitchell A and Sirois J:
Expression of prostaglandin G/H synthase type 1, but not type 2, in
human ovarian adenocarcinomas. J Histochem Cytochem. 46:77–84.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Daikoku T, Wang D, Tranguch S, Morrow JD,
Orsulic S, DuBois RN and Dey SK: Cyclooxygenase-1 is a potential
target for prevention and treatment of ovarian epithelial cancer.
Cancer Res. 65:3735–3744. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Daikoku T, Tranguch S, Trofimova IN,
Dinulescu DM, Jacks T, Nikitin AY, Connolly DC and Dey SK:
Cyclooxygenase-1 is overexpressed in multiple genetically
engineered mouse models of epithelial ovarian cancer. Cancer Res.
66:2527–2531. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Daikoku T, Tranguch S, Chakrabarty A, Wang
D, Khabele D, Orsulic S, Morrow JD, Dubois RN and Dey SK:
Extracellular signal-regulated kinase is a target of
cyclooxygenase-1-peroxisome proliferator-activated receptor-delta
signaling in epithelial ovarian cancer. Cancer Res. 67:5285–5292.
2007. View Article : Google Scholar
|
|
15
|
Gupta RA, Tejada LV, Tong BJ, Das SK,
Morrow JD, Dey SK and DuBois RN: Cyclooxygenase-1 is overexpressed
and promotes angiogenic growth factor production in ovarian cancer.
Cancer Res. 63:906–911. 2003.PubMed/NCBI
|
|
16
|
Reese J, Zhao X, Ma WG, Brown N, Maziasz
TJ and Dey SK: Comparative analysis of pharmacologic and/or genetic
disruption of cyclooxygenase-1 and cyclooxygenase-2 function in
female reproduction in mice. Endocrinology. 142:3198–3206.
2001.PubMed/NCBI
|
|
17
|
Williams CS, Watson AJM, Sheng H, Helou R,
Shao J and DuBois RN: Celecoxib prevents tumor growth in vivo
without toxicity to normal gut: lack of correlation between in
vitro and in vivo models. Cancer Res. 60:6045–6051. 2000.PubMed/NCBI
|
|
18
|
Seki A, Kodama J, Miyagi Y, Kamimura S,
Yoshinouchi M and Kudo T: Amplification of the mdm-2 gene and p53
abnormalities in uterine sarcomas. Int J Cancer. 73:33–37. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li W, Xu RJ, Jiang LH, Shi JF, Long X and
Fan B: Expression of cyclooxygenase-2 and inducible nitric oxide
synthase correlates with tumor angiogenesis in endometrial
carcinoma. Med Oncol. 22:63–70. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis: a new significant and independent prognostic
indicator in early-stage breast carcinoma. J Natl Cancer Inst.
84:1875–1887. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kitamura T, Itoh M, Noda T, Matsuura M and
Wakabayashi K: Combined effects of cyclooxygenase-1 and
cyclooxygenase-2 selective inhibitors on intestinal tumorigenesis
in adenomatous polyposis coli gene knockout mice. Int J Cancer.
109:576–580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ferrara N, Leung DW, Cachianes G, Winer J
and Henzel WL: Purification and cloning of vascular endothelial
growth factor secreted by pituitary folliculostellate cells.
Methods Enzymol. 198:391–405. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bernard MP, Bancos S, Sime PJ and Phipps
RP: Targeting cyclooxygenase-2 in hematological malignancies:
rationale and promise. Curr Pharm Des. 14:2051–2060. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ali-Fehmi R, Che M, Khalifeh I, Malone JM,
Morris R, Lawrence WD and Munkarah AR: The effect of
cyclooxygenase-2 expression on tumor vascularity in advanced stage
ovarian serous carcinoma. Cancer. 98:1423–1429. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khunnarong J, Tangjitgamol S,
Manusirivithaya S, Suekwattana P and Leelahakorn S: Expression of
cyclooxygenase-1 in epithelial ovarian cancer: a
clinicopathological study. Asian Pac J Cancer Prev. 9:757–762.
2008.PubMed/NCBI
|
|
26
|
Urick ME, Giles JR and Johnson PA: VEGF
expression and the effect of NSAIDs on ascites cell proliferation
in the hen model of ovarian cancer. Gynecol Oncol. 110:418–424.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li W, Wang J, Jiang HR, Xu XL, Zhang J,
Liu ML and Zhai LY: Combined effects of cyclooxygenase-1 and
cyclooxygenase-2 selective inhibitors on ovarian carcinoma in vivo.
Int J Mol Sci. 12:668–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harlozinska A, Sedlaczek P, Kulpa J,
Grybos M, Wójcik E, Van Dalen A and Einarsson R: Vascular
endothelial growth factor (VEGF) concentration in sera and tumor
effusions from patients with ovarian carcinoma. Anticancer Res.
