Introduction
Chronic myeloid leukemia (CML) is a clonal malignant
disorder of pluripotent hematopoietic stem cells, characterized by
the presence of the Philadelphia (Ph) chromosome in more than 90%
of patients. The Ph chromosome results from a reciprocal
translocation of chromosomes 9 and 22, which leads to the transfer
of the 3′ portion of the proto-oncogene ABL from 9q34 to the 5′
portion of the breakpoint cluster region (BCR) on 22q11. This
results in a fused BCR/ABL gene and the production of an abnormal
tyrosine kinase protein that causes the altered and disease-causing
myelopoiesis found in CML. Since tyrosine kinase activity is
required for the transforming function of the BCR/ABL fusion
protein, the specific inhibitor of the kinase Imatinib is an
effective treatment for CML patients. In a previous study, the
5-year estimated overall survival rate of 89% for patients who
received Imatinib as an initial therapy was higher than that
reported in any previously published prospective study of the
treatment of CML and only 7% of all patients progressed to the
accelerated phase or blast crisis (1).
In this study, we present a previously unreported
translocation of chromosomes 2 and 7 being present with the Ph
chromosome in a CML patient successfully treated with Imatinib. The
additional rearrangement was characterized in detail by
fluorescence in situ hybridization (FISH) and high
resolution array-proven multicolor banding (aMCB) as
t(2;7)(p13.1;p21.3).
Materials and methods
Case report
A 45-year-old female was diagnosed as suffering from
CML in chronic phase (CP). In December 2008, the white blood cell
count (WBC) was 15.3×109/l with 58.3% neutrophils, 34.8%
lymphocytes, 5.2% monocytes, 0.8% eosinophiles and 0.9% basophiles.
The platelet count was 978×109/l and the hemoglobin
level was 11.5g/dl. The previous physical examination revealed
hepatosplenomegaly. The patient was treated with imatinib mesylate
at 400 mg/day for eight months and the relevant symptoms
disappeared. In July 2009, she passed away under the treatment due
to unknown reasons. The study was approved by the ethics committee
of the Atomic Energy Commission of Syria and patient consent was
obtained.
Cytogenetic analysis
Chromosome analysis using GTG-banding was performed
according to standard procedures (2). A total of 20 metaphases derived from
the unstimulated bone marrow of the patient were analyzed.
Karyotypes were described according to the international system for
human cytogenetic nomenclature (3).
Molecular cytogenetics
FISH using a whole chromosome painting (WCP) probe
for chromosomes 2 and 7 (MetaSystems, Altlussheim, Germany) and
subtelomeric probes for 7pter and 7qter (Abbott Molecular/Vysis,
Des Plaines, IL, USA) were applied according to the manufacturer’s
instructions together with a chromosome enumeration probe (CEP) for
chromosome 2 (Abbott Molecular/Vysis) (2). aMCB sets based on
microdissection-derived region-specific libraries for chromosomes 2
and 7 were applied as previously described (4). Twenty metaphase spreads were analyzed
using a fluorescence microscope (AxioImager.Z1 mot, Carl Zeiss
Ltd., Hertfordshire, UK) equipped with appropriate filter sets to
discriminate between a maximum of five fluorochromes and the
counterstain DAPI (4′,6-diamino-2-phenylindole). Image capturing
and processing were carried out using an ISIS mFISH imaging system
(MetaSystems) for the evaluation of the MCB.
