Introduction
Bladder cancer is the fourth most common cancer in
males in the USA (1). For
muscle-invasive bladder cancer, radical cystectomy (RC) and urinary
diversion are the gold standard of therapy in many parts of the
world (1–3). Neoadjuvant chemotherapy (NC) with
cisplatin-based combination chemotherapy significantly improved the
5-year survival rate in two meta-analysis studies (4,5) and
pathological complete response (pCR) with NC was correlated with
survival (6,7). Although a number of studies have
discussed factors which predict pCR or a favorable survival rate
(8–10), none of the factors has been proven
in a clinical study.
The excision repair cross-complementing group 1
(ERCC1) gene is located on chromosome 19q13.2-q13.3. The ERCC1
protein is crucial in the nucleotide excision repair pathway
(11,12). In a previous study, we reported that
ERCC1 may predict the prognosis of chemoradiotherapy for bladder
cancer and that it was correlated with radiation rather than
cisplatin resistance in an in vitro study (13). A correlation between ERCC1 and
bladder cancer has been reported (14–17),
but whether ERCC1 is capable of predicting a favorable survival
rate in patients with advanced bladder cancer treated with
cisplatin-based chemotherapy is controversial.
The endothelial-mesenchymal transition (EMT) is
significant in invasive bladder cancer (18,19).
Mesenchymal markers, including N-cadherin, Zeb1, Snail and Slug,
suppress the expression of E-cadherin and are correlated with
radiation and cisplatin resistance in numerous types of cancer
(20–22). In their study, Hsu et al
reported that Snail regulated the expression of ERCC1 and that the
co-expression of Snail and ERCC1 was a poor prognostic factor for
head and neck cancer treated with cisplatin-based chemotherapy
(21). In bladder cancer, however,
there have been no studies, to the best of our knowledge,
concerning the expression of ERCC1 and Snail as predictors of
prognosis for cisplatin-based chemotherapy.
In this study, we investigated the predictive and
prognostic roles of the expression of ERCC1 and Snail to determine
the response to chemotherapy in bladder cancer treated with
cisplatin-based NC and RC.
Materials and methods
Patients and samples
In total, 58 patients (50 men, 8 women; median age,
66.0 years; range, 34–78 years) diagnosed with bladder cancer
without organ metastasis and treated with NC and RC at Osaka
University or Osaka Rosai Hospital between 1997 and 2010 were
enrolled in this study. All 58 patients were clinically staged by
computerized tomography (CT) or magnetic resonance imaging (MRI) of
the chest, abdomen and pelvis and, following transurethral
resection of the bladder tumor, the stage and histological grade of
the tumors were determined according to the 5th edition of the TNM
classification. Patients were generally followed up postoperatively
every 3–4 months for 5 years following RC and every 6 months or
annually thereafter. Follow-up consisted of physical examination,
routine blood tests, abdominopelvic CT or MRI and chest
radiography. Bone scan and chest CT were performed according to the
decision of the physician. Approval for this study was obtained
from the local institutional review boards of Osaka University and
Osaka Rosai Hospital.
Analysis of immunohistochemical
staining
Expression of the ERCC1 and Snail proteins was
determined by immunohistochemical staining of paraffin-embedded
tissue sections of TUR specimens of the bladder tumor just prior to
the initiation of NC. Polyclonal anti-ERCC1 antibody was purchased
from Santa Cruz Biotechnology, Inc. (#FL297, Santa Cruz, CA, USA)
and the polyclonal anti-Snail antibody was purchased from Abcam
(#ab-63371, Cambridge, MA, USA). Briefly, sections (5-μm) were
deparaffinized, rehydrated using xylene and alcohol and incubated
with 0.3% H2O2 to block endogenous peroxidase
activity. Prior to ERCC1 immunostaining, antigen retrieval was
performed by immersing the sections in 10 mmol/l citrate buffer (pH
6.0) and boiling in steam for 20 min. Prior to Snail
immunostaining, antigen retrieval was performed by immersing the
sections in 10 mmol/l citrate buffer (pH 6.0) and antigen retrieval
was performed using a pressure chamber (Pascal, Dako, Kyoto, Japan)
in which tissues were maintained at 125˚C for 30 sec and cooled to
90˚C for 10 sec. The sections were then cooled at room temperature
for 10 min prior to incubation with the primary antibodies.
