Introduction
Melanoma is the most aggressive form of skin cancer,
and is resistant to the currently used cancer therapeutic
modalities (1). Early diagnosis
followed by surgical resection improves the prognosis of patients
with melanoma. However, despite careful follow-up and treatment
with combination chemotherapy or adjuvant therapy, patients
frequently develop both local and distant metastases. Patients with
distant metastases almost always have a poor clinical outcome
(2).
Angiogenesis has a key role in the process of growth
and metastasis of primary solid tumors. A tumor usually begins
small and is localized, due to the lack of a vascular supply. Thus,
depriving a tumor of its vascular supply by means of
anti-angiogenic agents has been of great interest since its
proposal in the 1970s (3). Pigment
epithelium-derived factor (PEDF), a 50-kDa secreted glycoprotein
from the serine protease inhibitor superfamily, was described as
the most potent endogenous inhibitor of angiogenesis (4). PEDF exerts its anti-angiogenic
activity by inducing apoptosis in endothelial cells as well as by
inhibiting endothelial cell proliferation and migration even in the
presence of VEGF (5). The potential
of PEDF as a purified protein or using gene transfer approaches
with viral and non-viral vectors has been tested in several tumor
models including melanoma in previous studies. However, a
suboptimal half-life in plasma or its side effects reduce its
possible therapeutic effects (6,7). Thus,
a more efficient and safer approach is required.
Mesenchymal stem cells (MSCs), which have the
potential to differentiate along osteogenic, adipogenic and
chondrogenic lineages, were described as novel and efficient
therapeutic tools for the targeted delivery and local production of
biological agents in tumors (8).
The most significant source of mesenchymal stem cells is currently
bone marrow. However, cells from the bone marrow may only be
obtained through an invasive procedure, and stem cell numbers
decrease significantly with the age of the individual (9). For this reason, alternative sources
from where MSCs may be isolated have been sought. One significant
source is the placenta (10).
Several studies indicate that placenta-derived MSCs (PDMSCs) are
similar to stem cells from the bone marrow with respect to their
cell characteristics and multilineage differentiation potential
(11–13). The placenta fulfills two main
desiderata of cell therapy: obtaining as high as possible number of
cells and use of non-invasive methods for their harvesting
(14). Moreover, since
placenta-derived multipotent cells are fetal in origin, they may
generate less of an immune response than adult bone marrow MSCs
(15). These characteristics make
PDMSCs potential candidates for clinical application in cell-based
therapies.
In this study, we evaluated the antitumor activity
of human PDMSC transduced with a recombinant adenovirus expressing
PEDF in a mouse melanoma model. The results demonstrated that
treatment with PEDF-secreting PDMSCs (PDMSC-PEDF) led to a notable
inhibition of tumor growth associated with a decreased number of
microvessels and an increased apoptotic index of tumor cells.
Materials and methods
Cell lines and culture
B16-F10 mouse melanoma cell lines and human
embryonic kidney 293 cell lines were purchased from the American
Type Culture Collection (ATCC, Rockville, MD, USA). Cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL,
Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal
bovine serum (FBS; Gibco, Auckland, NZ), 2 mM L-glutamine and 100
μg/ml amikacin. Human umbilical vein endothelial cells (HUVECs)
were isolated from human umbilical cord veins as previously
described (16), and grown in EBM-2
medium with SingleQuots (Lonza Cologne GmbH, Walkersville, MA, USA)
containing VEGF and other growth factors. HUVECs were used between
passages 2 and 6.
Isolation, expansion and characterization
of human placenta-derived MSCs
After receiving informed consent, placenta was
obtained by vaginal delivery or caesarean sections from women
following uncomplicated full-term pregnancies. The MSCs were
isolated from the placenta as described previously (13). Briefly, placental tissue was
dissected following the drainage of umbilical cord blood. Following
mechanical and enzymatic treatment, the homogenate was cultured in
low-glucose DMEM (Gibco) supplemented with 10% FBS, 50 U/ml
penicillin and 50 μg/ml streptomycin, and incubated at 37°C in a 5%
CO2 atmosphere. After 48 days, the non-adherent
hematopoietic cells were discarded and the adherent MSCs were
preserved for further expansion. Medium changes were performed
twice per week. PDMSCs between passages 5 and 8 were used in the
experiments. Phenotype characteristics of the PDMSCs were analyzed
by flow cytometry (BD Biosciences, San Jose, CA, USA) using CD34,
CD44, CD45, CD73, CD90 and CD105 (BD Biosciences).
