Introduction
Keratins are cytoskeletal intermediate filaments,
which are present in normal and malignant epithelial cells. Various
keratins are expressed in a tissue- and differentiation-specific
manner; therefore, every epithelial cell can be categorized by the
specific pattern of its keratin expression profile (1). Keratin 20 (K20) is consistently
expressed in primary and metastatic colorectal carcinoma (CRC) and
demonstrates variable reactivity in gastric and pancreatic cancer
(2). Immunohistochemical analysis
for K7, K19 and K20 is considered useful when making a differential
diagnosis of primary and metastatic carcinomas of the liver
(3,4). In one study, 97% of cholangiocarcinoma
(CC) and 3% of metastatic CRC tissues were diffusely positive for
K7, 77% of CC and 64% of metastatic CRC tissues were diffusely
positive for K19, and 10% of CC and 74% of metastatic CRC tissues
were diffusely positive for K20 (3). It was identified that the
K7+/K20− profile has a 100% positive
predictive value (PPV) for CC and the
K7−/K20+ profile has a 93% PPV for metastatic
CRC (4). In previous studies, a
proportion of typical intrahepatic cholangiocarcinoma (ICC)
retained K20 expression (3–5); however, the frequency and
clinicopathological significance of K20 expression in ICC remains
unclear.
In this study, we evaluated the expression of K7,
K19 and K20 in 46 ICCs and 20 metastatic CRCs of the liver and 20
corresponding primary CRCs, and analyzed the clinicopathological
characteristics of K20+ ICC. We also examined the
correlation between K20 expression and mucin phenotype in ICC.
Patients and methods
Patients
We examined 66 surgically resected liver tumors
consisting of 46 ICCs obtained from 1998 to 2010, and 20 metastatic
CRCs of the liver and the corresponding primary CRCs obtained from
1998 to 2005 at Chonbuk National University Hospital, Jeonju,
Korea. In each case, clinicopathological features, including
patient age at diagnosis, gender, vessel and neural invasion, and
follow-up data were obtained from hospital records. Tumors were
staged according to the 2010 American Joint Committee on Cancer
tumor-node-metastasis (TNM) classification (6). Grade and phenotype of ICCs were
classified according to WHO classification (7) and mucin expression profiles. The
follow-up period was determined from the date of initial surgery to
the date of the last follow-up or mortality. This study was
approved by the ethics committees of Chonbuk National
University.
Immunohistochemical staining
A formalin-fixed, paraffin-embedded, representative
4-μm section was obtained from each of the 46 ICC, 20 primary CRC
and 20 metastatic CRC specimens. Immunohistochemical staining was
performed by polymer intense detection system using the Bond-Max
Automatic stainer (Leica Microsystems Inc., Bannockburn, IL, USA)
in accordance with the manufacturer's instructions. Following
antigen retrieval in a microwave oven for 10 min in 0.01 mol
citrate buffer (pH 9.0), cells were incubated with anti-K7
(Novocastra, Wetzlar, Germany), anti-K19 (Dako, Glostrup, Denmark),
anti-K20, anti-MUC2, anti-MUC 5AC, anti-MUC 6 (Novocastra) and
anti-CD10 (Cell Marque, Rocklin, CA, USA) antibody for 30 min.
Immunohistochemical analysis and
classification of epithelial phenotypes
The samples that were subjected to immunostaining
were rated according to a score calculated by multiplying the
cancer area of the stain with the intensity of the stain. The area
of staining was scored as follows: 0 (<10%), 1 (10–69%) or 2
(≥70%). The intensity of the cell cytoplasmic staining was grouped
into four categories: 0, no immunostaining; 1, weak; 2, moderate;
3, strong. If the score was ≥1, the tumor was considered positive;
otherwise, the tumor was considered negative. The classification of
the epithelial phenotypes was based on morphological features of
the tumor cells in addition to mucin expression patterns, which
were defined as follows: a) intestinal type, characterized by
positive staining for MUC2 or CD10; b) gastric type, characterized
by positive staining for MUC5AC or MUC6; c) mixed type,
characterized by positive staining for both gastric and intestinal
mucin; d) undifferentiated type, characterized by no staining for
any applied markers.
