Introduction
Similar to moderately differentiated hypervascular
hepatocellular carcinoma (HCC), poorly differentiated HCC is
generally rich in vasculature. Accordingly, these types of HCCs
have often been classified together as ‘hypervascular HCC’ and as a
consequence, ultrasonography (US) studies focusing exclusively on
poorly differentiated HCC are rare. Here, we study a case of poorly
differentiated HCC for which we conducted a detailed analysis of US
images. The study was performed with the approval of the ethics
committee at Toho University Omori Medical Center and with the
consent of the patient.
Case report
A 60-year-old female patient was diagnosed as being
a hepatitis B virus (HBV) carrier at approximately 30 years of age.
Routine annual physical examinations since the initial diagnosis
detected no abnormality until 2007 when US findings indicated the
presence of a hepatic mass in the S5 region. The patient was
referred to the Toho University Omori Medical Center (Tokyo, Japan)
for a complete physical examination in November 2007. No subjective
symptoms were observed. The patient had no history of alcohol
drinking or smoking; however, the mother of the patient had
succumbed to hepatic cirrhosis B. Additionally, with the exception
of hospitalization due to a gastric ulcer at the age of 20 years,
the patient had no previous history of major illness. Physical
examination findings at presentation included height (151 cm),
weight (45 kg), blood pressure (124/80 mmHg) and pulse rate (60
bpm). In addition, no anemia, jaundice, leg edema, palpable
superficial lymph nodes, heart murmurs or pulmonary rales on
auscultation were detected. The patient’s abdomen was soft and flat
with no tenderness or splenomegaly, and neurological examinations
revealed no abnormal findings.
Hematological findings revealed that the patient was