Results
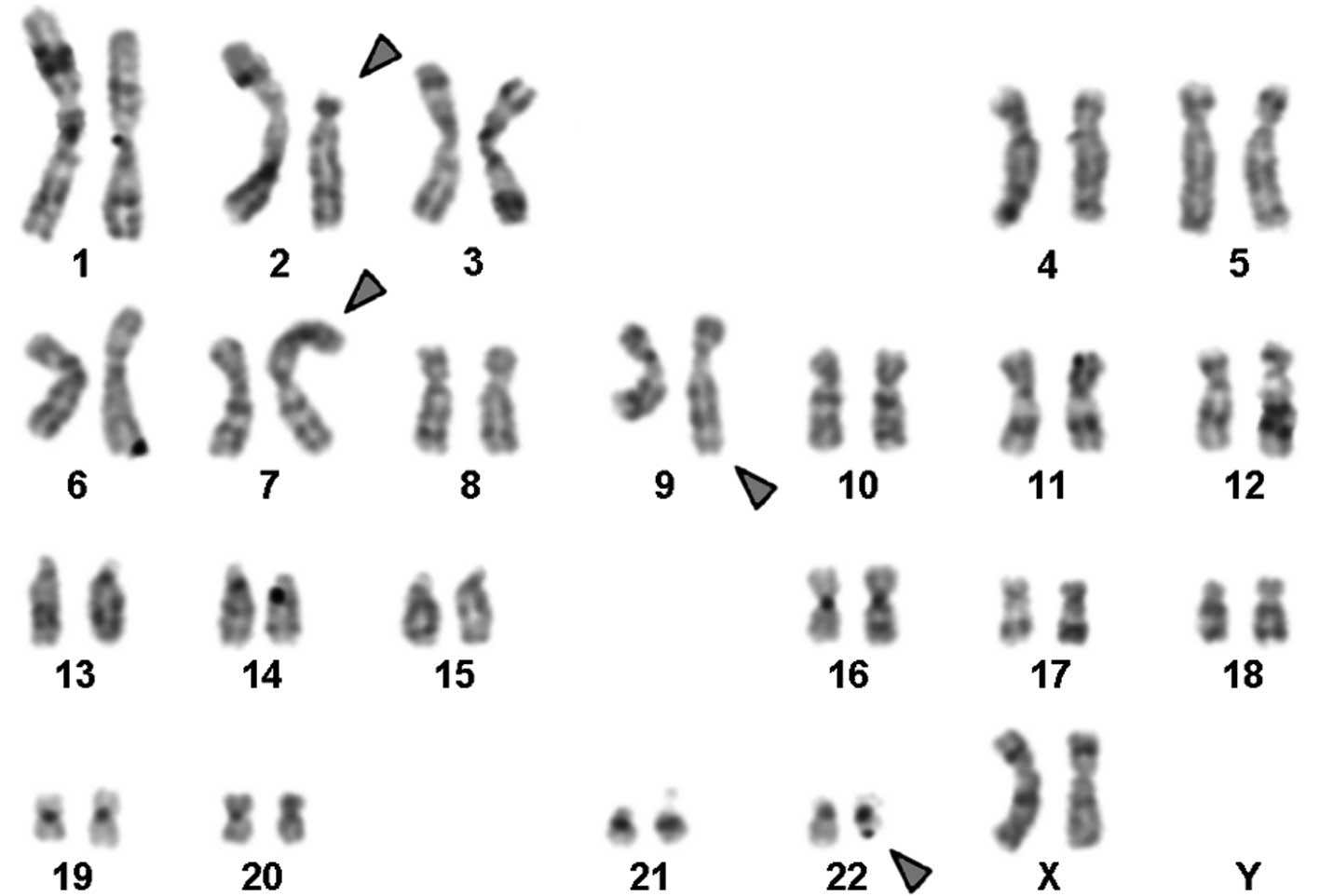
Karyotyping was performed prior to and following the
initiation of chemotherapy treatment. The result prior to
chemotherapy was 46,XX,t(9;22)[18]/46,XX[2] and following therapy
it was 46,XX,t(2;7),t(9;22)[20] in GTG-banding (Fig. 1). It was further specified by
molecular cytogenetic studies (Fig.
2). A dual-color-FISH applying probes specific to BCR and ABL
revealed a typical Ph chromosome with a BCR/ABL fusion gene
(Fig. 2A). The corresponding WCP
probes confirmed a Ph-independent translocation between chromosomes
2 and 7 (Fig. 2B), also
substantiated by subtelomeric probes for 7pter and 7qter (Fig. 2C). aMCB narrowed down the
breakpoints to 2p13.1 and 7p21.3.
Discussion
The present study identified one additional
chromosomal alteration in a Ph-positive CML-CP case as
t(2;7)(p13.1;p21.3). To the best of our knowledge, this
translocation has not been previously observed in CML (5). Moreover, neither breakpoint has been
reported to be involved in variant Ph-rearrangement in CML
(6).
The breakpoint 2p13 has been reported in patients
with acute bilineal leukemia (7,8) and
B-cell chronic lymphocytic leukemia (9,10). The
gene BCL11A may be involved in these diseases (10,11).
However, in Hodgkin’s lymphoma, breaks at 2p13 (12) have been reported to be associated
with the oncogene REL (13).
Finally, 2p13 has previously been reported in a translocation
t(2;14) in a Ph-positive acute lymphoblastic lymphoma (ALL) case
(14).