Statistical analysis
The SPSS version 15.0 statistical software program
(SPSS, Chicago, IL, USA) was used for the statistical analyses. The
clinicopathological characteristics were compared with the
expression of K7, K19 and K20 using the Chi-square test. A Cox
proportional hazard regression analysis was conducted to estimate
the impact of clinicopathological factors on patient survival.
Survival curves were calculated using the Kaplan-Meier method and
the differences between the curves were analyzed using the log-rank
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinicopathological data
The 46 ICC patients consisted of 31 (67.4%) males
and 15 (32.6%) females. According to the location of the tumor in
the biliary tract, 16 (34.8%) ICCs were classified as hilar and 30
(65.2%) as peripheral types. Based on gross morphology, 27 (58.7%)
ICCs were classified as mass-forming, 11 (23.9%) as intraductal
papillary and eight (17.4%) as periductal infiltrative type. A
total of 14 (30.4%) were well-differentiated, 20 (43.5%) were
moderately differentiated and 12 (26.1%) were poorly
differentiated. Additionally, 5 of the 46 ICC patients had
clonorchiasis and 5 had intrahepatic bile duct stones.
Immunohistochemical results
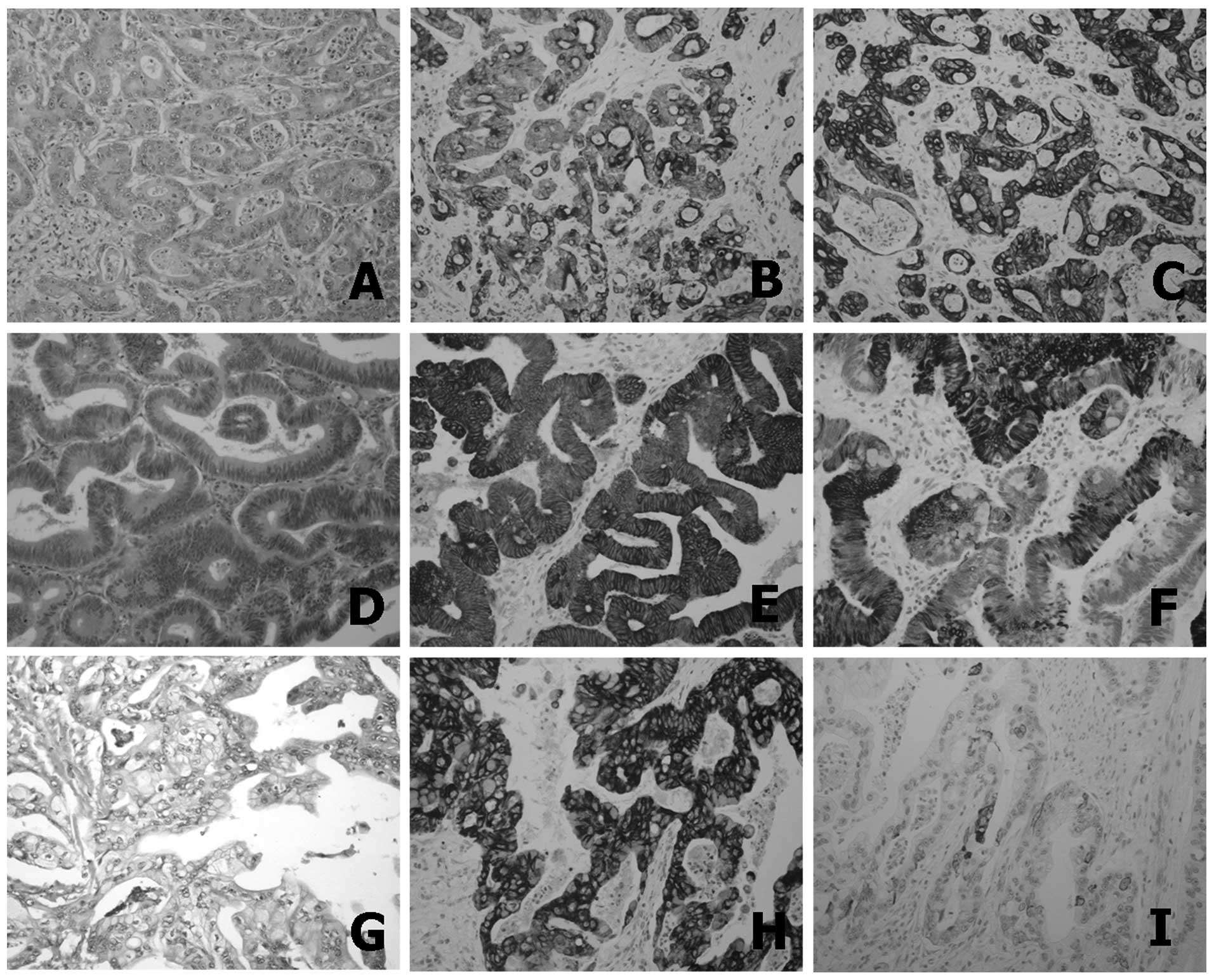
K7, K19 and K20 were expressed in 40 (87.0%), 45
(97.8%) and 16 (34.8%) of the 46 ICCs, respectively (Fig. 1). CD10, MUC2, MUC5AC and MUC6 were
expressed in 13 (28.3%), 10 (21.7%), 19 (41.3%) and 6 (13.0%) of
the 46 ICCs, respectively. K7, K19 and K20 were expressed in 1
(5%), 20 (100%) and 16 (80%) of the 20 primary CRCs and 2 (10%), 20
(100%) and 16 (80%) of the 20 metastatic CRCs, respectively. One
K7-negative primary CRC changed to positive in metastatic CRC,
while 4 K20-negative CRCs changed to K20-positive in metastatic
CRCs. According to the morphology of tumor cells and mucin
phenotype, the 46 ICCs were divided into 32 (69.6%)
pancreatobiliary, 8 (17.4%) intestinal, 2 (4.3%) gastric, 3 (6.5%)
mixed and 1 (2.2%) unclassified type.
Correlation between K20 expression and
clinicopathological features of ICC
The correlations between K20 expression in ICCs and
clinicopathological features are summarized in Table I. K20 expression in ICC was