Immunohistochemistry for ERCC1 and Snail was performed with
anti-ERCC1 antibody (1:250 dilution) and anti-Snail antibody (1
μg/ml dilution) using the EnVision + detection system (Dako)
according to the manufacturer’s instructions. Primary antibody was
incubated for 60 min at room temperature and the slides were
counterstained with hematoxylin. Two independent investigators
(A.K. and H.T.), well-trained in genitourinary pathology and blind
to the clinical data, independently evaluated the immunostained
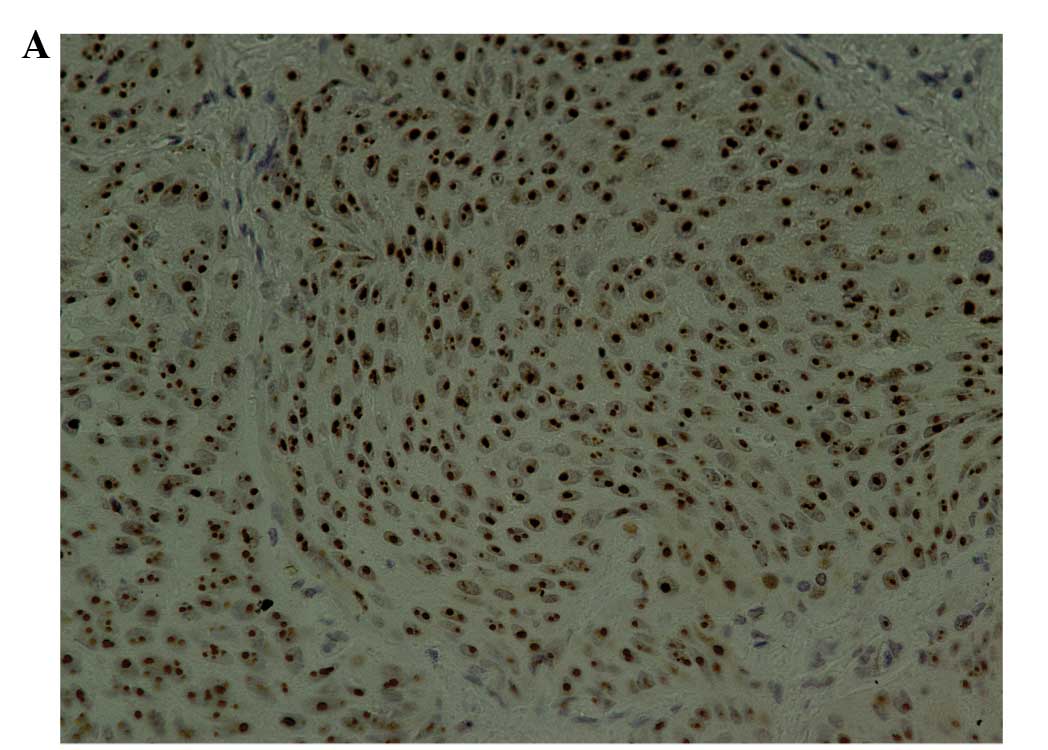
slides. ERCC1 nuclear expression was classified into four scoring
categories: 0, no expression in tumor cells; 1+, faint/barely
perceptible nuclear expression in <10% of tumor cells; 2+, weak
to moderate expression of the entire nucleus in >10% of tumor
cells; 3+, strong expression of the entire nucleus in >10% of
tumor cells. Scores of 2+ and 3+ were regarded as positive for
ERCC1 staining, as we previously reported (Fig. 1A) (13). Snail nuclear expression was
classified using the same four scoring categories as ERCC1
expression. The percentage of positive tumor nuclei was calculated
for each specimen and a proportion score was assigned (0 if 0%, 0.1
if 1–9%, 0.5 if 10–49% and 1.0 if ≥50%). This proportion score was
multiplied by the staining intensity of nuclei to obtain a final
semi-quantitative H score. Scores of 2+ and 3+ were regarded as
positive for Snail staining as previously reported (Fig. 1B) (21).
Statistical analysis
The primary outcomes of the patients with bladder
cancer were pathological response, overall survival (OS) and
disease-free survival (DFS). Estimates of OS and DFS were
calculated using the Kaplan-Meier method. The cohorts were defined
by age (<66 or ≥66 years), gender (male or female), clinical T
stage (T1, T2 or T3, T4), clinical N stage (negative or positive),
pathological histology (urothelial carcinoma only or other),
pathological tumor grade (grades 1, 2 or 3), chemotherapy regimen
[methotrexate, Adriamycin, vinblastine and cisplatin (M-VAC) or
gemcitabine and cisplatin (GC)], Snail expression (negative or
positive), ERCC1 expression (negative or positive) and
co-expression of Snail and ERCC1 (both positive or not both
positive). The associations between clinical response status and
the clinicopathological characteristics were evaluated using the
Fisher’s exact test and Pearson’s Chi-square test. For the
univariate analysis, survival rates were compared according to the
clinicopathological parameters previously mentioned. Prognostic
factors related to OS and DFS were analyzed with the Cox regression
analysis using a step-wise forward selection, with P<0.05 as the
criterion for model entry or stay for the multivariate analysis.
