Introduction
Colorectal cancer (CRC) is one of the most common
malignant diseases worldwide, affecting men and women approximately
equally. There are several biological mechanisms by which folate
deficiency increases the risk of CRC (1–3).
Folate may modulate DNA methylation, which is a significant
epigenetic determinant in gene expression, the maintenance of DNA
integrity and stability, chromosomal modifications and the
development of mutations. A growing body of evidence from in
vitro, animal and human studies indicates that folate
deficiency is associated with DNA strand breaks, impaired DNA
repair, increased mutations and aberrant DNA methylation, and that
folate supplementation corrects some of these defects (4).
Findings of previous epidemiological studies
suggested that the elevation of plasma homocysteine (Hcy) levels is
an independent risk factor for CRC (5,6),
although the mechanism remains unknown. A defect in the
methylenetetrahydrofolate reductase (MTHFR) gene and a deficiency
of folate may lead to the accumulation of Hcy in tissues and
blood.
The association between the MTHFR C677T gene
polymorphism and genetic susceptibility to CRC has been widely
evaluated in recent studies, but with controversial conclusions.
Several studies have reported that a homozygous variant genotype of
the polymorphism of MTHFR C677T was associated with an increased
risk of CRC. Certain studies have reported that individuals with
the MTHFR 677TT genotype had a decreased risk of CRC (8), whereas other authors observed no
association between the MTHFR C677T genotype and genetic
susceptibility to CRC (9–11). Considering the number of studies
performed thus far, inconsistent results have been reported on the
association of MTHFR C677T gene polymorphisms with genetic
susceptibility to CRC. We designed a case-control study to
determine the Hcy level and evaluate the potential role of the
MTHFR C677T gene polymorphism in CRC.
Materials and methods
Subjects
We conducted a hospital-based case-control study to
assess the association between Hcy levels, the MTHFR C677T gene
polymorphism and genetic susceptibility to CRC. The cases were 370
patients diagnosed with CRC attending Shandong Provincial Hospital,
Shandong University (Jinan, China), between March 2008 and December
2010. These patients had CRC at stages A, B or C, according to
Dukes’ classification, and all underwent elective and curative
surgery. Of the 370 patients, 204 were male and 166 were female.
The mean age was 49.3±8.2 years (range, 35–78). The subsite
distribution of the 93 CRCs showed 19 in the right colon, 6 in the
transverse colon, 45 in the left colon and 23 in the rectum.
Diagnosis of adenocarcinoma was confirmed in all patients by
histology and cytological investigations. Histopathological grading
of adenocarcinoma was: 5% well-differentiated, 74% moderately
differentiated and 21% poorly differentiated. A total of 370
healthy unrelated subjects were recruited into the control group
after being interviewed with regard to whether they had been
diagnosed with colorectal carcinoma or associated diseases, using
age and gender as frequency-matching criteria. The mean age of the
normal donors was 47.3±8.1 years (range, 32–74); 196 (53.0%) of the
healthy subjects were male and 174 (47.0%) were female. The
characteristics of the study population are shown in Table I. Informed consent was obtained from
all study subjects following an explanation of the nature of the
study. The study was approved by the Shandong University Research
Ethics Committee, China.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Controls
(n=370)
n (%) | Cases
(n=370)
n (%) | χ2 | P-value |
|---|
| Age (years) |
| <45 | 67 (18.1) | 51 (13.8) | 4.523 | 0.104 |
| 45–60 | 167 (45.1) | 158 (42.7) | | |
| >60 | 136 (36.8) | 161 (43.5) | | |
| Gender |
| Male | 196 (53.0) | 204 (55.1) | 0.348 | 0.555 |
| Female | 174 (47.0) | 166 (44.9) | | |
| Location |
| Colon | | 237 (64.1) | | |
| Rectum | | 133 (35.9) | | |
| Dukes’ stage |
| A | | 33 (8.9) | | |
| B | | 131 (35.4) | | |
| C | | 206 (55.7) | | |
Hcy determination and genotyping
Peripheral venous blood was obtained from each
subject and genomic DNA was extracted using a DNA extraction kit
(Qiagen, Crawley, UK) according to the manufacturer’s instructions.
