Introduction
Due to an increased rate in the accurate diagnosis
of early gastric cancer (EGC), in which invasion is confined to
either the mucosa or submucosa, regardless of the presence or
absence of regional lymph node metastasis (LNM), and subsequently
improved prognosis, increased interest has focused on improving the
quality of life and minimizing invasive procedures. Endoscopic
mucosal resection (EMR) has been widely used for the treatment of
ECG (1,2). EMR is now considered to be sufficient
treatment for histopathologically differentiated, non-ulcerated
intramucosal gastric cancer smaller than 2 cm (3), as such cancer rarely metastasizes to
the lymph nodes (4,5). However, in certain cases of EGC,
submucosal gastric cancer (SGC) is later revealed upon
histopathological examination of the specimen obtained by EMR
(6–8). In such cases, additional gastrectomy
with lymphadenectomy is considered the standard therapy (9,10),
even if the gastric tumor lesion has been completely excised by EMR
in consideration of the high rate (approximately 20%) of LNM
(11,12). However, LNM is not present in
approximately 80% of surgical cases of SGC (11,12),
thus gastrectomy with lymphadenectomy may be overtreatment for
these cases.
Therefore, we conducted a retrospective study to
determine the clinicopathological characteristics predictive of LNM
in differentiated SGC. Furthermore, we established a simple
criterion to indicate additional surgical treatment in
differentiated SGC cases revealed following EMR.
Patients and methods
Patients
In this retrospective study, patients who had
undergone radical surgery due to EGC in the Department of Surgical
Oncology of the Affiliated Xingtai People’s Hospital of Hebei
Medical University, Xingtai, China, between January 1985 and
December 2006 were screened for the identification of SGC cases
revealed following EMR.
The inclusion criteria for this study were: i) lymph
node dissection beyond limited (D1) dissection (D1 dissection and
dissection of lymph nodes along the left gastric artery, D1
dissection and dissection of lymph nodes along the common hepatic
artery, D1 dissection and dissection of lymph nodes along the
celiac artery), or extended (D2) dissection was performed; ii)
resected specimens and lymph nodes that had been pathologically
analyzed and diagnosed as SGC; iii) histopathologically-classified
SGC as the differentiated type, according to the Japanese
Classification of Gastric Carcinoma (JCGC) (13); and iv) availability of the patient’s
medical records.
During the period mentioned, radical surgery was
performed in 293 patients with EGC. Of these patients, 163 patients
were histologically diagnosed as SGC; 73 as differentiated SGC and
90 as undifferentiated SGC. Among the 73 patients with
differentiated SGC, medical records were not completely available
for three cases. Thus, 70 patients (60 males and 10 females; mean
age, 59 years; range, 33–80 years) with histopathologically
differentiated-type SGC met the inclusion criteria for the study.
The study protocol was approved by the Ethics Committee of Hebei
Medical University.
Surgical dissection of lymph nodes
The lymph nodes of each case were meticulously
dissected from the en bloc specimens, and the classification of the
dissected lymph nodes was determined by a surgeon following
examination of the excised specimens based on the JCGC (13). The resected lymph nodes were then
sectioned, stained with hematoxylin and eosin, and examined by
pathologists for metastasis and lymphatic vessel involvement.
Association between clinicopathological
parameters and LNM
Clinicopathological parameters investigated in this
study were selected according to the JCGC (13). These characteristics included,
gender (male and female), age (<60 years and ≥60 years), family
medical history of gastric cancer, number of tumors (single or
multitude), location of the tumor (upper, middle or lower section
of the stomach), tumor size (maximum dimension, <2 or ≥2 cm),
macroscopic type [protruded (type I)], superficial elevated (type
IIa), flat (type IIb), superficial depressed (type IIc) or
excavated (type III)], histopathological type (well-differentiated,
moderately differentiated or papillary adenocarcinoma), lymphatic
vessel involvement, presence of intermingled components of
undifferentiated cancer cells (poorly differentiated
adenocarcinoma, signet-ring-cell carcinoma or mucinous
adenocarcinoma).
Statistical analysis
Data were analyzed using SPSS 13.0 statistical
software (SPSS Inc., Chicago, IL, USA). The differences in the
clinicopathological parameters between patients with and without
LNM were determined by the χ2 test. A multivariate
stepwise logistic regression analysis was performed to identify
independent risk factors of LNM. The hazard ratio and 95%
confidence interval (CI) were calculated. P<0.05 was
considered to indicate a statistically significant difference.