P<0.05 was considered to indicate a statistically significant
result. Statistical analyses were performed using the Statistical
Package for the Social Sciences software, version 16.0 (SPSS, Inc.,
Chicago, IL, USA).
Results
Patient characteristics
The clinical and pathological characteristics of the
58 patients with bladder cancer in this study are shown in Table I. Fifty-four patients had pure
urothelial carcinoma. Histological grade 3 cancer was present in 42
patients and the clinical T stage was T1 in 6 patients, T2 in 13,
T3 in 34 and T4 in 5. Twelve patients had pelvic lymph node
metastasis. Fifty patients were treated with M-VAC therapy (median
number of courses, 2) and 8 were treated with GC therapy (median
number of courses, 2). We assessed the efficacy of NC according to
the results of RC. A complete response (CR) was defined as pT0 (no
evidence of tumor). Twenty patients achieved CR.
 | Table IClinical and pathological
characteristics of 58 patients with bladder cancer treated with
neoadjuvant chemotherapy and radical cystectomy. |
Table I
Clinical and pathological
characteristics of 58 patients with bladder cancer treated with
neoadjuvant chemotherapy and radical cystectomy.
| Characteristics | Total (n=58) | Snail positive
(n=43) | Snail negative
(n=15) | ERCC1 positive
(n=25) | ERCC1 negative
(n=33) | Snail and ERCC1
positive (n=24) | Snail and/or ERCC1
negative (n=34) |
|---|
| Age, years
(median) | | P=0.357 | | P=0.332 | | P=0.254 | |
| 34–78 (66) | 34–78 (66) | 37–75 (63) | 42–78 (67) | 34–77 (64) | 42–78 (68) | 34–77 (64) |
| Gender | | P=0.357 | | P=0.671 | | P=0.594 | |
| Male | 50 | 36 | 14 | 21 | 29 | 20 | 30 |
| Female | 8 | 7 | 1 | 4 | 4 | 4 | 4 |
| Histology | | P=0.967 | | P=0.182 | | P=0.157 | |
| UC only | 54 | 40 | 14 | 22 | 32 | 21 | 33 |
| Others | 4 | 3 | 1 | 3 | 1 | 3 | 1 |
| Clinical T
stage | | P=0.463 | | P=0.320 | | P=0.096 | |
| T1 | 6 | 3 | 3 | 1 | 5 | 0 | 6 |
| T2 | 13 | 9 | 4 | 6 | 7 | 6 | 7 |
| T3 | 34 | 27 | 7 | 17 | 17 | 17 | 17 |
| T4 | 5 | 4 | 1 | 1 | 4 | 1 | 4 |
| Clinical N
stage | | P=0.507 | | P=0.588 | | P=0.496 | |
| N0 | 46 | 35 | 11 | 19 | 27 | 18 | 28 |
| N+ | 12 | 8 | 4 | 6 | 6 | 6 | 6 |
| Highest histology
grade | | P=0.563 | | P=0.261 | | P=0.118 | |
| G3 | 42 | 32 | 10 | 20 | 22 | 20 | 22 |
| G2 | 16 | 11 | 5 | 5 | 11 | 4 | 12 |
| Chemotherapy
regimen | | P=0.418 | | P=0.730 | | P=0.810 | |
| M-VAC | 50 | 38 | 12 | 22 | 28 | 21 | 29 |
| GC | 8 | 5 | 3 | 3 | 5 | 3 | 5 |
| Chemotherapy
response | | P=0.074 | | P=0.366 | | P=0.202 | |
| CR | 20 | 12 | 8 | 7 | 13 | 6 | 14 |
| Non-CR | 38 | 31 | 7 | 18 | 20 | 18 | 20 |
| ERCC1
expression | | P=0.001 | | | | | |
| Positive | 25 | 24 | 1 | | | | |
| Negative | 33 | 19 | 14 | | | | |
Snail was positively expressed in 43 patients
(74.1%) and ERCC1 was positively expressed in 25 patients (43.1%).