The specimens were stored at −70°C until use. Total Hcy was
quantified using the fluorescence polarization immunoassay (FPIA)
on the IMx analyzer from Abbott Laboratories (Abbott Park, IL,
USA). The IMx Hcy assay is based on the reduction of the plasma
samples with dithiothreitol and subsequent conversion of free Hcy
to S-adenosyl homocysteine by hydrolase in the presence of added
adenosine. The sample and the tracer compete in binding to the
monoclonal antibody. This reaction is followed by the detection of
S-adenosyl homocysteine by an FPIA. The concentration of Hcy in
plasma is inversely correlated with the intensity of the polarized
light.
Genomic DNA was detected by real-time polymerase
chain reaction (RT-PCR) amplification followed by digestion with
the restriction enzyme HinFI, as described by Frosst et
al (12). The primers were
designed as previously reported, with the following sequences for
sense, 5′-GCCCAGCCACTCACTGTTTTA-3′, and antisense,
5′-AGGACGGTGCGGTGAGAGTG-3′, that were used in a 25-μl mixture for
amplification. The cycling conditions for PCR were an initial
denaturation of 5 min at 94°C, followed by 30 cycles of 40 sec at
94°C, annealing at 56°C for 4 sec and extension at 72°C for 12 sec.
A final extension step for 7 min at 72°C was also carried out. The
products were stored at 4°C. Amplified products were later mixed
and buffered with restriction endonuclease HinFI and
sustained in the water bath kettle overnight for digestion. The
products were observed following polyacrylamide gel
electrophoresis.
Statistical analysis
Statistical analyses were performed using SPSS 13.0
statistical software (SPSS Inc., Chicago, IL, USA) and data were
shown as the mean ± standard deviation. Comparisons between the two
groups were performed by the independent t-test; χ2
analysis was applied to determine the difference in the genotype
and gene frequency. Odds ratios (ORs) and 95% confidence intervals
(CIs) were calculated from unconditional logistic regression
models. P<0.05 was considered to indicate a statistically
significant result.
Results
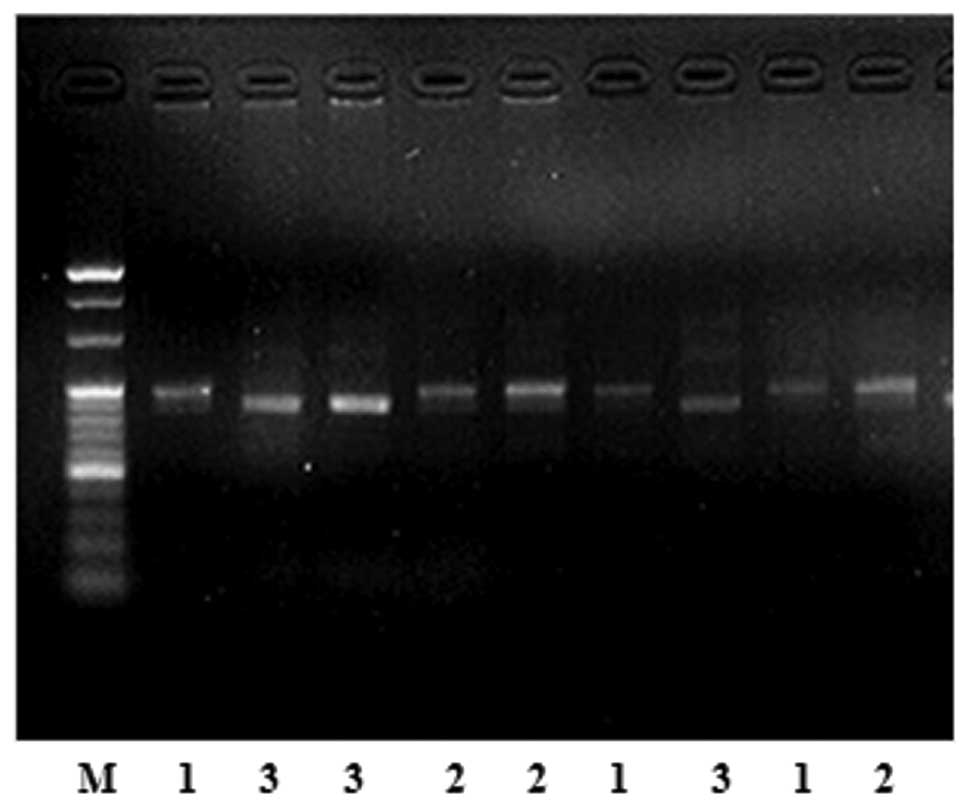
Fig. 1 shows
polyacrylamide gel electrophoresis with restriction endonuclease
HinFI. There are 3 genotypes, CC, CT and TT, in the MTHFR
gene at position 677 in the two groups. Table II shows that the distribution of
the MTHFR C677T gene polymorphisms in the controls was in
Hardy-Weinberg equilibrium. Table
III shows the MTHFR C677T genotype frequencies and allele
frequencies between the two groups (χ2=4.690; P=0.030).