Results
Association between the
clinicopathological parameters and LNM
The association between various clinicopathological
characteristics and LNM was first analyzed using the χ2
test (Table I). Tumor size,
lymphatic vessel involvement and presence of intermingled
components of undifferentiated cancer cells were significantly
associated with a higher rate of LNM (P<0.05). However, gender,
age, family medical history of gastric cancer, number of tumors,
location, macroscopic type and histological type of the tumor were
not found to be associated with LNM.
 | Table IUnivariate analysis of potential risk
characteristics for lymph node metastasis. |
Table I
Univariate analysis of potential risk
characteristics for lymph node metastasis.
| Characteristics | No. of cases | Positive no. (%) of
lymph node metastasis | P-value |
|---|
| Gender |
| Male | 60 | 8 (13.3) | 0.180 |
| Female | 10 | 3 (30) | |
| Age (years) |
| <60 | 33 | 3 (9.1) | 0.150 |
| ≥60 | 37 | 8 (21.6) | |
| Family medical
history |
| Positive | 13 | 3 (23.1) | 0.419 |
| Negative | 57 | 8 (14.0) | |
| No. of tumors |
| Single | 67 | 10 (14.9) | 0.391 |
| Multitude | 3 | 1 (33.3) | |
| Location |
| Upper | 4 | 1 (25) | 0.419 |
| Middle | 11 | 3 (27.3) | |
| Lower | 55 | 7 (12.7) | |
| Tumor size in
diameter (cm) |
| <2 | 35 | 2 (5.7) | 0.022 |
| ≥2 | 35 | 9 (25.7) | |
| Macroscopic type |
| I | 3 | 1 (33.3) | 0.514 |
| II | 41 | 5 (12.2) | |
| III | 26 | 5 (19.2) | |
| Histological
type |
|
Well-differentiated | 33 | 5 (15.2) | 0.450 |
| Moderately
differentiated | 31 | 4 (12.9) | |
| Papillary
adenocarcinoma | 6 | 2 (33.3) | |
| Lymphatic vessel
involvement |
| Negative | 61 | 5 (8.2) | <0.001 |
| Positive | 9 | 6 (66.7) | |
| Undifferentiated
componenta |
| Absence | 51 | 3 (5.9) | <0.001 |
| Presence | 19 | 8 (42.1) | |
Multivariate analysis of potential
independent clinicopathological risk factors for LNM
Of the three characteristics that were significantly
associated with LNM by univariate analysis, lymphatic vessel
involvement and presence of intermingled components of
undifferentiated cancer cells were found to be significant and
independent risk factors for LNM by multivariate analysis
(P<0.05) (Table II).
 | Table IIMultivariate analysis of potential
risk factors for lymph node metastasis. |
Table II
Multivariate analysis of potential
risk factors for lymph node metastasis.
| Characteristics | Hazard ratio | 95% Confidence
interval | P-value |
|---|
| Tumor size (cm) |
| <2 vs. ≥2 | 1.375 | 0.146–12.980 | 0.781 |
| Lymphatic vessel
involvement | | | |
| Negative vs.
positive | 392.269 | 1.380–1115.032 | 0.038 |
| Undifferentiated
component |
| Absence vs.
presence | 98.515 | 2.687–3612.400 | 0.012 |
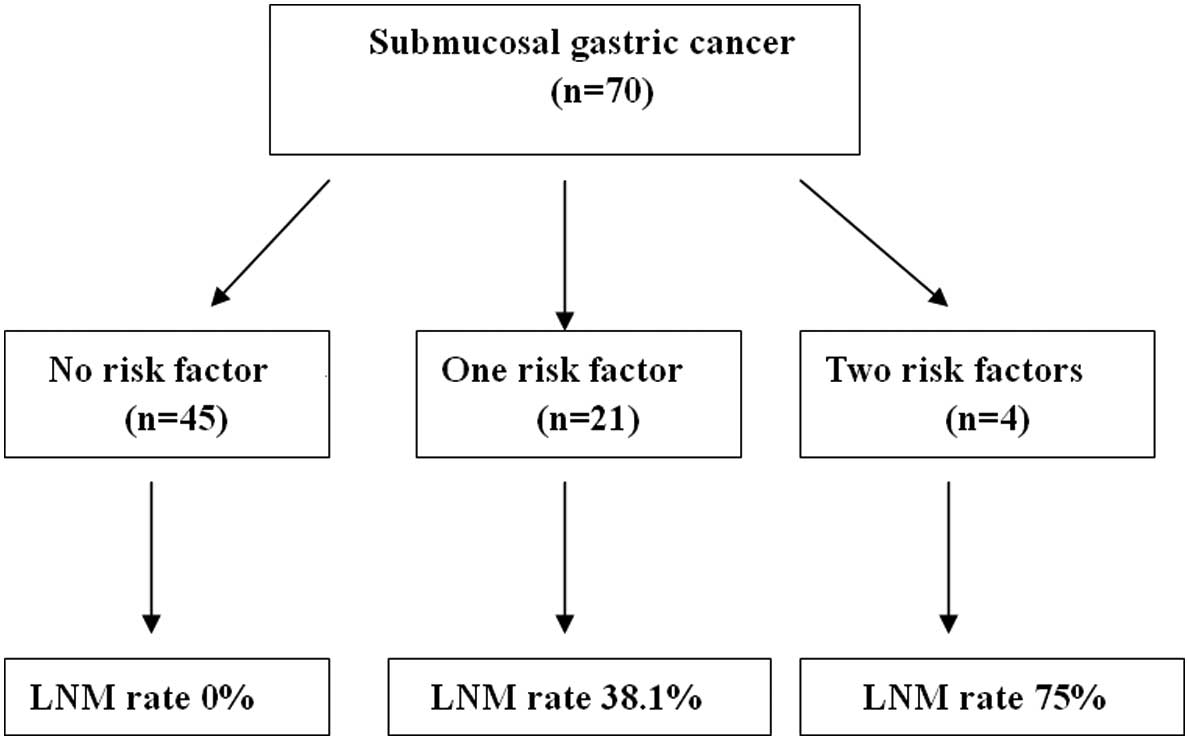
LNM in differentiated SGC
Of the 70 cases, LNM was histologically confirmed in
11 (15.7%) patients. The correlation between the two positive risk
clinicopathological characteristics and LNM were studied in
differentiated SGC. There was no LNM in 45 patients without the two
pathological risk factors, whereas LNM was present in 38.1% (8/21)
of patients with only one pathological risk characteristics. LNM
occurred in 75.0% (3/4) of the patients with two risk factor