With regard to clinicopathological factors, there was no bias
between the patients with a positive and negative expression of
both markers. Notably, 24 of the 43 patients (55.8%) with a
positive Snail expression also had a positive ERCC1 expression and
14 patients (93.3%) with a negative Snail expression also had a
negative ERCC1 expression. A marked correlation was found between
Snail and ERCC1 expression (P=0.001).
The co-expression of Snail and ERCC1 was observed in
24 patients (41.4%), none of whom was at clinical T stage T1. There
was no bias between patients with and without co-expression. The
co-expression of Snail and ERCC1 was not able to predict pCR
(P=0.202). Similarly, the individual expression of either Snail or
ERCC1 (P=0.074 and P=0.366, respectively) was not able to predict
pCR.
Univariate analysis of predictive factors
for DFS and OS
Five-year DFS and OS rates were 60.4 and 66.3%,
respectively. In patients with a negative Snail expression, 5-year
DFS and OS rates were 84.0 and 88.9%, respectively, and those of
patients with a positive expression were 51.0 and 58.3%,
respectively (P=0.019 and P=0.023, respectively). Five-year DFS and
OS rates of patients with a negative expression of ERCC1 were 69.9
and 78.3%, respectively, whereas those of patients with a positive
expression were 47.3 and 49.8%, respectively (P=0.055 and 0.070,
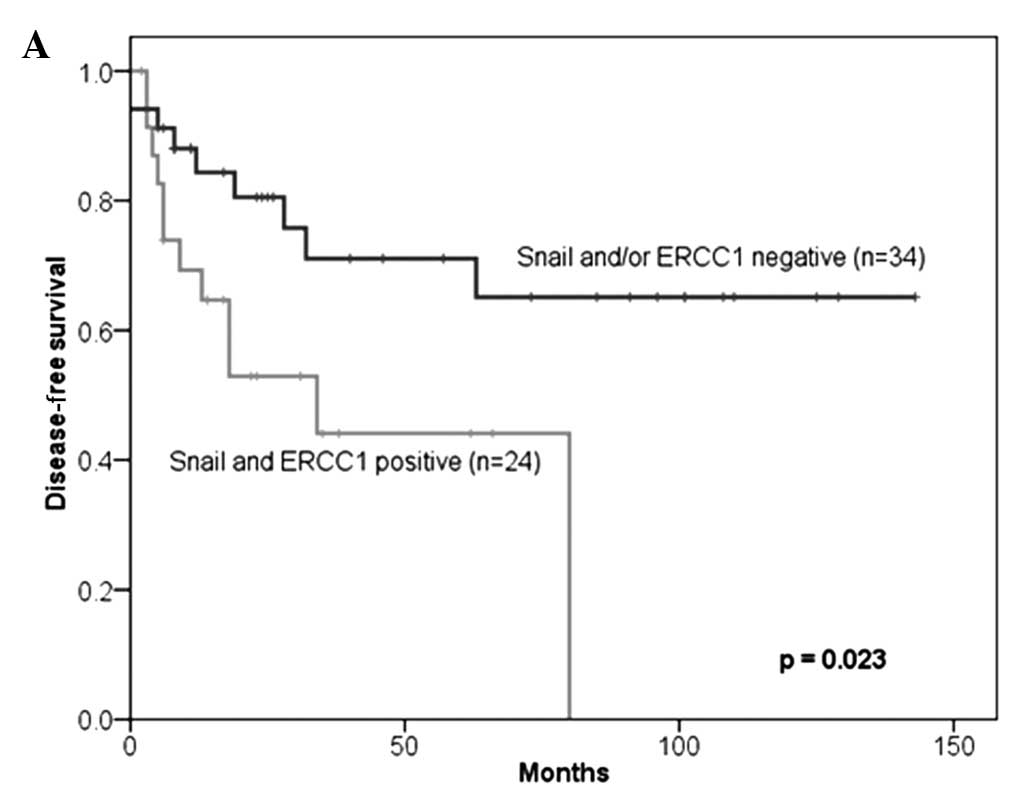
respectively). For patients with a co-expression of Snail and
ERCC1, 5-year DFS and OS rates were 44.1 and 46.3%, respectively,
and the median progression-free survival (PFS) and OS times were
34.0 and 37.0 months, respectively. The co-expression of Snail and