significantly associated with male gender (P=0.034), hilar location
(P=0.026), intraductal papillary type (P=0.006) and intestinal
epithelial type (P<0.001). No significant correlation was
identified between K20 expression and differentiation, invasion
depth, lymph node metastasis, distant metastasis, overall stage,
neural invasion and vessel invasion. On comparison with the mucin
phenotype of ICC, K20 expression was significantly associated with
MUC2 expression (P=0.008) (Table
II). Although there was no statistical significance between
MUC2 expression and the intraductal papillary type, five of the 11
(45%) intraductal papillary types displayed MUC2 expression,
indicating a close correlation between these two factors.
 | Table ICorrelation between K20 expression and
clinicopathological factors in ICC. |
Table I
Correlation between K20 expression and
clinicopathological factors in ICC.
| Clinicopathological
factors | K20+
(%)
n=16 | K20−
(%)
n=30 | Total (%)
n=46 | P-value |
|---|
| Age, mean ± SD
(years) | 62.3±8.4 | 61.7±9.1 | 61.9±8.8 | 0.832 |
| Gender |
| Female | 2 (12.5) | 13 (43.3) | 15 (32.6) | 0.034 |
| Male | 14 (87.5) | 17 (56.7) | 31 (67.4) | |
| Location |
| Hilar | 9 (56.3) | 7 (23.3) | 16 (34.8) | 0.026 |
| Peripheral | 7 (43.8) | 23 (76.7) | 30 (65.2) | |
| Macroscopic type |
| Intraductal
papillary | 8 (50.0) | 3 (10.0) | 11 (23.9) | 0.006 |
| Periductal
infiltrative | 3 (18.8) | 5 (16.7) | 8 (17.4) | |
| Mass-forming | 5 (31.3) | 22 (73.3) | 27 (58.7) | |
| Differentiation |
| Well | 8 (50.0) | 6 (20.0) | 14 (30.4) | 0.081 |
| Moderate | 6 (37.5) | 14 (46.7) | 20 (43.5) | |
| Poor | 2 (12.5) | 10 (33.3) | 12 (26.1) | |
| T category |
| Tis, 1 | 9 (56.3) | 12 (40.0) | 21 (45.7) | 0.292 |
| T2, 3, 4 | 7 (43.2) | 18 (60.0) | 25 (54.3) | |
| N category |
| N0 | 14 (87.5) | 26 (86.7) | 40 (87.0) | 0.936 |
| N1 | 2 (12.5) | 4 (13.3) | 6 (13.0) | |
| M category |
| M0 | 15 (93.8) | 28 (93.3) | 43 (93.5) | 0.957 |
| M1 | 1 (6.3) | 2 (6.7) | 3 (6.5) | |
| Stage |
| 0, I | 8 (50.0) | 12 (40.0) | 20 (43.5) | 0.514 |
| II, III, IV | 8 (50.0) | 18 (60.0) | 26 (56.5) | |
| Neural
invasion |
| Absence | 15 (93.8) | 23 (76.7) | 38 (82.6) | 0.145 |
| Presence | 1 (6.3) | 7 (23.3) | 8 (17.4) | |