Adenoviral transduction of PDMSCs
The adenoviruses were created using the AdEasy
system. The viruses were amplified in HEK293 cells and purified on
CsCl gradients according to standard methods (7). The PDMSCs were transduced with
recombinant adenovirus at a multiplicity of infection (MOI) of
1500. Prior to transduction, the growth medium was removed and the
cells washed once with serum-free medium. Virus infection was
performed for 4 h at 37°C and the infection medium was replaced
with complete medium. PDMSCs were also infected with adenovirus
LacZ (Ad-LacZ) at an MOI of 1500 as a control. After 48 h the
virus-infected PDMSCs were harvested for subsequent
experiments.
Western blot analysis and ELISA
assay
Western blot analysis was conducted as previously
described (17). Briefly, PDMSCs
were transduced with adenoviruses for 4 h and the virus-containing
medium was changed for serum-free low-glucose DMEM. After a further
48 h of incubation, the conditioned media (CM) were collected. The
CM were concentrated by super filter (10 kDa, Millipore, Billerica,
MA, USA), and western blot assay was performed using a mouse
anti-human PEDF monoclonal antibody (R&D Systems, Boston, MA,
USA). The concentration of the PEDF secreted in the CM was measured
using a sandwich enzyme-linked immunosorbent assay (ELISA) kit for
the human PEDF protein (GBD, San Diego, CA, USA) following the
manufacturer's instructions.
HUVEC migration inhibition assay
The Transwell migration assay was used to determine
the effect of PEDF secreted from Ad-PEDF-infected PDMSCs on HUVECs
and was performed as previously described (18,19).
Briefly, HUVECs (2×104 per well) were suspended in 200
μl of the CM derived from PDMSCs, PDMSC-LacZ and PDMSC-PEDF,
respectively, and seeded in the upper chamber which was coated with
50 μl Matrigel. The lower well of the Transwell plate was filled
with 600 μl EBM-2 medium containing various growth factors. After
24 h of incubation, non-migrated cells were scraped. The cells that
had migrated to the opposite side of the membrane were fixed with
100% methanol, stained with 0.05% crystal violet, sealed on slides,
and counted by microscopy (Olympus; magnification, ×100) with 5
fields.
HUVEC proliferation inhibition assay
Anti-angiogenic activity of PEDF produced by
PDMSC-PEDF was also confirmed by a HUVEC proliferation inhibition
assay as described previously (20). The CM were obtained from PDMSCs,
PDMSC-LacZ and PDMSC-PEDF, respectively. HUVECs (8×103)
had been seeded on 24-well plates the previous day. At 50%
confluence, the cells were washed with phosphate-buffered saline
(PBS) following the removal of the media, and then 500 μl CM was
added. The cells were incubated at 37°C in 5% CO2 for 72
h. The cells were then trypsinized, and the number of viable cells
was counted using a trypan blue assay.
In vivo experiments
Female C57BL/6 mice, 6 to 8 weeks old, were
purchased from the West China Experimental Animal Center of Sichuan
University, China, and were maintained in pathogen-free conditions
with sterile chow. All animal procedures were conducted according
to guidelines provided by the Animal Care and Use Committee of West
China Hospital Cancer Center. B16-F10 melanoma cells
(1×105) were injected into the right flank of each mouse
subcutaneously. When tumor diameters reached 3 mm, mice were
randomly divided into four groups: i) mice treated with PBS, ii)
mice treated with 5×105 PDMSCs, iii) mice treated with
5×105 PDMSC-LacZ, and iv) mice treated with
5×105 PDMSC-PEDF. The tumors were treated twice by
intratumoral injection at a 4-day interval. Tumor growth was
monitored every 3 days by caliper and the volume was calculated as
0.52 × length × width2 (21). When any mice began to moribund they
were sacrificed. Subcutaneous tumors from sacrificed mice were
removed and the weight was recorded.