ERCC1 more accurately predicted shorter DFS and OS than the
negative expression of Snail and/or ERCC1 (P=0.023 and 0.031,
respectively; Fig. 2). Moreover, in
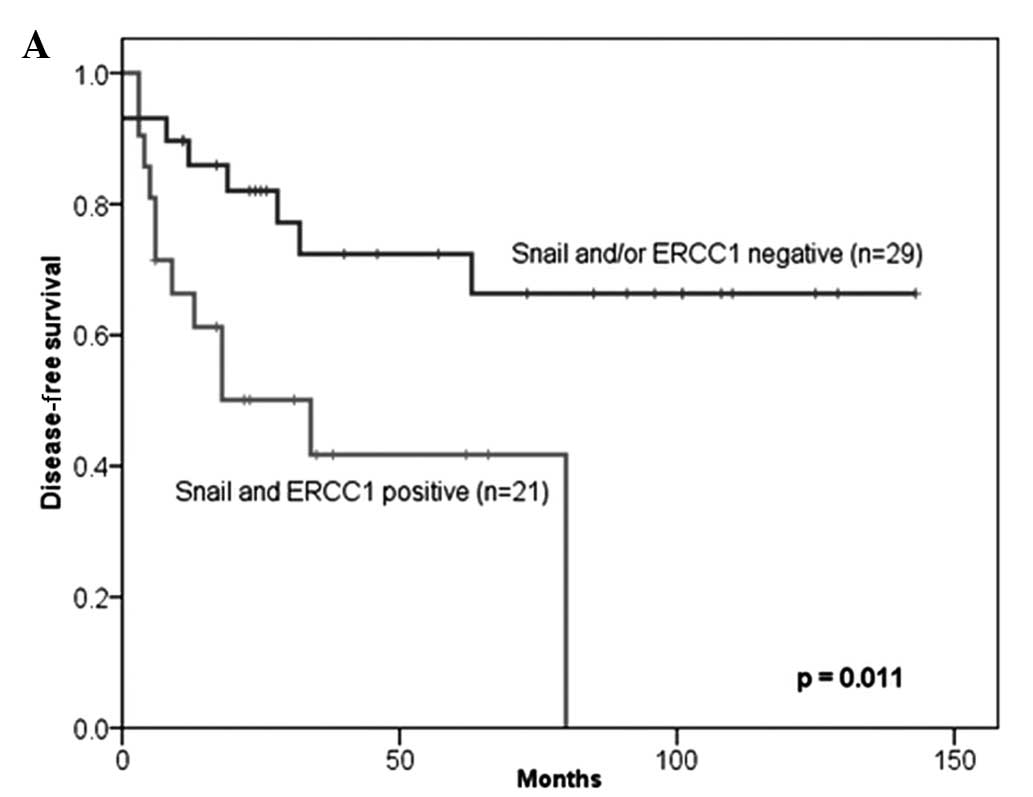
the patients treated only with the M-VAC regimen, the co-expression
of Snail and ERCC1 more accurately predicted shorter DFS and OS
than Snail and/or ERCC1 negative expression (P=0.011 and 0.029,
respectively; Fig. 3). Of the
remaining clinicopathological factors, only clinical T stage was a
significant prognostic factor for longer DFS and no factor
predicted the prognosis for OS (Table
II).
 | Table IIUnivariate and multivariate analysis
of predictive factors for DFS and OS in 58 patients treated with
cisplatin-based chemotherapy and radical cystectomy. |
Table II
Univariate and multivariate analysis
of predictive factors for DFS and OS in 58 patients treated with
cisplatin-based chemotherapy and radical cystectomy.
| DFS | OS |
|---|
|
|
|
|---|
| Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|---|
| Prognostic
factors | P-value | Exp | 95% CI | P-value | P-value | Exp | 95% CI | P-value |
|---|
| Age (years) |
| (<66 vs.
≥66) | 0.404 | - | - | - | 0.410 | - | - | - |
| Gender |
| (Female vs.
male) | 0.842 | - | - | - | 0.542 | - | - | - |
| Histology |
| (UC only vs.
others) | 0.571 | - | - | - | 0.404 | - | - | - |
| Clinical T
stage |
| (T1/T2 vs.
T3/T4) | 0.027 | - | - | - | 0.117 | - | - | - |
| Clinical N
stage |
| (N0 vs. N+) | 0.664 | - | - | - | 0.867 | - | - | - |
| Highest
histological grade |
| (Grade 3 vs. grade
2) | 0.250 | - | - | - | 0.668 | - | - | - |
| Chemotherapy
regimen |
| (M-VAC vs.