positive for hepatitis B surface antigen (s) and e-antibody (e),
but not for hepatitis C virus (HCV) antibody, and the HBV-DNA
concentration was 5.2 LGE/ml. The levels of α-fetoprotein (AFP),
protein induced by vitamin K absence or antagonist (PIVKA) II and
carcinoembryonic antigen (CEA) tumor markers were all within the
normal range (Table I). B-mode US
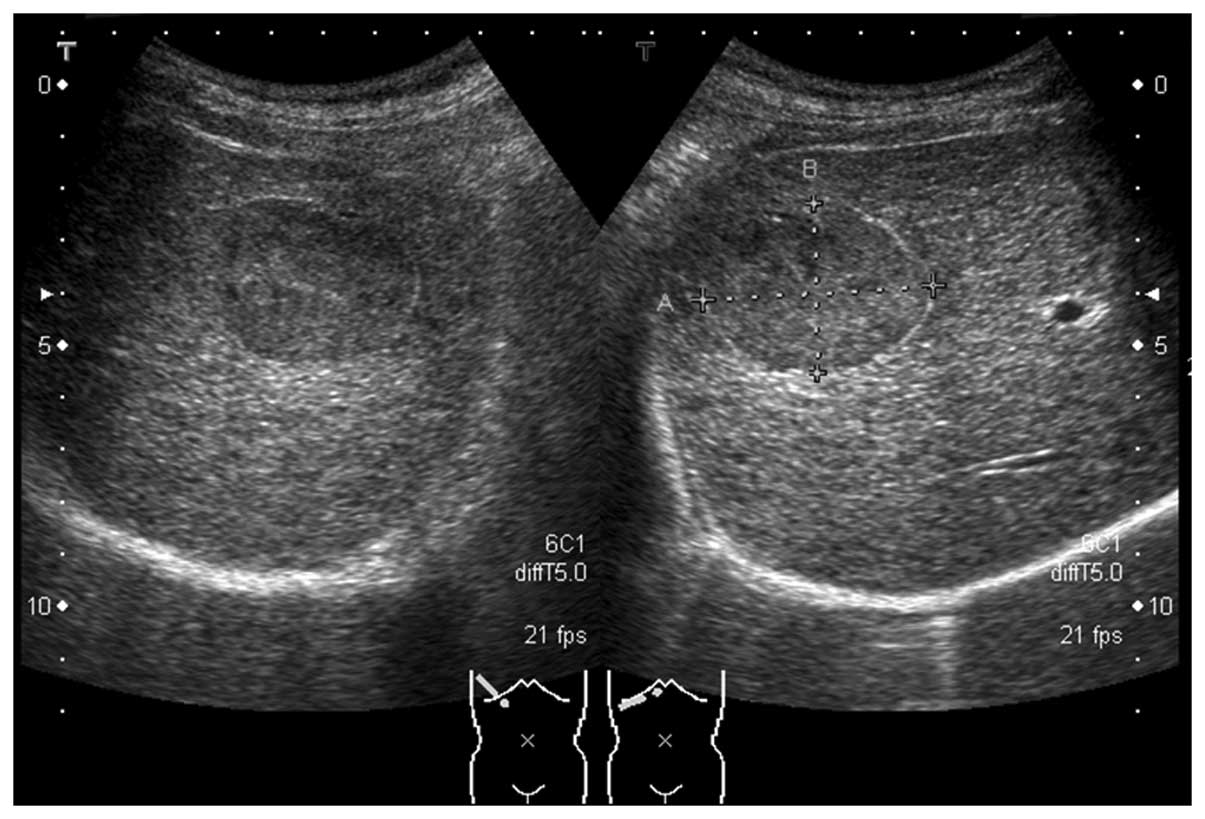
revealed the presence of an oval-shaped mass (44×32 mm in diameter)
in the S5 region of the liver. The mass had a hyperechoic rim-like
high-echo band along the margin and an internal echo pattern, which
was homogeneous and isoechoic to the surrounding hepatic tissue
(Fig. 1). Contrast-enhanced US
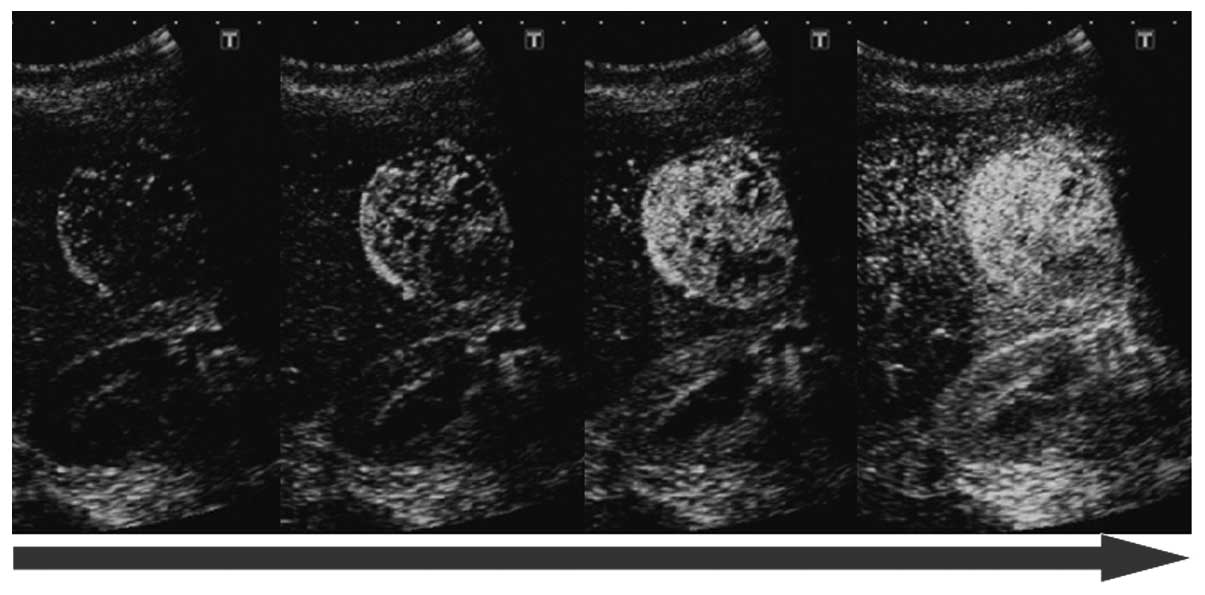
(CEUS) with Sonazoid demonstrated an early enhancement pattern
extending from the outside to the inside of the mass in the
vascular phase, and an enhancement pattern similar to that of the