Immunohistochemical analysis and TUNEL
assay
To determine the effect of anti-angiogenesis
treatment on vessel density, frozen sections were fixed in acetone,
incubated and probed with an anti-CD31 antibody (BD Biosciences) as
previously described (22). The
sections were then visualized and microvessels were calculated with
a microscope (Olympus) at a magnification of ×400.
The analysis of apoptotic cells in tumor tissue was
performed by terminal deoxynucleotidyl transferase-mediated dUTP
nick-end labeling (TUNEL) staining using the DeadEnd Fluorometric
TUNEL system (Promega, Madison, WI, USA) following the
manufacturer's guide. Images of the sections were captured using a
fluorescence microscope (Olympus). The apoptotic index was
calculated by dividing the number of TUNEL-positive cells by the
total number of cells in the field (5 high-power fields per
slide).
Statistical analysis
Values were shown as the means ± SEM (standard error
of the mean), and SPSS 17.0 was used for statistical analysis. The
statistical significances among the different groups were evaluated
using one-way analysis of variance (ANOVA). P<0.05 was
considered to indicate a statistically significant result.
Results
Adenoviral transduction of PDMSCs and
confirmation of PEDF expression in vitro
The phenotype characteristics of the isolated and
expanded PDMSCs were confirmed by flow cytometry. PDMSCs were
cultured to reach to ~90% confluence and incubated with
adenoviruses at a MOI of 1500 for 4 h. After 48 h, the secreted
PEDF in the CM was confirmed by western blot and ELISA. Western
blot showed that PEDF was only detected in the CM from
Ad-PEDF-transduced PDMSCs, but not in Ad-LacZ-transduced PDMSCs nor
in untransduced PDMSCs (Fig. 1A).
These results indicate that our recombinant adenovirus successfully
transferred the PEDF gene into PDMSCs and produced secretory
protein. ELISA revealed that PDMSC-PEDF cells had secreted PEDF