GC) | 0.731 | - | - | - | 0.649 | - | - | - |
| ERCC1/Snail
expression |
| (ERCC1 and Snail
positive vs. ERCC1 and/or Snail negative) | 0.023 | 2.688 | 1.106–6.529 | 0.029 | 0.031 | 2.864 | 1.050–7.806 | 0.040 |
Multivariate analysis of predictive
factors for PFS and OS
Snail expression was a predictive factor for DFS
[hazard ratio (HR), 4.893; 95% confidence interval (CI),
1.130–21.192; P=0.034] but not for OS (P=0.053; data not shown).
The co-expression of ERCC1 and Snail was a predictive factor for
DFS (HR, 2.688; 95% CI, 1.106–6.529; P=0.029) and OS (HR, 2.864;
95% CI, 1.050–7.806; P=0.040; Table
II). Moreover, in the patients treated only with the M-VAC
regimen, the co-expression of ERCC1 and Snail was also a predictive
factor for DFS (HR, 3.108; 95% CI, 1.237–7.807; P=0.016) and OS
(HR, 2.892; 95% CI, 1.062–7.876; P=0.038; Table III). The co-expression of Snail
and ERCC1 was the only significant factor involved in the
prediction of shorter DFS and OS by multivariate analysis.
 | Table IIIUnivariate and multivariate analysis
of predictive factors for DFS and OS in 50 patients treated with
M-VAC regimen chemotherapy and radical cystectomy. |
Table III
Univariate and multivariate analysis
of predictive factors for DFS and OS in 50 patients treated with
M-VAC regimen chemotherapy and radical cystectomy.
| DFS | OS |
|---|
|
|
|
|---|
| Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|---|
| Prognostic
factors | P-value | Exp | 95% CI | P-value | P-value | Exp | 95% CI | P-value |
|---|
| Age (years) |
| (<66 vs.
≥66) | 0.474 | - | - | - | 0.412 | - | - | - |
| Gender |
| (Female vs.
male) | 0.466 | - | - | - | 0.551 | - | - | - |
| Histology |
| (UC only vs.
others) | 0.599 | - | - | - | 0.417 | - | - | - |
| Clinical T
stage |
| (T1/T2 vs.
T3/T4) | 0.037 | - | - | - | 0.120 | - | - | - |
| Clinical N
stage |
| (N0 vs. N+) | 0.923 | - | - | - | 0.850 | - | - | - |
| Highest
histological grade |
| (Grade 3 vs. grade
2) | 0.355 | - | - | - | 0.687 | - | - | - |
| ERCC1/Snail
expression |
| (ERCC1 and Snail
positive vs. ERCC1 and/or Snail negative) | 0.011 | 3.108 | 1.237–7.807 | 0.016 | 0.029 | 2.892 | 1.062–7.876 | 0.038 |
Discussion
In the present study, we examined the expression of
Snail and ERCC1 in bladder cancer and found that the co-expression
of Snail and ERCC1 was the only significant factor for predicting
prognosis following NC and RC against bladder cancer.
ERCC1 is crucial in the nucleotide excision repair
pathway and an association of different cancer cell lines with
resistance to platinum compounds has been suggested (11,12).
In a clinical study using immunohistochemistry, Olaussen et
al reported that patients with ERCC1-negative non-small-cell
lung cancer appeared to benefit from adjuvant cisplatin-based
chemotherapy, whereas patients with ERCC1-positive tumors did not
(23). In a clinical study of
bladder cancer, a number of studies addressed the correlation
between ERCC1 and prognosis (14–17).
However, the role of ERCC1 in predicting the prognosis for advanced
bladder cancer was controversial. In particular, no studies have
discussed whether ERCC1 was capable of predicting CR and prognosis
for NC against bladder cancer. Previously, we reported that ERCC1
might be more resistant to radiation exposure than cisplatin and
may be a predictive factor for chemoradiotherapy against
muscle-invasive bladder cancer (13). In the present study, the expression
of ERCC1 alone was not found to be either a predictive or
prognostic factor for NC and RC against bladder cancer by
univariate and multivariate analyses (Table I). This result was supported by our
earlier in vitro results as the majority of the patients in
the present study were not treated with radiation therapy.