surrounding hepatic tissue in the post-vascular phase (Figs. 2 and 3). B-mode findings suggested that the
lesion was a hemangioma, and due to the early enhancement pattern
observed in the vascular phase of CEUS, the lesion was suspected to
be a high-flow hemangioma. As the patient was a HBV carrier, we
conducted magnetic resonance imaging (MRI) and computed tomography
(CT) to eliminate the possibility of HCC. The mass was detected as
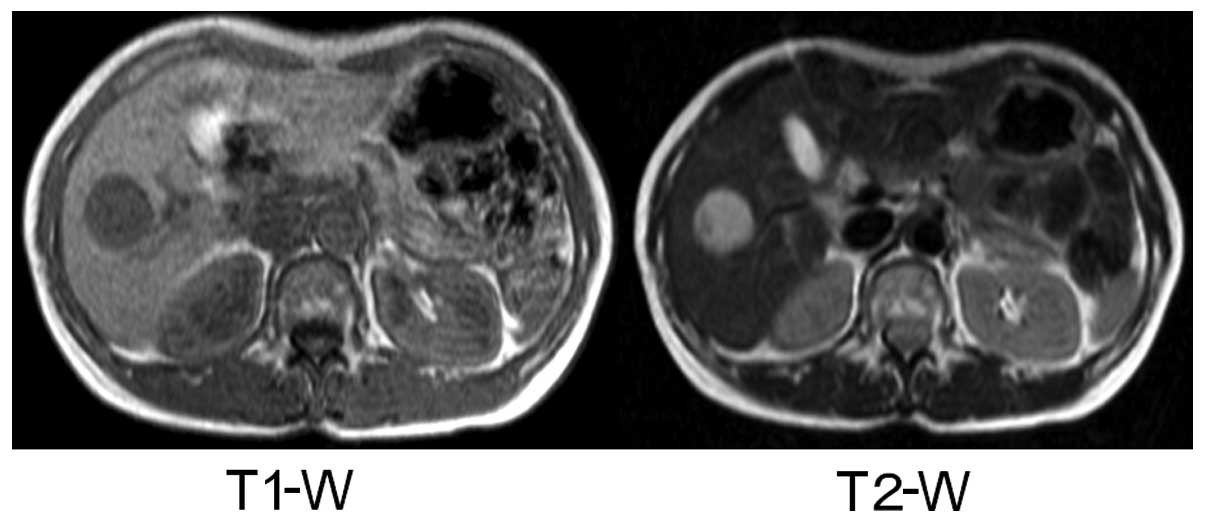
low- and high-intensity signals on T1- and T2-weighted MRI images,
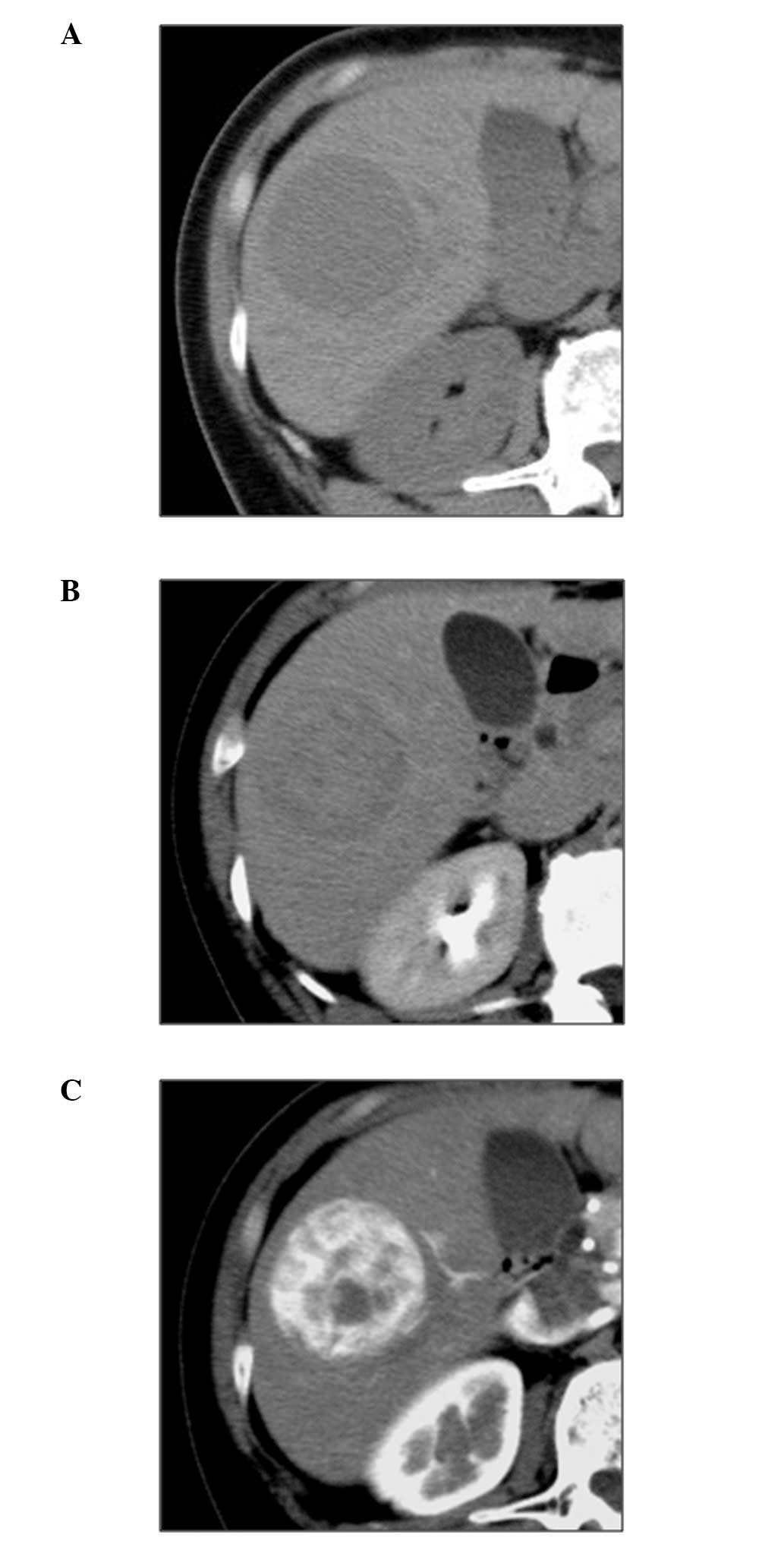
respectively (Fig. 4). An abdominal
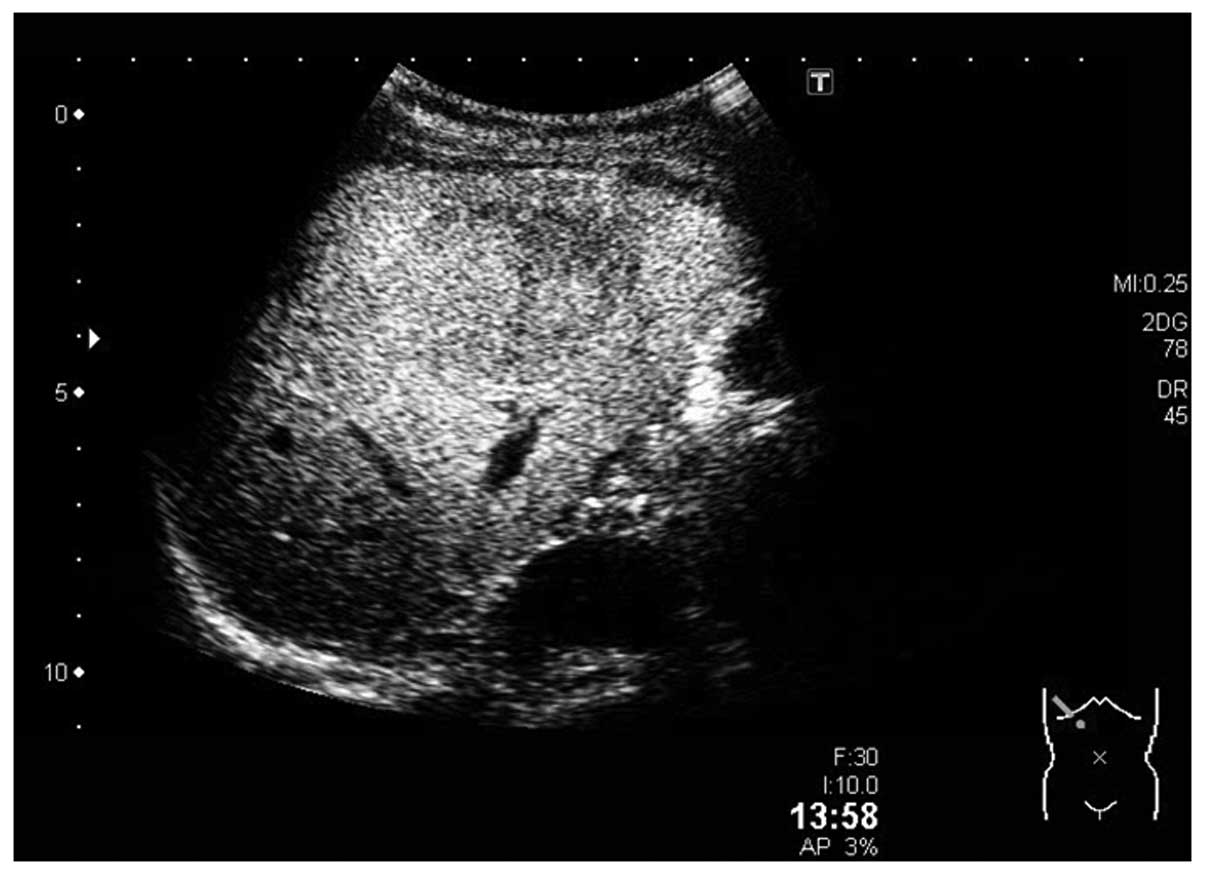
CT scan revealed a low-density lesion in the plain phase, a
heterogeneous high-density lesion in the early phase and a
low-density lesion in the equilibrium phase (Fig. 5). Based on the collected results,
the patient was diagnosed with hypervascular HCC and was subjected
to laparoscopic right hepatic lobectomy.