into the CM at a concentration of 65.2±4.9 ng/ml; however, only a
minimal amount of PEDF was detected in the CM from
Ad-LacZ-transduced and untransduced PDMSCs (Fig. 1B).
PEDF from PDMSC-PEDF inhibited the
migration and proliferation of HUVECs in vitro
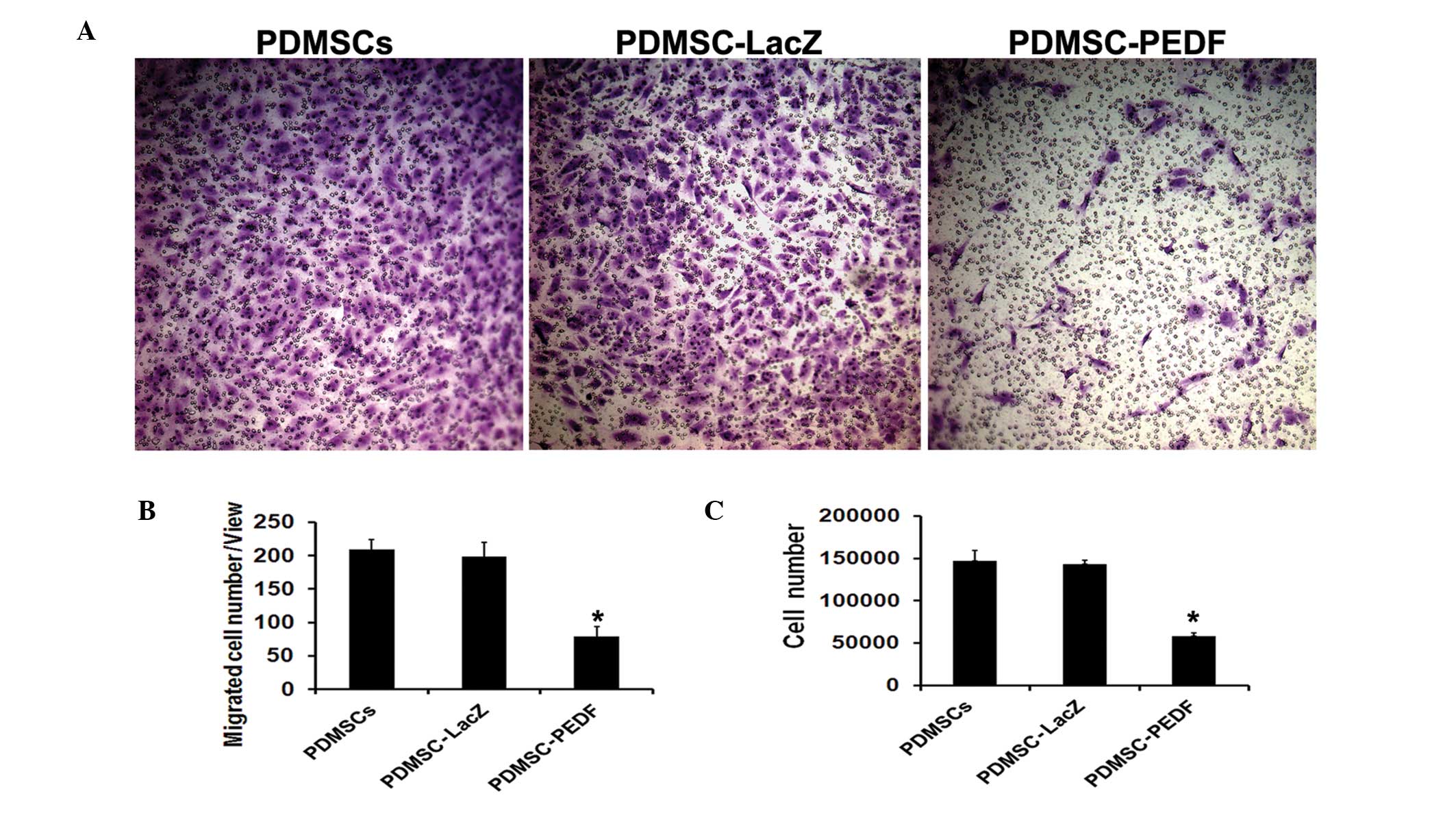
The bioactivity of PEDF expressed by PDMSC-PEDF was
verified by HUVEC migration inhibition assay and proliferation
inhibition assay. The CM from PDMSC-PEDF markedly reduced
endothelial cell migration, but the control CM from
Ad-LacZ-transduced and untransduced PDMSCs had no inhibitory effect
on it (P<0.05) (Fig. 2A and B).
The CM from PDMSCs-PEDF significantly inhibited HUVEC proliferation
compared with that from PDMSCs or PDMSC-LacZ (P<0.05) (Fig. 2C). These results indicate that the
secretory PEDF was functional.
PDMSC-PEDF inhibited the growth of
B16-F10 melanoma in vivo
To examine the therapeutic effect of PEDF
gene-modified PDMSCs in vivo, C57BL/6 mice bearing B16-F10
subcutaneous tumors were treated with PBS, 5×105 PDMSCs,
5×105 PDMSC-LacZ, or 5×105 PDMSC-PEDF two
times at a 4-day interval by intratumoral injection. The tumor
volume in the PDMSC-PEDF-treated group was significantly smaller
than that in the control groups (P<0.05). The mean tumor volume
(± SD) in PDMSC-PEDF-treated mice was 1287.1±284.3 mm3
versus 3439.1±417 mm3 in PDMSCs-LacZ-treated mice,
3620.4±279.7 mm3 in PDMSC-treated mice and 3782.4±315.3
mm3 in PBS-treated mice (Fig. 3A). There was no significant
difference between the PDMSC-treated group and the PBS-treated
group (P>0.05). The tumor weight was measured when the mice were
sacrificed. The mean tumor weights were 2.89±0.19, 2.76±0.41,
2.56±0.42 and 0.98±0.13 g in the PBS-, PDMSC-, PDMSC-LacZ- and
PDMSC-PEDF-treated groups, respectively (Fig. 3B). Taken together, the data
demonstrate that PDMSC-PEDF has a significant and prolonged
inhibitory effect on the tumor growth of B16-F10 melanoma in
vivo.