EMT has been reported to be significant in invasive
bladder cancer (18,19). Snail, one of the markers of EMT, has
been reported to predict the intravesical recurrence of superficial
bladder cancer (24). However,
there have been no studies, to the best of our knowledge,
concerning the chemoresistance of Snail in bladder cancer. Thus, we
examined Snail expression in bladder cancer patients treated with
NC and RC and found that Snail was highly expressed in bladder
cancer (74.1%). This expression may be due to most patients having
invasive bladder cancer, although there was no bias between Snail
expression and clinical T stage. A negative Snail expression was
also a significant predictive factor of longer DFS and OS in the
univariate analysis. In the multivariate analysis, however, Snail
expression was able to predict longer DFS but not OS. One possible
reason for this is that the rate of positive expression of Snail
was high. Another is that Snail may be correlated with angiogenesis
and occult metastasis. In urothelial carcinoma, Kosaka et al
reported that Snail may be correlated with the angiogenesis and
prognosis of invasive upper urinary tract carcinoma (25). Therefore, Snail expression may be
significant in the prediction of DFS in bladder cancer, as the
results of the present study indicate.
Hsu et al have reported that Snail directly
regulates ERCC1 expression and that the co-expression of Snail and
ERCC1 is a poor prognostic factor in head and neck cancer (21). Therefore, we examined the
correlation between the co-expression of Snail and ERCC1 and the
prognosis of bladder cancer. Notably, Snail expression was markedly
correlated with ERCC1 expression (P=0.001; Table I) and the co-expression of Snail and
ERCC1 was a significant prognostic factor to predict longer DFS and
OS in univariate and multivariate analysis (Table II). This co-expression was also a
significant prognostic factor in the 50 patients treated with M-VAC
therapy (Table III).
NC and RC have been recommended for invasive bladder
cancer (4,5) and it is crucial to be able to predict
the CR or prognosis of these therapies. CR to NC has been reported
to be a significant prognostic factor in several studies (6,7).
Takata et al reported that 14 genes were found to be
predictive of pCR and may be prognostic factors in patients with
bladder cancer treated with neoadjuvant M-VAC therapy (10,26).
With regard to DNA repair genes, BRCA1 mRNA was reported to predict
the efficacy of cisplatin-based NC (8). In the present study, although the
co-expression of Snail and ERCC1 was not a significant factor for
the prediction of CR to NC, notably, the co-expression of the
proteins, and not just the individual expression of ERCC1 or Snail,
was identified as significant prognostic factors for shorter DFS
and OS in the patients treated with a cisplatin-based regimen and
also those treated with the M-VAC regimen.
One limitation of the present study is that we did
not examine the correlation between Snail and ERCC1 in an in
vitro study and did not examine other markers such as ERK1/2,
which has been reported to regulate Snail expression (27). Another limitation is that the sample
size for immunohistochemical analysis was small and the study was
retrospective. More detailed studies are needed in the future to
address these limitations.
The results of the present study suggest that the
expression of Snail was correlated with that of ERCC1 and the
co-expression of Snail and ERCC1 was a significant factor for
predicting shorter DFS and OS in bladder cancer treated with NC and
RC. Moreover, Snail may be crucial in the progression of bladder
cancer treated with cisplatin-based chemotherapy. Further
prospective studies are required to confirm the results of this
retrospective study.
Acknowledgements
This study was supported by a Young Researcher
Promotion Grant in 2011 to A. Kawashima.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics. CA Cancer J Clin. 59:225–249. 2009.
|
|
2
|
Committee for Establishment of the
Clinical Practice Guidelines for the Management of Bladder Cancer
and the Japanese Urological Association. Evidence-based clinical
practice guidelines for bladder cancer (summary - JUA 2009
Edition). Int J Urol. 17:102–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stenzl A, Cowan NC, De Santis M, et al:
The updated EAU guidelines on muscle-invasive and metastatic
bladder cancer. Eur Urol. 55:815–825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Advanced Bladder Cancer (ABC)
Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive
bladder cancer: update of a systematic review and meta-analysis of
individual patient data. Eur Urol. 48:202–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winquist E, Kirchner TS, Segal R, Chin J
and Lukka H: Neoadjuvant chemotherapy for transitional cell
carcinoma of the bladder: a systematic review and meta-analysis. J
Urol. 171:561–569. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grossman HB, Natale RB, Tangen CM, et al:
Neoadjuvant chemotherapy plus cystectomy compared with cystectomy
alone for locally advanced bladder cancer. N Engl J Med.
349:859–866. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsui Y, Nishiyama H, Watanabe J, et al:
The current status of perioperative chemotherapy for invasive
bladder cancer: a multiinstitutional retrospective study in Japan.