| Vessel
invasion |
| Absence | 11 (68.8) | 17 (56.7) | 28 (60.9) | 0.424 |
| Presence | 5 (31.3) | 13 (43.3) | 18 (39.1) | |
| Epithelial
type |
| I | 8 (50.0) | 0 (0.0) | 8 (17.4) | <0.001 |
| G + mixed | 0 (0.0) | 5 (16.7) | 5 (10.8) | |
| Pb +
undifferentiated | 8 (50.0) | 25 (83.3) | 33 (71.8) | |
 | Table IICorrelation between K20 expression
and mucin phenotype of ICC. |
Table II
Correlation between K20 expression
and mucin phenotype of ICC.
| Mucin
phenotype | No. (%)
n=46 | K20 expression
(%) | P-value |
|---|
|
|---|
| Negative, n=30 | Positive, n=16 |
|---|
| CD10 |
| Negative | 33 (71.7) | 21 (70.0) | 12 (75.0) | 0.720 |
| Positive | 13 (28.3) | 9 (30.0) | 4 (25.0) | |
| MUC2 |
| Negative | 36 (78.3) | 27 (90.0) | 9 (56.3) | 0.008 |
| Positive | 10 (21.7) | 3 (10.0) | 7 (43.8) | |
| MUC5AC |
| Negative | 27 (58.7) | 16 (53.3) | 11 (68.8) | 0.312 |
| Positive | 19 (41.3) | 14 (46.7) | 5 (31.3) | |
| MUC6 |
| Negative | 40 (87.0) | 27 (90.0) | 13 (81.3) | 0.401 |
| Positive | 6 (13.0) | 3 (10.0) | 3 (18.8) | |
K7/K20 profiles in differential diagnosis
of ICC and metastatic CRC of the liver
The sensitivity, specificity and PPV of the
different K7/K20 immunophenotypes for ICC and metastatic CRC are
demonstrated in Table III. The
K7+/K20− immunophenotype had a 100% PPV for
the diagnosis of ICC and the K7−/K20+
immunophenotype had an 84.2% PPV for the diagnosis of metastatic
CRC. The K7+/K20+ immunophenotype had an
86.7% and 13.3% PPV for the diagnosis of ICC and metastatic CRC,
respectively.
 | Table IIISensitivity, specificity and PPV of
K7/20 profiles in ICC and metastatic CRC. |
Table III
Sensitivity, specificity and PPV of
K7/20 profiles in ICC and metastatic CRC.
| K7/K20
expression | ICC | Metastatic CRC |
|---|
|
|
|---|
| Sensitivity
(%) | Specificity
(%) | PPV | Sensitivity
(%) | Specificity
(%) | PPV |
|---|
|
K7+/K20− | 58.7 | 100.0 | 100.0 | 0.0 | 41.3 | 0.0 |
|
K7−/K20+ | 6.5 | 20.0 | 15.8 | 80.0 | 93.5 | 84.2 |
|
K7+/K20+ | 28.3 | 90.0 | 86.7 | 20.0 | 71.7 | 13.3 |
Patient outcome
The median follow-up period for patients with ICC
was 28.8 months and the median survival time was 30.0 months. There
was a total of six mortalities from ICC and one from pancreatitis.