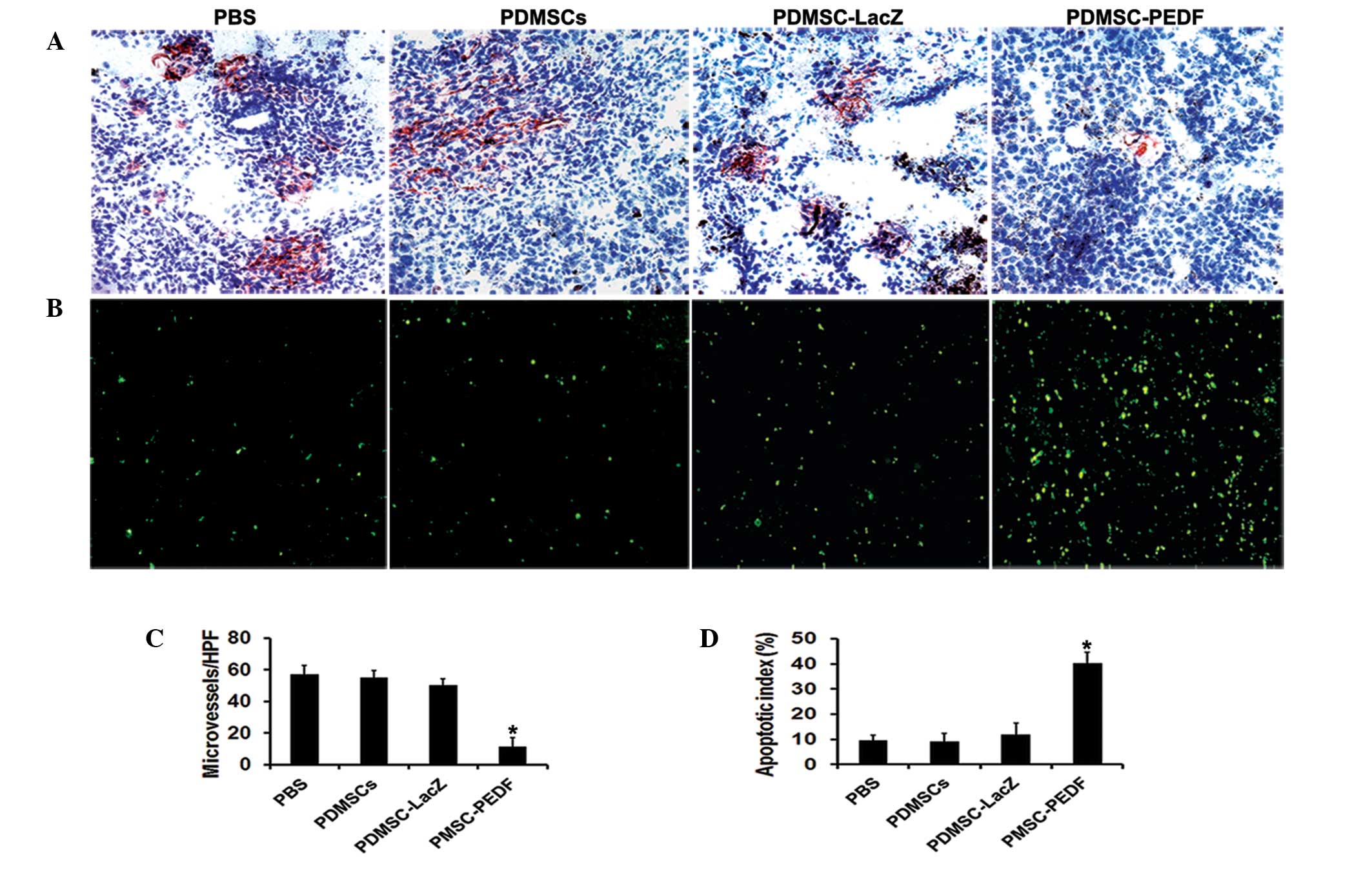
PDMSC-PEDF inhibited angiogenesis and
induced apoptosis in vivo
Angiogenesis within the tumor tissue was estimated
by counting the number of microvessels on the section stained with
an anti-CD31 antibody (Fig. 4A).
The microvessel density was significantly reduced in the
PDMSC-PEDF-treated group compared with the other groups (P<0.05)
(Fig. 4C). Apoptotic cells in
tumors were determined by TUNEL assay (Fig. 4B). The number of apoptotic cells in
the PDMSC-PEDF-treated group was found to be significantly higher
than that of the other groups (P<0.05) (Fig. 4D).