 | Table ILaboratory data of patient on
admission. |
Table I
Laboratory data of patient on
admission.
| Hematological
findings | Result |
|---|
| Peripheral blood |
| Hb (g/dl) | 13.1 |
| Ht (%) | 39.8 |
| RBC
(/μl) |
407×104 |
| WBC
(/μl) | 5500 |
| Plt
(/μl) |
24.5×104 |
| Coagulation test |
| PT (%) | 100.0 |
| Blood chemistry |
| BUN (mg/dl) | 14 |
| Cr (mg/dl) | 0.57 |
| TP (g/dl) | 7.4 |
| Alb (g/dl) | 4.5 |
| AST (IU/l) | 49 |
| ALT (IU/l) | 43 |
| LDH (IU/l) | 385 |
| ALP (IU/l) | 240 |
| γ-GTP (IU/l) | 11 |
| T-bil (mg/dl) | 0.8 |
| ChE (IU/l) | 330 |
| AMY (IU/l) | 104 |
| FBS (mg/dl) | 74 |
| HbA1c (%) | 5.1 |
| CRP (mg/dl) | 0.2 |
| Virus markers |
| HBsAg
(expression) | + |
| HBsAb
(expression) | − |
| HBeAg
(expression) | − |
| HBeAb
(expression) | + |
| HBV-DNA
(LGE/ml) | 5.2 |
| HCV-Ab
(expression) | − |
| Tumor markers |
| AFP (mg/ml) | 2.7 |
| PIVKA II (U/ml) | 11 |
| CEA (U/ml) | 2.9 |
Macroscopic findings revealed a brownish colour and
an irregular surface of the liver (Fig.
6). No enlargement or atrophy was observed and the liver edge
was reasonably sharp. Additionally, the tumor was a 40×28 mm
grayish-white solid mass with a relatively clear margin and a
capsule-like structure.