24:1149–1157. 2004.PubMed/NCBI
|
|
29
|
Prager GW and Poettler M: Angiogenesis in
cancer. Basic mechanisms and therapeutic advances. Hamostaseologie.
32:Aug 12–2011.(Epub ahead of print).
|
|
30
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Senger DR, Van de Water L, Brown LF, Nagy
JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM and Dvorak HF:
Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer
Metastasis Rev. 12:303–324. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li L, Wang L, Zhang W, Tang B, Zhang J,
Song H, Yao D, Tang Y, Chen X, Yang Z, et al: Correlation of serum
VEGF levels with clinical stage, therapy efficacy, tumor metastasis
and patient survival in ovarian cancer. Anticancer Res.
24:1973–1979. 2004.PubMed/NCBI
|
|
33
|
Yamamoto S, Konishi I, Mandai M, Kuroda H,
Komatsu T, Nanbu K, Sakahara H and Mori T: Expression of vascular
endothelial growth factor (VEGF) in epithelia ovarian neoplasms:
correlation with clinicopathology and patient survival and analysis
of serum VEGF levels. Br J Cancer. 76:1221–1227. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
von Rahden BH, Stein HJ, Pühringer F, Koch
I, Langer R, Piontek G, Siewert JR, Höfler H and Sarbia M:
Coexpression of cyclooxygenases (COX-1, COX-2) and vascular
endothelial growth factors (VEGF-A, VEGF-C) in esophageal
adenocarcinoma. Cancer Res. 65:5038–5044. 2005.PubMed/NCBI
|
|
35
|
Li W, Xu RJ, Lin ZY, Zhuo GC and Zhang HH:
Effects of a cyclooxygenase-1-selective inhibitor in a mouse model
of ovarian cancer, administered alone or in combination with
ibuprofen, a nonselective cyclooxygenase inhibitor. Med Oncol.
26:170–177. 2009. View Article : Google Scholar
|
|
36
|
Li Y, Guo Z, Han YP and Guo XY:
Expressions of MVD, VEGF, Ki67 in residual prostate cancer after
cryoablation. Clin Oncol Cancer Res. 8:27–32. 2011.
|
|
37
|
Zhou YJ, Xiong YX, Wu XT, Shi D, Fan W,
Zhou T, Li YC and Huang X: Inactivation of PTEN is associated with
increased angiogenesis and VEGF overexpression in gastric cancer.
World J Gastroenterol. 10:3225–3229. 2004.PubMed/NCBI
|
|
38
|
Wang J, Lv F, Fei X, Cui Q, Wang L, Gao X,
Yuan Z, Lin Q, Lv Y and Liu A: Study on the characteristics of
contrast-enhanced ultrasound and its utility in assessing the
microvessel density in ovarian tumors or tumor-like lesions. Int J
Biol Sci. 7:600–606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Goodheart MJ, Ritchie JM, Rose SL,
Fruehauf JP, De Young BR and Buller RE: The relationship of
molecular markers of p53 function and angiogenesis to prognosis of
stage I epithelial ovarian cancer. Clin Cancer Res. 11:3733–3742.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guşet G, Costi S, Lazăr E, Dema A,
Cornianu M, Vernic C and Păiuşan L: Expression of vascular
endothelial growth factor (VEGF) and assessment of microvascular
density with CD34 as prognostic markers for endometrial carcinoma.
Rom J Morphol Embryol. 51:677–682. 2010.PubMed/NCBI
|
|
41
|
Cantu De León D, Lopez-Graniel C, Frias
Mendivil M, Chanona Vilchis G, Gomez C and De La Garza Salazar J:
Significance of microvascular density (MVD) in cervical cancer
recurrence. Int J Gynecol Cancer. 13:856–862. 2003.PubMed/NCBI
|
|
42
|
Meresman G: Relevance of apoptosis in the
female reproductive system. Invest Clin. 52:274–290.
2011.PubMed/NCBI
|
|
43
|
Forones NM, Carvalho AP, Giannotti-Filho
O, Lourenço LG and Oshima CT: Cell proliferation and apoptosis in
gastric cancer and intestinal metaplasia. Arq Gastroenterol.
42:30–34. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Munkarah AR, Morris R, Baumann P, Deppe G,
Malone J, Diamond MP and Saed GM: Effects of prostaglandin E(2) on
proliferation and apoptosis of epithelial ovarian cancer cells. J
Soc Gynecol Investig. 9:168–173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kumar S, Mahdi H, Bryant C, Shah JP, Garg
G and Munkarah A: Clinical trials and progress with paclitaxel in
ovarian cancer. Int J Womens Health. 2:411–427. 2010. View Article : Google Scholar : PubMed/NCBI
|