Univariate Cox survival analysis of the 46 ICCs identified that
invasion depth (P=0.005), lymph node metastasis (P=0.012), tumor
stage (P=0.004) and vessel invasion (P=0.023) were significantly
associated with poor patient survival, and that MUC6 expression
(P=0.044) had a strong correlation with patient survival. Tumor
stage (P=0.002) was associated with poor patient survival, while
MUC6 expression (P=0.036) was correlated with good patient survival
as revealed by multivariate Cox survival analysis. The median
survival time of patients with K20-positive ICC was 22.9±7.7
months. The median survival time of patients with K20-negative ICC
was 42.9±18.9 months. The 1-, 3- and 5-year survival rates in
patients with K20-positive ICC were lower (41, 33 and 33%,
respectively) than those of patients with K20-negative ICC (54, 42
and 34%, respectively). However, no significant survival difference
was observed between patients with K20-positive and K20-negative
ICC as demonstrated by a Kaplan-Meier analysis (Table IV).
 | Table IVUnivariate and multivariate Cox
proportional hazard analysis of the factors associated with 46 ICC
patients. |
Table IV
Univariate and multivariate Cox
proportional hazard analysis of the factors associated with 46 ICC
patients.
| ICC associated
factors | No. (%) | Univariate
model | Multivariate
model |
|---|
|
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
|
Differentiation |
| Well | 14 (30.4) | 1 | | | | | |
| Moderate | 20 (43.5) | 1.5 | 0.58–3.92 | 0.405 | | | |
| Poor | 12 (26.1) | 3.49 | 1.22–9.97 | 0.020 | | | |
| T category |
| Tis, 1 | 21 (45.7) | 1 | | | | | |
| T2, 3, 4 | 25 (54.3) | 3.32 | 1.44–7.63 | 0.005 | | | |
| N category |
| N0 | 40 (87.0) | 1 | | | | | |
| N1 | 6 (13.0) | 3.41 | 1.31–8.87 | 0.012 | | | |
| Stage |
| 0, I | 20 (43.5) | 1 | | | 1 | | |
| II, III, IV | 26 (56.5) | 3.57 | 1.51–8.43 | 0.004 | 3.83 | 1.61–9.15 | 0.002 |
| Vessel
invasion |
| Absence | 28 (60.9) | 1 | | | | | |
| Presence | 18 (39.1) | 2.51 | 1.13–5.56 | 0.023 | | | |
| MUC6 |
| Positive | 6 (13.0) | 0.12 | 0.02–0.95 | 0.044 | 0.11 | 0.02–0.87 | 0.036 |
| Negative | 40 (87.0) | 1 | | | 1 | | |
| K7 |
| Positive | 40 (87.0) | 0.81 | 0.30–2.16 | 0.674 | | | |
| Negative | 6 (13.0) | 1 | | | | | |
| K19 |
| Positive | 45 (98.7) | 0.32 | 0.04–2.45 | 0.271 | | | |
| Negative | 1 (1.3) | 1 | | | | | |
| K20 |
| Positive | 16 (34.8) | 1.18 | 0.54–2.57 | 0.685 | | | |
| Negative | 30 (65.2) | 1 | | | | | |
Discussion
Previous studies have investigated the use of K
immunostaining in differentiating ICC from metastatic malignant
tumor from other primary sites (2–4,8).
However, with respect to the clinicopathological and biological
significance, immunohistochemical studies of K expression remain
insufficient. K20 expression in ICC is significantly associated
with gender, tumor location, intraductal papillary type, intestinal
phenotype and MUC2 expression. Although a proportion of ICC cases
express K20, combined immunostaining for K7 and K20 has been
identified to be useful in differentiating ICC from metastatic CRC.
Advanced tumor stage is a poor independent prognostic indicator,
while MUC6 expression is a good independent prognostic indicator.
Additionally, K20 expression was significantly associated with
intraductal papillary growth type and MUC2 expression.