characteristics (Fig. 1).
Discussion
Several studies have been conducted to evaluate
predictive factors for LNM in SGC and to establish the most
appropriate treatment strategy (13–16).
Factors including depth of invasion, tumor size, gross appearance
and histological type have been observed to be predictors of LNM in
SGC (17,18). In the present study, lymphatic
vessel involvement and presence of intermingled components of
undifferentiated cancer cells were significantly associated with
differentiated LNM.
A significant issue is whether additional treatment,
including gastrectomy with lymph node dissection, is necessary when
SGC is revealed by pathological examination following EMR since LNM
is known to occur in approximately 20% of SGC cases. In the present
study, LNM was not observed in the patients without the two
pathological risk characteristics. This finding may indicate that
EMR is sufficient in treating these cases, and that additional
surgery is unnecessary. However, 38.1 and 75.0% of patients with
one or two of the pathological risk factors had LNM, respectively,
and the survival rate of patients with one or two of the
pathological risk factors was significantly lower than that of the
patients without any of the risk factors. Therefore, gastrectomy
with lymphadenectomy is inevitable for patients with the risk
factors.
In addition to conventional open gastrectomy with
lymphadenectomy, laparoscopic gastrectomy with lymphadenectomy may
be an alternative approach (19).
When compared with conventional open gastrectomy, laparoscopic
gastrectomy has several clinical advantages, including less pain,
milder inflammatory response, faster recovery of the
gastrointestinal function, shorter hospital stay and improved
quality of life (20). Moreover,
over the past 17 years significant advances in laparoscopic
surgical techniques and instruments, such as laparoscopic
coagulating shears, have been observed (21). It is now possible to perform total
gastrectomy and extended lymph node dissection (D2)
laparoscopically (22,23).
In this study, all cases underwent conventional open
gastrectomy and all metastatic lymph nodes were within N2. Thus,
for patients with pathological risk characteristics laparoscopic
gastrectomy coupled with lymph node dissection simultaneously may
enable curability and improve the quality of life. However, this
hypothesis requires further clinical verification.
Based on our findings, we propose a treatment
strategy for patients with SGC that is revealed after EMR for EGC.
For patients without any of the risk factors, EMR without
lymphadenectomy is sufficient. However, for patients with a
pathological risk factor, additional radical gastrectomy should be
recommended (Fig. 2).
Lymphatic vessel involvement and presence of
intermingled components of undifferentiated cancer cells are
independently associated with LNM in differentiated SGC. Thus, the
two risk factors may be used to establish a simple criterion to
guide further surgical procedures in cases with SGC revealed by
EMR. EMR alone may be sufficient for the majority of cases without
the two risk factors. However, for patients with either of these
pathological risk factors, additional radical gastrectomy is
recommended.