The 7p21.3 region also merits further study. This
region includes the ETS variant gene 1 (ETV1), which encodes an ETS
translocation variant 1 protein in humans (15,16).
The ETS genes (including FLI, ERG, ETV4 and ETV1) encode a family
of eukaryotic transcription factors that includes more than 30
members that are found in organisms from sponges to humans
(17). The ETS genes are involved
in multiple processes, including cell proliferation and cancer cell
invasion (18). Some of these genes
become oncogenic by retroviral insertional mutagenesis (17) [as in gastric cancer (18), prostate cancer (19) and breast cancer metastasis (18)] or by chromosomal translocations [as
in myeloid leukemia and Ewing’s sarcomas (17)]. For example, ETV1 has been mapped at
position 7p21.3 and has been found to be subject to a translocation
between chromosomes 7 and 22 in a human Ewing’s sarcoma (17).
The 2p13.1 region includes the Anthrax toxin
receptor 1 gene (ATR1) (20). ATR1
is a transmembrane protein and a tumor-specific endothelial marker
(TEM) that is involved in colorectal cancer (21).
TEM-8 belongs to a family of TEMs that were
identified as being predominantly expressed in tumor endothelium
(22). Moreover, a high TEM-8
expression appears to be correlated with advanced tumor stage in
breast and colorectal cancer (23).
An overexpression of ATR1 has also been found in several
neuroblastoma (NB) cell lines (24).
TEM-8 has been shown to function as a cell surface
TEM that is highly conserved in mice and human tumor endothelium,
thus making TEM-8 an attractive marker for the establishment of an
in vivo model system in an attempt to assess anti-angiogenic
strategies in combating tumor growth (22).
In conclusion, in this study we reported a unique
case of a Ph chromosome-positive CML in CP with a new
translocation, possibly therapy-initiated, involving the two
chromosomal aberrations 2p13.1 and 7p21.3, which has not previously
been described. Notably, the reported patient had a good response
to Imatinib.
Acknowledgements
We thank Professor I. Othman, the Director General
of the Atomic Energy Commission of SYRIA (AECS), and Dr N. Mirali,
the Head of Molecular Biology and Biotechnology Department, for
their support. This study was supported by the AECS and in parts by
the Stefan-Morsch-Stiftung, Monika-Kutzner-Stiftung and the DAAD
(D/07/09624).
References
|
1
|
O’Brien S, Thall PF and Siciliano MJ:
Cytogenetics of chronic myelogeneous leukemia. Baillieres Clin
Hematol. 10:259–276. 1997.
|
|
2
|
Al-Achkar W, Wafa A and Nweder MS: A
complex translocation t(5;9;22) in Philadelphia cells involving the
short arm of chromosome 5 in a case of chronic myelogenous
leukemia. J Exp Clin Cancer Res. 26:411–415. 2007.PubMed/NCBI
|
|
3
|
Shaffer L, Slovak M and Campbell L: An
International System for Human Cytogenetic Nomenclature. S Karger;
Basel: 2009
|
|
4
|
Liehr T, Heller A, Starke H, Rubtsov N,
Trifonov V, Mrasek K, Weise A, Kuechler A and Claussen U:
Microdissection based high resolution multicolor banding for all 24
human chromosomes. Int J Mol Med. 9:335–339. 2002.PubMed/NCBI
|
|
5
|
Mitelman F, Johansson B and Mertens F:
Mitelman Database of Chromosome Aberrations in Cancer. 2009,
http://cgap.nci.nih.gov/Chromosomes/Mitelman.
|
|
6
|
Johansson B, Fioretos T and Mitelman F:
Cytogenetic and molecular genetic evolution of chronic myeloid
leukemia. Acta Haematol. 107:76–94. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gale RP and Ben-Bassat I: Hybrid acute
leukaemia. Br J Haematol. 65:261–264. 1987. View Article : Google Scholar
|
|
8
|
Weir EG, Ali Ansari-Lari M, Batista DA,
Griffin CA, Fuller S, Smith BD and Borowitz MJ: Acute bilineal
leukemia: a rare disease with poor outcome. Leukemia. 21:2264–2270.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Richardson AL, Humphries CG and Tucker PW:
Molecular cloning and characterization of the t(2;14) translocation
associated with childhood chronic lymphocytic leukemia. Oncogene.