The frequencies of the C and T allele were 56.1 and 43.9% in the
CRC group, and 61.6 and 38.4% in the controls, respectively. The
MTHFR C677T frequencies of the CC, CT and TT genotypes were 33.5,
45.1 and 21.4% among the CRC patients and 37.6, 48.1 and 14.3% in
the controls, respectively (χ2=6.327; P=0.042). Table IV shows that, compared with the CC
genotype, the TT genotype was significantly correlated with an
increased risk of CRC (OR, 1.671; 95% CI, 1.094–2.553;
P=0.018).
 | Table IIMTHFR C677T genotype distribution in
Hardy-Weinberg equilibrium. |
Table II
MTHFR C677T genotype distribution in
Hardy-Weinberg equilibrium.
| Gene | Genotype | Predicted value | Observed value | χ2 | P-value |
|---|
| MTHFR C677T | CC | 140 (37.9) | 139 (37.6) | 0.066 | 0.967 |
| CT | 175 (47.4) | 178 (48.1) | | |
| TT | 55 (14.7) | 53 (14.3) | | |
 | Table IIIMTHFR C677T genotype frequency and
allele frequency between the two groups. |
Table III
MTHFR C677T genotype frequency and
allele frequency between the two groups.
| Genotypes and
alleles | Controls
(n=370)
n (%) | Cases
(n=370)
n (%) | χ2 | P-value |
|---|
| C | 456 (61.6) | 415 (56.1) | 4.690 | 0.030 |
| T | 284 (38.4) | 325 (43.9) | | |
| CC | 139 (37.6) | 124 (33.5) | 6.327 | 0.042 |
| CT | 178 (48.1) | 167 (45.1) | | |
| TT | 53 (14.3) | 79 (21.4) | | |
 | Table IVMTHFR C677T genotype frequencies and
the CRC risk. |
Table IV
MTHFR C677T genotype frequencies and
the CRC risk.
| Genotypes | Controls
(n=370)
n (%) | Cases
(n=370)
n (%) | OR (95% CI) | P-value |
|---|
| CC | 139 (37.6) | 124 (33.5) | 1 | |
| CT | 178 (48.1) | 167 (45.1) | 1.052
(0.763–1.450) | 0.758 |
| TT | 53 (14.3) | 79 (21.4) | 1.671
(1.094–2.553) | 0.018 |
| CT+TT | 231 (62.4) | 246 (66.5) | 1.194
(0.883–1.614) | 0.249 |
The plasma levels of Hcy in the group of patients
with CRC (12.63±3.11 μmol/l) was significantly higher than that in
the control group (10.87±2.42 μmol/l; P<0.05). The levels of Hcy
among the different genotypes are shown in Table V. In the CRC group (F=37.346;
P<0.001), the level of Hcy in subjects with the CT or TT
genotypes (14.47±3.92 μmol/l) was significantly higher than that in
subjects with the CC genotype (11.67±2.03 μmol/l; P<0.05). In
the control group (F=46.241; P<0.001), the level of Hcy in
subjects with the CT or TT genotypes (12.08±3.53 μmol/l) was
significantly higher than that in subjects with the CC genotype
(9.54±1.72 μmol/l; P<0.05). In each genotype, the level of Hcy
was higher in the CRC group than in the control group (t=22.179 for
CC, P<0.001; t=10.966 for CT, P<0.001; t=2.449 for TT,
P=0.016; t=7.654 for CT+TT, P<0.001).
 | Table VPlasma Hcy levels among the different
genotypes. |
Table V
Plasma Hcy levels among the different
genotypes.