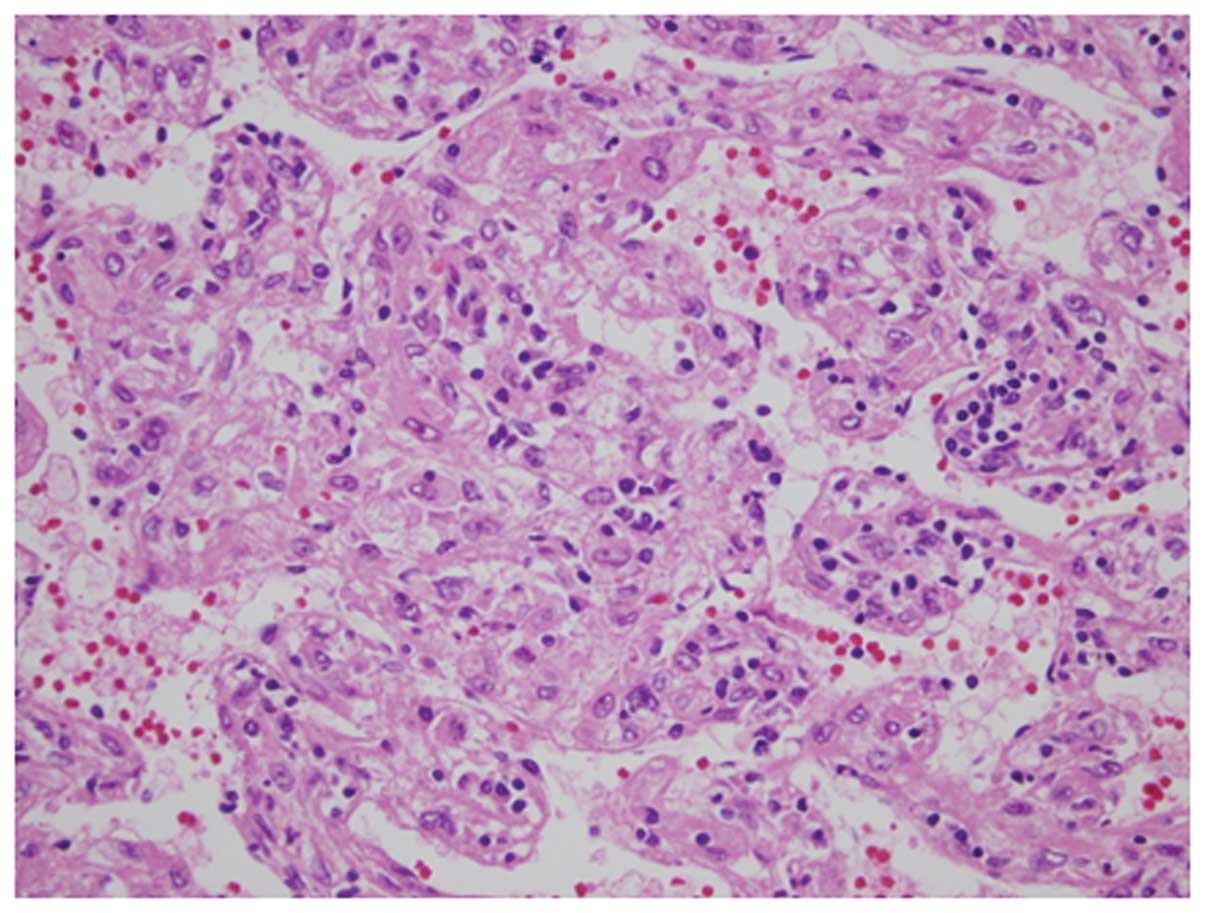
Histopathological findings identified solid tumor
cells in the mass region (Figs. 7
and 8). Slit-like, or in certain
parts, blood sinus-like spaces composed of endothelial cells were
observed to be arranged in slightly ambiguous five-to-seven-layer
cords. Tumor cells were not markedly eosinophilic and had an
increased nuclear/cytoplasmic (N/C) ratio. Additionally, the cells
had polymorphic and chromatin-rich nuclei. The mass was surrounded
by a fibrous capsule, which was internally lined with a layer of
lipid droplets as thick as the fibrous capsule, spreading along
almost the entire circumference of the tumor. The pathological
diagnosis was poorly differentiated HCC. The non-cancerous area was
diagnosed as chronic hepatitis (F2/A1).
Discussion
Poorly differentiated HCC has a worse prognosis
compared to well- and moderately differentiated HCC (1). Poorly differentiated HCC is also
reported to have poor outcomes following living donor liver
transplantation, which has increased in recent years (2). However, as poorly and moderately
differentiated HCC have been grouped together as ‘hypervascular
HCC’, the imaging characteristics of the former have rarely been
studied in detail.
According to previous studies, hypervascular HCCs
with a diameter greater than 15 mm observed using US are often
associated with a hypoechoic halo (3), and a CEUS with Sonazoid signal that is
weaker than that in the surrounding liver during the post-vascular
phase (4). However, in the present
case, we observed imaging features that are uncharacteristic of
hypervascular HCC, including a hyperechoic (not hypoechoic) band
around the mass on B-mode US, and the absence of perfusion defects
on CEUS with Sonazoid images in the post-vascular phase.
Possible reasons for the atypical US finding of a
hyperechoic band are that, from the histological standpoint, the
tumor was not only enclosed by a fibrous outer capsule, but also
covered by a layer of lipid droplets of a width similar to that of
the capsule. This lipid droplet layer is suggested to be the
hyperechoic rim observed on B-mode US. It is unclear why the tumor
was covered with a layer of lipid droplets; however, it is possible
that these droplets were pushed outwards in the late maturation
phase as the biological malignancy of the tumor increased.