ICC can be categorized into three macroscopic growth
types: mass-forming, periductal infiltrative and intraductal
papillary. Intraductal papillary ICC differs from other types as it
has better prognosis and secreted mucin subtypes (7). It is considered to be the biliary
counterpart of intraductal mucinous neoplasms of the pancreas
(9,10). Immunohistochemically, papillary CCs
are characterized by the frequent co-expression of MUC2, CDX2 and
K20 (9). Zen et al proposed
three carcinogenetic pathways characterized by different
immunophenotypes of mucin and K expression. Intraductal papillary
neoplasms of the bile duct were characterized by an intestinal
phenotype (MUC2+/K20+), and by carcinogenesis
leading to tubular adenocarcinoma with increasing MUC1 expression
(11). Genetic alterations and
molecular changes vary between papillary ICC and non-papillary ICC
(12–14). The close correlation between ICC of
intraductal papillary type and K20+/MUC2+ in
this study supports the hypothesis that the intraductal papillary
type may be different from other types of ICC. In this study, we
also identified that K20-positive ICC was closely associated with
tumor location. This is in accordance with previous studies, which
demonstrated that K20 expression correlated with hilar type ICC
(4,5). The K20-positive rate varies according
to the sites of origin of CC and appears to increase from
peripheral to large extrahepatic bile ducts CC (4). Guedj et al revealed that hilar
and peripheral CC demonstrate different morphological features and
display specific protein profiles, suggesting that hilar and
peripheral CC may be considered to be distinct tumors that follow
specific molecular pathways of carcinogenesis (15).
Differentiating between ICC and metastatic CRC of
the liver may be difficult by means of conventional histological
examination. The use of K7 and K20 immunostaining is relevant for
the differential diagnosis of ICC and metastatic CRC, due to the
specific K profile of metastatic CRC
(K7−/K20+), which differs from that of ICC
(K7+/K20−) (2–5). In
the present study, K20 expression was observed in 35% of the 46 ICC
patients, which was similar to earlier studies of K20 expression in
10–50% of ICCs (2,3,5,8).
However, this result differs from other studies, in which K20
expression was evident in up to 71% of ICC tissues (4). This discrepancy may be explained in
part by the varied criteria for positivity, the different
antibodies used and detection methods applied. In our study, K19
was expressed in 97.8% of ICC and 100% of primary and metastatic
CRC cases. K19 is normally expressed in the lining of the
gastroenteropancreatic and hepatobiliary tracts (16). Therefore, K19 may not be useful in
the differential diagnosis of ICC from metastatic CRC. Similar to
previous studies (2–5), K7 was rarely positive, while in the
present study K20 was usually positive in metastatic CRC. We
identified that the combined K7/K20 immunophenotype was useful when
making a differential diagnosis of ICC and metastatic CRC. The
K7+/K20− profile was specific for ICC (100%),
when compared with that of metastatic CRC, and the PPV of this
phenotype for ICC diagnosis was 100%. In comparison, the
K7−/K20+ profile was specific for metastatic
CRC (93.5%), when compared with that of ICC, and the PPV of this
phenotype for metastatic CRC diagnosis was 84.2%. However, a
precise analysis of clinicopathological features and the use of
additional relevant markers are also required in cases of
K7+/K20+ tumors for correct diagnosis.
ICC is the second most common type of primary
malignant tumor, which demonstrates an extremely poor prognosis,
despite combined therapeutic strategies (17,18). A
recent large-scale study reported that factors associated with
adverse prognosis in ICC included positive margin status, multiple
lesions, T category, lymph node metastasis and vascular invasion
(18). Similarly, we identified
that T category, lymph node metastasis, tumor stage and vessel
invasion were significantly associated with patient survival.
Developments in molecular techniques have improved
our understanding of carcinogenesis in CC and confirmed the role of
biomarkers, including mucins and Ks, in predicting a poor patient
outcome (19). Ks are intermediate
filaments that form part of the cytoskeleton in epithelial cells;
there is increasing interest in their application as prognostic
biomarkers (19,20). Aishima et al identified that
patients with ICC characterized by reduced K903 reactivity, which
detects K1, K5, K10 and K14, displayed a significantly more
favorable survival rate compared to those with preserved K903
reactivity (21). A high serum K19
fragment is associated with tumor progression and poor outcome in
patients with ICC (22). The
expression of K20 and its significance as a prognostic factor in
ICC has not been elucidated. In the present study, the survival
rate of patients with K20-positive ICC was lower than that of
patients with K20-negative ICC; however, the difference was not
significant. A longer term follow-up with a larger cohort is
required to define the biological behavior of K20-positive ICC.