| Genotypes | Cases (μmol/l) | Controls
(μmol/l) | t | P-value |
|---|
| CC | 11.67±2.03 | 9.54±1.72 | 22.179 | <0.001 |
| CT | 12.94±2.68a | 9.98±2.33 | 10.966 | <0.001 |
| TT | 15.36±3.44a,b | 13.79±3.85a,b | 2.449 | 0.016 |
| CT+TT | 14.47±3.29a,b,c | 12.08±3.53a,b,c | 7.654 | <0.001 |
| F | 37.346 | 46.241 | - | - |
| P | <0.001 | <0.001 | - | - |
Discussion
In the general population, hyperhomocysteinemia has
been found to be associated with the occurrence of adenomas and CRC
(6). However, in the absence of low
serum folate, the presence of a mutation of the MTHFR gene which is
responsible for hyperhomocysteinemia has not been identified as an
oncogenic risk factor. Hcy is formed from methionine and is either
catabolized in the vitamin B6-dependent transsulfuration pathway or
remethylated into methionine (13).
This latter reaction is catalyzed by methionine synthase, which
requires 5-methyltetrahydrofolate as a substrate and vitamin B12 as
a co-factor; 5-methyltetrahydrofolate is formed by the reduction of
5,10-methylenetetrahydrofolate by MTHFR, which is a regulating
enzyme in Hcy metabolism. A 677C-T mutation was detected in the
MTHFR gene and homozygosity for this genotype was found to be
associated with a decreased specific enzyme activity and elevated
Hcy.
MTHFR is an essential enzyme in the metabolism of
folic acid and catalyzes the irreversible reduction of
5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate
(14). A change of C to T at
nucleotide 677 in MTHFR C677T results in an amino acid sequence
change of an alanine to valine, and this protein is associated with
reduced enzyme activity that leads to reduced plasma folate levels.
The low enzyme activity of MTHFR C677T variant genotypes is
associated with DNA hypomethylation, which may induce genomic
instability and thereby affect the expression of oncogenes or tumor
suppressor genes.
It has been shown that Hcy levels are positively
associated with the proliferation rates of cells in a variety of
tumors (15,16), as well as with oxidative damage to
cells. Hcy has been considered to possess antioxidant properties
through its rate-limiting role in the biosynthesis of glutathione,
the intracellular antioxidant and detoxifying agent (17,18).
However, evidence from in vivo and in vitro studies
suggests that Hcy acts as a pro-oxidant agent that causes DNA
oxidative damage as a result of the overproduction of free radicals
and hydrogen peroxide, leading to gene mutation and subsequent
cancer development. Elevated levels of Hcy are also associated with
several metabolic disorders, including high body mass index, high
plasma triglyceride levels, hypertension and the abnormal oxidation
of low-density lipoproteins (19),
which may lead to the development of several types of cancer,
including CRC.
A number of studies concerning the molecular
mechanisms of hyperhomocysteinemia in oncogenesis have been
performed. It is known that Hcy levels are raised in colic tissue
in inflammatory bowel disease (20). The increase in homocysteinemia may
present an oncogenic risk in two ways. Firstly, it increases
cellular oxidative stress by stimulating and increasing the
secretion of proinflammatory cytokines, including TNF and IL-12
(21). Chronic inflammation is now
known to increase carcinogenic risk. Secondly, hyperhomocysteinemia
is responsible for a fault in cellular methylation (22). Hypomethylated DNA is unstable and
subject to breakage of DNA strands. In the case of the
hypomethylation of DNA, there is an increase in the number of
non-methylated cytosines, which are deaminated in uracyls and
therefore are incorporated more easily. Uracyl DNA glycosylase
repairs these errors by excising the abnormally present uracyls in
the DNA, thus creating temporary breaks (23). The likelihood of the two DNA strands
breaking is increased, thus increasing carcinogenic risk.