The absence of perfusion defects on CEUS with
Sonazoid images in the post-vascular phase may have been caused by
portal vein tumor thrombus in the vicinity of the tumor. The number
of Kupffer cells reduces as the biological malignancy of HCC
progresses (5–7). Furthermore, the post-vascular phase of
Sonazoid-enhanced US imaging mainly reflects the number and
functional role of the Kupffer cells in the liver, indicating that,
in the case of hypervascular HCC, defects are present during the
post-vascular phase of US (4).
However, in the present case, the tumor did not appear as a defect,
but as an isoechoic mass compared to the surrounding liver in that
phase. A reason for this may be the presence of a portal vein tumor
thrombus, which appears progressively as HCC develops (8). If a tumor thrombus is present in the
portal vein, which serves as the exit for tumor blood flow, the
contrast agent that enters the tumor will be prevented from exiting
the tumor. This would force the contrast agent to remain in the
tumor and not cause a perfusion defect. According to the 17th
Annual Survey and Follow-up Study of Primary Liver Cancer conducted
by the Liver Cancer Study Group of Japan, poorly differentiated HCC
has a higher incidence of portal vein tumor thrombus compared to
moderately differentiated HCC (9).
The study also revealed that poorly differentiated HCC had fewer
defects on post-vascular phase Sonazoid-enhanced US images compared
to moderately differentiated HCC.
Poorly differentiated HCC has a worse prognosis than
well- and moderately differentiated HCC. Clinically, it is
extremely important to make a precise diagnosis of the disease
using diagnostic imaging techniques, particularly non-invasive US.
Here, we studied an notable case of poorly differentiated HCC which
offers an insight into the relatively unknown imaging
characteristics of poorly differentiated HCC.
Abbreviations:
|
US
|
ultrasonography
|
|
CEUS
|
contrast-enhanced ultrasonography
|
References
|
1
|
Nakajima Y, Shimamura T, Kamiyama T,
Kimura J, Sato N, Matsushita M, Une Y and Uchino J: Evaluation of
surgical resection for small hepatocellular carcinomas. Am J Surg.
171:360–363. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jonas S, Bechstein WO, Steinmüller T,
Herrmann M, Radke C, Berg T, Settmacher U and Neuhaus P: Vascular
invasion and histopathologic grading determine outcome after liver
transplantation for hepatocellular carcinoma in cirrhosis.
Hepatology. 33:1080–1086. 2001. View Article : Google Scholar
|
|
3
|
Ohkuma K: Ultrasound diagnosis. Jpn J
Cancer Clin. 47:987–994. 2001.(In Japanese).
|
|
4
|
Shunichi S, Hiroko I, Fuminori M and Waki
H: Definition of contrast enhancement phases of the liver using a
perfluoro-based microbubble agent, perflubutane microbubbles.
Ultrasound Med Biol. 35:1819–1827. 2009. View Article : Google Scholar
|
|
5
|
Tobe K, Tsuchiya T and Fujiwara R: Kupffer
cells in well-differentiated tissue of hepatocellular carcinoma.
Acta Hepatol Jpn. 26:630–637. 1985.(In Japanese with English
abstract).
|
|
6
|
Sugihara S, Nakashima O and Kiyomatsu K:
Pathomorphologic study on macrophages in hepatocellular carcinoma.
Acta Hepatol Jpn. 31:12–18. 1999.(In Japanese with English
abstract).
|
|
7
|
Tanaka M, Nakashima O, Wada Y, Kage M and
Kojiro M: Pathomorphological study of Kupffer cells in
hepatocellular carcinoma and hyperplastic nodular lesions in the
liver. Hepatology. 24:807–812. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yabuuchi I, Matuda Y and Ifuji T:
Classification of contrast enhanced US images of small
hepatocellular carcinoma using Levovist. Kan Tan Sui. 47:175–182.
2003.(In Japanese with English abstract).
|
|
9
|
Ikai I, Arii S, Okazaki M, Okita K, Omata
M, Kojiro M, Takayasu K, Nakanuma Y, Makuuchi M, Matsuyama Y,
Monden M and Kudo M: Report of the 17th Nationwide Follow-up Survey
of Primary Liver Cancer in Japan. Hepatol Res. 37:676–691. 2007.
View Article : Google Scholar : PubMed/NCBI
|