Int J Clin Oncol. 10:133–138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Font A, Taron M, Gago JL, et al: BRCA1
mRNA expression and outcome to neoadjuvant cisplatin-based
chemotherapy in bladder cancer. Ann Oncol. 22:139–144. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pinho MB, Costas F, Sellos J, et al: XAF1
mRNA expression improves progression-free and overall survival for
patients with advanced bladder cancer treated with neoadjuvant
chemotherapy. Urol Oncol. 27:382–390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takata R, Katagiri T, Kanehira M, et al:
Validation study of the prediction system for clinical response of
M-VAC neoadjuvant chemotherapy. Cancer Sci. 98:113–117. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gossage L and Madhusudan S: Current status
of excision repair cross complementing-group 1 (ERCC1) in cancer.
Cancer Treat Rev. 33:565–577. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park CH, Bessho T, Matsunaga T and Sancar
A: Purification and characterization of the XPF-ERCC1 complex of
human DNA repair excision nuclease. J Biol Chem. 270:22657–22660.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawashima A, Nakayama M, Kakuta Y, et al:
Excision repair cross-complementing group 1 may predict the
efficacy of chemoradiation therapy for muscle-invasive bladder
cancer. Clin Cancer Res. 17:2561–2569. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bellmunt J, Paz-Ares L, Cuello M, et al:
Gene expression of ERCC1 as a novel prognostic marker in advanced
bladder cancer patients receiving cisplatin-based chemotherapy. Ann
Oncol. 18:522–528. 2007. View Article : Google Scholar
|
|
15
|
Hoffmann AC, Wild P, Leicht C, et al: MDR1
and ERCC1 expression predict outcome of patients with locally
advanced bladder cancer receiving adjuvant chemotherapy. Neoplasia.
12:628–636. 2010.PubMed/NCBI
|
|
16
|
Kim KH, Do IG, Kim HS, et al: Excision
repair cross-complementation group 1 (ERCC1) expression in advanced
urothelial carcinoma patients receiving cisplatin-based
chemotherapy. APMIS. 118:941–948. 2010. View Article : Google Scholar
|
|
17
|
Matsumura N, Nakamura Y, Kohjimoto Y, et
al: The prognostic significance of human equilibrative nucleoside
transporter 1 expression in patients with metastatic bladder cancer
treated with gemcitabine-cisplatin-based combination chemotherapy.
BJU Int. Dec 16–2010.(E-pub ahead of print). View Article : Google Scholar
|
|
18
|
Baumgart E, Cohen MS, Silva Neto B, et al:
Identification and prognostic significance of an
epithelial-mesenchymal transition expression profile in human
bladder tumors. Clin Cancer Res. 13:1685–1694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McConkey DJ, Choi W, Marquis L, et al:
Role of epithelial-to-mesenchymal transition (EMT) in drug
sensitivity and metastasis in bladder cancer. Cancer Metastasis
Rev. 28:335–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arumugam T, Ramachandran V, Fournier KF,
et al: Epithelial to mesenchymal transition contributes to drug
resistance in pancreatic cancer. Cancer Res. 69:5820–5828. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsu DS, Lan HY, Huang CH, et al:
Regulation of excision repair cross-complementation group 1 by
Snail contributes to cisplatin resistance in head and neck cancer.
Clin Cancer Res. 16:4561–4571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sayan AE, Griffiths TR, Pal R, et al: SIP1
protein protects cells from DNA damage-induced apoptosis and has
independent prognostic value in bladder cancer. Proc Natl Acad Sci
USA. 106:14884–14889. 2009. View Article : Google Scholar
|
|
23
|
Olaussen KA, Dunant A, Fouret P, et al:
DNA repair by ERCC1 in non-small-cell lung cancer and
cisplatin-based adjuvant chemotherapy. N Engl J Med. 355:983–991.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bruyere F, Namdarian B, Corcoran NM, et
al: Snail expression is an independent predictor of tumor
recurrence in superficial bladder cancers. Urol Oncol. 28:591–596.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kosaka T, Kikuchi E, Mikami S, et al:
Expression of snail in upper urinary tract urothelial carcinoma:
prognostic significance and implications for tumor invasion. Clin
Cancer Res. 16:5814–5823. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takata R, Katagiri T, Kanehira M, et al:
Predicting response to methotrexate, vinblastine, doxorubicin, and
cisplatin neoadjuvant chemotherapy for bladder cancers through
genome-wide gene expression profiling. Clin Cancer Res.
11:2625–2636. 2005. View Article : Google Scholar
|
|
27
|
Ko JC, Su YJ, Lin ST, et al: Suppression
of ERCC1 and Rad51 expression through ERK1/2 inactivation is
essential in emodin-mediated cytotoxicity in human non-small cell
lung cancer cells. Biochem Pharmacol. 79:655–664. 2010. View Article : Google Scholar : PubMed/NCBI
|