References
|
1
|
Koeda K, Nishizuka S and Wakabayashi G:
Minimally invasive surgery for gastric cancer: the future standard
of care. World J Surg. 35:1469–1477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ishikawa S, Togshi A, Inoue M, et al:
Indications for EMR/ESD in cases of early gastric cancer:
relationship between histological type, depth of wall invasion, and
lymph node metastasis. Gastric Cancer. 10:35–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ono H, Kondo H, Gotoda T, et al:
Endoscopic mucosal resection for treatment of early gastric cancer.
Gut. 48:225–229. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamao T, Shirao K, Ono H, et al: Risk
factors for lymph node metastasis from intramucosal gastric
carcinoma. Cancer. 77:602–606. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takagawa R, Nagahori Y, Takahashi M, et
al: Lymph node status in patients with submucosal gastric cancer.
Ann Surg Oncol. 13:1364–1371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohashi S, Segawa K, Okumura S, et al: The
utility of endoscopic ultrasonography and endoscopy in the
endoscopic mucosal resection of early gastric cancer. Gut.
45:599–604. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park YD, Chung YJ, Chung HY, et al:
Factors related to lymph node metastasis and the feasibility of
endoscopic mucosal resection for treating poorly differentiated
adenocarcinoma of the stomach. Endoscopy. 40:7–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akakoshi K, Chijiiwa Y, Hamada S, et al:
Endoscopic ultrasonography: a promising method for assessing the
prospects of endoscopic mucosal resection in early gastric cancer.
Endoscopy. 29:614–619. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kito T, Yamamura Y and Kobayashi S:
Surgical treatment of early gastric cancer. Anticancer Res.
8:335–338. 1988.PubMed/NCBI
|
|
10
|
Itoh H, Oohata Y, Nakamura K, et al:
Complete ten-year postgastrectomy follow-up of early gastric
cancer. Am J Surg. 158:14–16. 1989.PubMed/NCBI
|
|
11
|
Gotoda T, Sasako M, Ono H, et al:
Evaluation of the necessity for gastrectomy with lymph node
dissection for patients with submucosal invasive gastric cancer. Br
J Surg. 88:444–449. 2001. View Article : Google Scholar
|
|
12
|
Abe N, Watanabe T, Suzuki K, et al: Risk
factors predictive of lymph node metastasis in depressed early
gastric cancer. Am J Surg. 183:168–172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Japanese Gastric Cancer Association.
Japanese classification of gastric carcinoma - 2nd English edition.
Gastric Cancer. 1:10–24. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamada H, Nihei Z, Yamashita T, et al: Is
lymphadenectomy needed for all submucosal gastric cancers? Eur J
Surg. 167:199–203. 2001. View Article : Google Scholar
|
|
15
|
Park DJ, Lee HK, Lee HJ, et al: Lymph node
metastasis in early gastric cancer with submucosal invasion:
feasibility of minimally invasive surgery. World J Gastroenterol.
10:3549–3552. 2004.PubMed/NCBI
|
|
16
|
Kim DY, Joo JK, Ryu SY, Kim YJ and Kim SK:
Factors related to lymph node metastasis and surgical strategy used
to treat early gastric carcinoma. World J Gastroenterol.
10:737–740. 2004.PubMed/NCBI
|
|
17
|
Nasu J, Nishina J, Hirasaki S, et al:
Predictive factors of lymph node metastasis in patients with
undifferentiated early gastric cancers. J Clin Gastroenterol.
40:412–415. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lo SS, Wu CW, Chen JH, et al: Surgical
results of early gastric cancer and proposing a treatment strategy.
Ann Surg Oncol. 14:340–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abe N, Mori T, Izumisato Y, et al:
Successful treatment of an undifferentiated early stage gastric
cancer by combined en bloc EMR and laparoscopic regional
lymphadenectomy. Gastrointest Endosc. 57:972–975. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adachi Y, Shiraishi N and Kitano S: Modern
treatment of early gastric cancer: review of the Japanese
experience. Dig Surg. 19:333–339. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Swanstrom LL and Pennings JL: Laparoscopic
control of short gastric vessels. J Am Coll Surg. 181:347–351.
1995.
|
|
22
|
Uyama I, Sugioka A, Fujita J, et al:
Complete laparoscopic extraperigastric lymph node dissection for
gastric malignancies located in the middle or lower third of the
stomach. Gastric Cancer. 2:186–190. 1999. View Article : Google Scholar
|
|
23
|
Goh PM, Khan AZ, So JB, et al: Early
experience with laparoscopic radical gastrectomy for advanced
gastric cancer. Surg Laparosc Endosc Percutan Tech. 11:83–87. 2001.
View Article : Google Scholar : PubMed/NCBI
|