The results of our study have shown that the
frequency of MTHFR TT homozygotes was 21.4% in our CRC group, which
was higher than in a UK study (14.0%) (24). Compared with wild-type individuals,
carriers of the MTHFR 677TT genotype are more likely to develop
CRC. The percentage of subjects with the T allele has been found to
be 43.9%. The results of MTHFR genotyping in different populations
showed an overall T allele prevalence of 32%. In North America
(25), this value was 35%. It is
thought that the TT genotype rarely occurs in African-Americans
(26), whereas among healthy
Japanese (27) its prevalence is
14.7%. Our study showed that, in controls, the prevalence of this
polymorphism was 14.3% and that of the T allele was 38.4%, markedly
higher than in a Polish study (28), with 4.4 and 21.5%, respectively. The
observed trend (overall TT versus CC: OR, 1.671; 95% CI,
1.094–2.553; P=0.018) indicates a pathogenic effect. The plasma
level of Hcy in the CRC group (12.63±3.11 μmol/l) was significantly
higher than that in the control group (10.87±2.42 μmol/l;
P<0.05). In the CRC group, the level of Hcy in subjects with the
CT or TT genotypes was significantly higher than in subjects with
the CC genotype. In the control group, the level of Hcy in subjects
with the CT or TT genotypes was significantly higher than in
subjects with the CC genotype. The plasma Hcy level was associated
with the polymorphism of MTHFR C677T. In our study, the frequency
of the MTHFR 677TT genotype in the CRC group was significantly
higher than that in the control group, suggesting an increased risk
for CRC in patients with the MTHFR 677TT genotype. The T allele
frequency in the CRC group was increased when compared with healthy
control subjects, which was consistent with the results of other
studies.
In conclusion, the results of this study suggest
that the MTHFR C677T polymorphism indicates susceptibility to CRC
and is correlated with CRC pathogenesis. The plasma Hcy level in
CRC patients was higher than that in healthy control subjects and
the frequency of the MTHFR 677TT genotype in CRC patients was
markedly increased when compared with healthy subjects. These
findings suggest that the MTHFR C677T polymorphism is a candidate
genetic risk factor for CRC.
References
|
1
|
Choi SW and Mason JB: Folate and
carcinogenesis: an integrated scheme. J Nutr. 130:129–132.
2000.PubMed/NCBI
|
|
2
|
Duthie SJ: Folic acid deficiency and
cancer: mechanisms of DNA instability. Br Med Bull. 55:578–592.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mason JB and Choi SW: The mechanisms by
which folate depletion enhances colorectal carcinogenesis: a
unified scheme. Nestle Nutr Workshop Ser Clin Perform Programme.
4:87–101. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Whiteside MA, Heimburger DC and Johanning
GL: Micronutrients and cancer therapy. Nutr Rev. 62:142–147. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peyrin-Biroulet L, Barraud H, Ancel D,
Petit-Laurent F, Bigard MA, Gueant JL and Bronowicki JP: Folate
metabolism and colorectal carcinogenesis. Gastroenterol Clin Biol.
28:582–592. 2004. View Article : Google Scholar
|
|
6
|
Kato I, Dnistrian AM, Schwartz M, et al:
Serum folate, homocysteine and colorectal cancer risk in women: a
nested case-control study. Br J Cancer. 79:1917–1922. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen K, Song L, Jin MJ, Fan CH, Jiang QT
and Yu WP: Association between genetic polymorphisms in folate
metabolic enzyme genes and colorectal cancer: a nested case-control
study. Zhonghua Zhong Liu Za Zhi. 28:429–432. 2006.(In
Chinese).
|
|
8
|
Le Marchand L, Wilkens LR, Kolonel LN and
Henderson BE: The MTHFR C677T polymorphism and colorectal cancer:
the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev.
14:1198–1203. 2005.PubMed/NCBI
|
|
9
|
Kim JK, Kim S, Han JH, et al:
Polymorphisms of 5,10-methylenetetrahydrofolate reductase and risk
of stomach cancer in a Korean population. Anticancer Res.
25:2249–2252. 2005.PubMed/NCBI
|
|
10
|
Zeybek U, Yaylim I, Yilmaz H, et al:
Methylenetetrahydrofolate reductase C677T polymorphism in patients
with gastric and colorectal cancer. Cell Biochem Funct. 25:419–422.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vollset SE, Igland J, Jenab M, et al: The
association of gastric cancer risk with plasma folate, cobalamin,
and methylenetetrahydrofolate reductase polymorphisms in the
European Prospective Investigation into Cancer and Nutrition.
Cancer Epidemiol Biomarkers Prev. 16:2416–2424. 2007. View Article : Google Scholar
|
|
12
|
Frosst P, Blom HJ, Milos R, et al: A
candidate genetic risk factor for vascular disease: a common
mutation in methylenetetrahydrofolate reductase. Nat Genet.