7:961–970. 1992.PubMed/NCBI
|
|
10
|
Satterwhite E, Sonoki T, Willis TG, Harder
L, Nowak R, Arriola EL, Liu H, Price HP, Gesk S, Steinemann D, et
al: The BCL11 gene family: involvement of BCL11A in lymphoid
malignancies. Blood. 98:3413–3420. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bezrookove V, van Zelderen-Bhola SL, Brink
A, Szuhai K, Raap AK, Barge R, Beverstock GC and Rosenberg C: A
novel t(6;14)(q25-q27;q32) in acute myelocytic leukemia involves
the BCL11B gene. Cancer Genet Cytogenet. 149:72–76. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gozzetti A, Davis EM, Espinosa R III,
Fernald AA, Anastasi J and Le Beau MM: Identification of novel
cryptic translocations involving IGH in B-cell non-Hodgkin’s
lymphomas. Cancer Res. 62:5523–5527. 2002.PubMed/NCBI
|
|
13
|
Barth TF, Martin-Subero JI, Joos S, Menz
CK, Hasel C, Mechtersheimer G, Parwaresch RM, Lichter P, Siebert R
and Möoller P: Gains of 2p involving the REL locus correlate with
nuclear c-Rel protein accumulation in neoplastic cells of classical
Hodgkin lymphoma. Blood. 101:3681–3686. 2003. View Article : Google Scholar
|
|
14
|
Inaba T, Oku N, Gotoh H, Murakami S, Oku
N, Itoh K, Ura Y, Nakanishi S, Shimazaki C, Nakagawa M, et al:
Philadelphia chromosome positive precursor B-cell acute
lymphoblastic leukemia with a translocation t(2;14)(p13;q32).
Leukemia. 5:719–722. 1991.PubMed/NCBI
|
|
15
|
Brown TA and McKnight SL: Specificities of
protein-protein and protein-DNA interaction of GABP alpha and two
newly defined ets-related proteins. Genes Dev. 6:2502–2512. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nambiar M, Kari V and Raghavan SC:
Chromosomal translocations in cancer. Biochim Biophys Acta.
1786:139–152. 2008.PubMed/NCBI
|
|
17
|
Coutte L, Monté D, Imai K, Pouilly L,
Dewitte F, Vidaud M, Adamski J, Baert JL and de Launoit Y:
Characterization of the human and mouse ETV1/ER81 transcription
factor genes: role of the two alternatively spliced isoforms in the
human. Oncogene. 18:6278–6286. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai C, Hsieh CL, Omwancha J, Zheng Z, Chen
SY, Baert JL and Shemshedini L: ETV1 is a novel androgen
receptor-regulated gene that mediates prostate cancer cell
invasion. Mol Endocrinol. 21:1835–1846. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
King JC, Xu J, Wongvipat J, Hieronymus H,
Carver BS, Leung DH, Taylor BS, Sander C, Cardiff RD, Couto SS, et
al: Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation
in prostate oncogenesis. Nat Genet. 41:524–526. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oberthuer A, Skowron M, Spitz R, Kahlert
Y, Westermann F, Mehler K, Berthold F and Fischer M:
Characterization of a complex genomic alteration on chromosome 2p
that leads to four alternatively spliced fusion transcripts in the
neuroblastoma cell lines IMR-5, IMR-5/75 and IMR-32. Gene.
363:41–50. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carson-Walter EB, Watkins DN, Nanda A,
Vogelstein B, Kinzler KW and St Croix B: Cell surface tumor
endothelial markers are conserved in mice and humans. Cancer Res.
61:6649–6655. 2001.PubMed/NCBI
|
|
22
|
Davies G, Rmali KA, Watkins G, Mansel RE,
Mason MD and Jiang WG: Elevated levels of tumour endothelial
marker-8 in human breast cancer and its clinical significance. Int
J Oncol. 29:1311–1317. 2006.PubMed/NCBI
|
|
23
|
Venanzi FM, Petrini M, Fiammenghi L, Bolli
E, Granato AM, Ridolfi L, Gabrielli F, Barucca A, Concetti A,
Ridolfi R and Riccobon A: Tumor endothelial marker 8 expression
levels in dendritic cell-based cancer vaccines are related to
clinical outcome. Cancer Immunol Immunother. 59:27–34. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen QR, Bilke S, Wei JS, Whiteford CC,
Cenacchi N, Krasnoselsky AL, Greer BT, Son CG, Westermann F,
Berthold F, et al: cDNA array-CGH profiling identifies genomic
alterations specific to stage and MYCN-amplification in
neuroblastoma. BMC Genomics. 5:702004. View Article : Google Scholar : PubMed/NCBI
|