There is a strong correlation between the expression
of mucin antigens and the survival of ICC patients (19). MUC1 is important in the invasiveness
and metastatic potential of CC, and usually correlates with a
decreased survival (23–25). In contrast to MUC1, MUC2 acts as a
protective protein and is associated with a more favorable
prognosis (26,27). MUC5AC is a gel-forming secreted
mucin and serum MUC5AC is likely to be a poor prognostic factor in
CC patients (28,29). In the present study, MUC6 expression
was a good independent prognostic factor in ICC, which is
consistent with previous findings (29,30).
Further investigation is required to clarify the mechanisms of
mucin expression associated with prognosis.
In conclusion, our study indicates that a proportion
of ICC (35%) retains K20 expression, and combined immunostaining
for K7 and K20 is useful when making a differential diagnosis of
ICC and metastatic CRC. K20 expression is also significantly
associated with gender, location and macroscopic growth pattern of
tumor, intestinal phenotype and MUC2 expression. Finally, we
identified that MUC6 expression in ICC is a good independent
prognostic factor.
Acknowledgements
This study was supported by the National Research
Foundation of Korea Grant funded by the Korea government (No.
2011-0028223).
References
|
1
|
Moll R, Franke WW, Schiller DL, Geiger B
and Krepler R: The catalog of human cytokeratins: patterns of
expression in normal epithelia, tumors and cultured cells. Cell.
31:11–24. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miettinen M: Keratin 20:
immunohistochemical marker for gastrointestinal, urothelial, and
Merkel cell carcinomas. Mod Pathol. 8:384–388. 1995.PubMed/NCBI
|
|
3
|
Maeda T, Kajiyama K, Adachi E, Takenaka K,
Sugimachi K and Tsuneyoshi M: The expression of cytokeratins 7, 19,
and 20 in primary and metastatic carcinomas of the liver. Mod
Pathol. 9:901–909. 1996.PubMed/NCBI
|
|
4
|
Rullier A, Le Bail B, Fawaz R, Blanc JF,
Saric J and Bioulac-Sage P: Cytokeratin 7 and 20 expression in
cholangiocarcinomas varies along the biliary tract but still
differs from that in colorectal carcinoma metastasis. Am J Surg
Pathol. 24:870–876. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimonishi T, Miyazaki K and Nakanuma Y:
Cytokeratin profile relates to histological subtypes and
intrahepatic location of intrahepatic cholangiocarcinoma and
primary sites of metastatic adenocarcinoma of liver.
Histopathology. 37:55–63. 2000. View Article : Google Scholar
|
|
6
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; New York, NY: 2010
|
|
7
|
Nakanuma Y, Curado MP, Franceschi S, Gores
G, Paradis V, Sripa B, Tsui WMS and Wee A: Intrahepatic
cholangiocarcinoma. WHO Classification Of Tumours Of The Digestive
System. Bosman FT, Carneiro F, Hruban RH and Theise ND: 4th
edition. IARC Press; Lyon: pp. 217–224. 2010
|
|
8
|
Moll R, Lowe A, Laufer J and Franke WW:
Cytokeratin 20 in human carcinomas. A new histodiagnostic marker
detected by monoclonal antibodies. Am J Pathol. 140:427–447.
1992.PubMed/NCBI
|
|
9
|
Zen Y, Fujii T, Itatsu K, et al: Biliary
papillary tumors share pathological features with intraductal
papillary mucinous neoplasm of the pancreas. Hepatology.
44:1333–1343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakanuma Y, Sasaki M, Ishikawa A, Tsui W,
Chen TC and Huang SF: Biliary papillary neoplasm of the liver.
Histol Histopathol. 17:851–861. 2002.PubMed/NCBI
|
|
11
|
Zen Y, Sasaki M, Fujii T, et al: Different
expression patterns of mucin core proteins and cytokeratins during
intrahepatic cholangiocarcinogenesis from biliary intraepithelial
neoplasia and intraductal papillary neoplasm of the bile duct - an
immunohistochemical study of 110 cases of hepatolithiasis. J
Hepatol. 44:350–358. 2006.
|
|
12
|
Sugimachi K, Taguchi K, Aishima S, et al:
Altered expression of beta-catenin without genetic mutation in
intrahepatic cholangiocarcinoma. Mod Pathol. 14:900–905. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hidaka E, Yanagisawa A, Seki M, Setoguchi
T and Kato Y: Genetic alterations and growth pattern in biliary
duct carcinomas: loss of heterozygosity at chromosome 5q bears a
close relation with polypoid growth. Gut. 48:656–659. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Isa T, Tomita S, Nakachi A, Miyazato, et
al: Analysis of microsatellite instability, K-ras gene mutation and
p53 protein overexpression in intrahepatic cholangiocarcinoma.