10:111–113. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Finkelstein JD: The metabolism of
homocysteine: pathways and regulation. Eur J Pediatr. 157:40–44.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gudnason V, Stansbie D, Scott J, Bowron A,
Nicaud V and Humphries S: C677T (thermolabile alanine/valine)
polymorphism in methylenetetrahydrofolate reductase (MTHFR): its
frequency and impact on plasma homocysteine concentration in
different European populations. Atherosclerosis. 136:347–354. 1998.
View Article : Google Scholar
|
|
15
|
Hultdin J, Van Guelpen B, Bergh A,
Hallmans G and Stattin P: Plasma folate, vitamin B12, and
homocysteine and prostate cancer risk: a prospective study. Int J
Cancer. 113:819–824. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martínez-Poveda B, Chavarría T,
Sánchez-Jiménez F, Quesada AR and Medina MA: An in vitro evaluation
of the effects of homocysteine thiolactone on key steps of
angiogenesis and tumor invasion. Biochem Biophys Res Commun.
311:649–653. 2003.PubMed/NCBI
|
|
17
|
Chavarría T, Rodríguez-Nieto S,
Sánchez-Jiménez F, Quesada AR and Medina MA: Homocysteine is a
potent inhibitor of human tumor cell gelatinases. Biochem Biophys
Res Commun. 303:572–575. 2003.PubMed/NCBI
|
|
18
|
Schroecksnadel K, Frick B, Fiegl M,
Winkler C, Denz HA and Fuchs D: Hyperhomocysteinaemia and immune
activation in patients with cancer. Clin Chem Lab Med. 45:47–53.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lever M, George PM, Dellow WJ, Scott RS
and Chambers ST: Homocysteine, glycine betaine, and
N,N-dimethylglycine in patients attending a lipid clinic.
Metabolism. 54:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morgenstern I, Raijmakers MT, Peters WH,
et al: Homocysteine, cysteine, and glutathione in human colonic
mucosa: elevated levels of homocysteine in patients with
inflammatory bowel disease. Dig Dis Sci. 48:2083–2090. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Poddar R, Sivasubramanian N, DiBello PM,
et al: Homocysteine induces expression and secretion of monocyte
chemoattractant protein-1 and interleukin-8 in human aortic
endothelial cells: implications for vascular disease. Circulation.
103:2717–2723. 2001. View Article : Google Scholar
|
|
22
|
Kim YI, Pogribny IP, Basnakian AG, et al:
Folate deficiency in rats induces DNA strand breaks and
hypomethylation within the p53 tumor suppressor gene. Am J Clin
Nutr. 65:46–52. 1997.PubMed/NCBI
|
|
23
|
Blount BC, Mack MM, Wehr CM, et al: Folate
deficiency causes uracil misincorporation into human DNA and
chromosome breakage: implications for cancer and neuronal damage.
Proc Natl Acad Sci USA. 94:3290–3295. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adams M, Smith PD, Martin D, Thompson JR,
Lodwick D and Samani NJ: Genetic analysis of thermolabile
methylenetetrahydrofolate reductase as a risk factor for myocardial
infarction. QJM. 89:437–444. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zuntar I, Topić E, Vukosavić D, et al:
Croatian population data for the C677T polymorphism in
methylenetetrahydrofolate reductase: frequencies in healthy and
atherosclerotic study groups. Clin Chim Acta. 335:95–100. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Motulsky AG: Nutritional ecogenetics:
homocysteine-related arteriosclerotic vascular disease, neural tube
defects, and folic acid. Am J Hum Genet. 58:17–20. 1996.PubMed/NCBI
|
|
27
|
Zhang L, Miyaki K, Araki J, Nakayama T and
Muramatsu M: The relation between nicotinamide N-methyltransferase
gene polymorphism and plasma homocysteine concentration in healthy
Japanese men. Thromb Res. 121:55–58. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goracy I, Cyrylowski L, Kaczmarczyk M, et
al: C677T polymorphism of the methylenetetrahydrofolate reductase
gene and the risk of ischemic stroke in Polish subjects. J Appl
Genet. 50:63–67. 2009. View Article : Google Scholar : PubMed/NCBI
|