Hepatogastroenterology. 49:604–608. 2002.PubMed/NCBI
|
|
15
|
Guedj N, Zhan Q, Perigny M, et al:
Comparative protein expression profiles of hilar and peripheral
hepatic cholangiocarcinomas. J Hepatol. 51:93–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jain R, Fischer S, Serra S and Chetty R:
The use of Cytokeratin 19 (CK19) immunohistochemistry in lesions of
the pancreas, gastrointestinal tract, and liver. Appl
Immunohistochem Mol Morphol. 18:9–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
18
|
de Jong MC, Nathan H, Sotiropoulos GC, et
al: Intrahepatic cholangiocarcinoma: an international
multi-institutional analysis of prognostic factors and lymph node
assessment. J Clin Oncol. 29:3140–3145. 2011.PubMed/NCBI
|
|
19
|
Briggs CD, Neal CP, Mann CD, Steward WP,
Manson MM and Berry DP: Prognostic molecular markers in
cholangiocarcinoma: a systematic review. Eur J Cancer. 45:33–47.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Linder S: Cytokeratin markers come of age.
Tumour Biol. 28:189–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aishima S, Asayama Y, Taguchi K, et al:
The utility of keratin 903 as a new prognostic marker in
mass-forming-type intrahepatic cholangiocarcinoma. Mod Pathol.
15:1181–1190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uenishi T, Yamazaki O, Tanaka H, et al:
Serum cytokeratin 19 fragment (CYFRA21-1) as a prognostic factor in
intrahepatic cholangiocarcinoma. Ann Surg Oncol. 15:583–589. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Higashi M, Yonezawa S, Ho JJ, et al:
Expression of MUC1 and MUC2 mucin antigens in intrahepatic bile
duct tumors: its relationship with a new morphological
classification of cholangiocarcinoma. Hepatology. 30:1347–1355.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park SY, Roh SJ, Kim YN, et al: Expression
of MUC1, MUC2, MUC5AC and MUC6 in cholangiocarcinoma: prognostic
impact. Oncol Rep. 22:649–657. 2009.PubMed/NCBI
|
|
25
|
Matsumura N, Yamamoto M, Aruga A, Takasaki
K and Nakano M: Correlation between expression of MUC1 core protein
and outcome after surgery in mass-forming intrahepatic
cholangiocarcinoma. Cancer. 94:1770–1776. 2002. View Article : Google Scholar
|
|
26
|
Hong SM, Cho H, Moskaluk CA, Frierson HF
Jr, Yu E and Ro JY: CDX2 and MUC2 protein expression in
extrahepatic bile duct carcinoma. Am J Clin Pathol. 124:361–370.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tamada S, Goto M, Nomoto M, et al:
Expression of MUC1 and MUC2 mucins in extrahepatic bile duct
carcinomas: its relationship with tumor progression and prognosis.
Pathol Int. 52:713–723. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boonla C, Wongkham S, Sheehan JK, et al:
Prognostic value of serum MUC5AC mucin in patients with
cholangiocarcinoma. Cancer. 98:1438–1443. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aishima S, Kuroda Y, Nishihara Y, et al:
Gastric mucin phenotype defines tumour progression and prognosis of
intrahepatic cholangiocarcinoma: gastric foveolar type is
associated with aggressive tumour behaviour. Histopathology.
49:35–44. 2006. View Article : Google Scholar
|
|
30
|
Thuwajit P, Chawengrattanachot W, Thuwajit
C, Sripa B, Paupairoj A and Chau-In S: Enhanced expression of mucin
6 glycoprotein in cholangiocarcinoma tissue from patients in
Thailand as a prognostic marker for survival. J Gastroenterol
Hepatol. 23:771–778. 2008. View Article : Google Scholar : PubMed/NCBI
|