Discussion
In this study, we focused on the possibility of
employing human xenogeneic PDMSCs as a vehicle for the delivery of
PEDF to mouse B16-F10 melanoma. The data showed that treatment with
PDMSCs expressing PEDF led to a considerable reduction of tumor
growth compared with the control groups.
For tumors to develop, grow and spread, angiogenesis
is the primary mechanism involved, whereby new blood vessels form
from preexisting ones (23).
Melanoma has been well-documented as an angiogenic tumor type,
clearly demonstrating new vessel formation as an essential step in
disease progression from atypical melanocytes, through radial
growth to the aggressive vertical growth phase. Thus,
anti-angiogenic therapy has been considered to be a new direction
to fight melanoma (24). PEDF was
described as the most potent endogenous inhibitor of angiogenesis
and is capable of inducing apoptosis in endothelial cells as well
as inhibiting endothelial cell proliferation and migration. A
number of studies demonstrate that a low level of PEDF is
associated with the increased incidence of metastasis and poor
malignancy prognosis in various tumors (25). Garcia et al and Abe et
al showed that overexpression of PEDF in malignant melanoma
cell lines by stable transfection with retrovirus and plasmids,
respectively, markedly reduced intratumoral microvessel density as
well as primary tumor growth and metastasis (26,27).
In our previous study, the potent antitumor activity of
adenovirus-mediated PEDF was demonstrated in B16-F10 melanoma.
However, the high immunogenicity of the adenovirus, which induces a
major humoral and cellular immune response, results in rapid
clearance of the virus as well as side effects such as inflammation
in vivo (7). To overcome
these problems, we focused on MSC-based gene therapy.
Recently, MSCs have been used as a new therapeutic
strategy for the targeted delivery and local production of
biological agents in tumors to improve the efficacy and minimize
the toxicity. This is because MSCs have tumor-targeting properties,
can be easily isolated and expanded to the numbers required for
use, and can be genetically manipulated with viral vectors
(8). Adult bone marrow (BM) is the
common source of MSCs used in clinical settings. However, invasive
isolation procedures and low yield for BM-MSCs are an obstacle to
their use in cellular therapy (12). As an alternative source, PDMSCs
exhibit clear advantages: placenta can be obtained at every
delivery and its use does not pose any ethical problems.
Furthermore, the recovery of cells from this tissue does not
involve any invasive procedures for the donor (28). MSCs derived from placenta have low
immunogenicity associated with a lack or low level of expression of
MHC class II molecules and co-stimulatory molecules in the same way
as bone marrow-derived MSCs (29).
Moreover, since placenta-derived cells are fetal in origin, they
may generate less of an immune response than BM-MSCs (30). It has been demonstrated that cells
isolated from amniotic and chorionic membranes do not induce an
allogeneic or xenogeneic immune response in mixed lymphocyte
reactions and are capable of actively suppressing the proliferation
of lymphocytes in vitro (15). Several studies have already reported
a prolonged survival of human placenta-derived cells following
xenogeneic transplantation into immunocompetent animals including
rats (31,32), swine (31), and bonnet monkeys (33), with no evidence of immunological
rejection.
In our study, we demonstrated that PDMSCs may be
genetically modified with Ad-PEDF and express high levels of PEDF
in vitro. Furthermore, we revealed that the PEDF produced by
these engineered PDMSCs is a functional protein with potent
inhibitory effects on HUVEC proliferation and migration. In the
in vivo study, we found that PDMSC-PEDF efficiently
inhibited the growth of B16-F10 melanoma. CD31 staining and TUNEL
assay revealed a significant reduction in microvessel density and
an increase in the apoptotic index in the tumor tissue of the
PDMSC-PEDF-treated group. The observed antitumoral effect was a
result of the expression of PEDF and not a result of an immune
response to PDMSCs, since the injection of control PDMSCs had no
effect on tumor growth.
In summary, our investigation demonstrated that
adenovirus-mediated anti-angiogenesis gene therapy based on
xenogeneic PDMSCs inhibits the growth of B16-F10 melanoma. Thus,
the use of PDMSCs as a delivery vehicle of therapeutic genes is
likely to be of great interest for the clinical application of stem
cell-based cancer therapy. Further studies should be carried out in
an allogeneic setting to detect whether PDMSCs survive longer due
to their low immunogenicity and exert a more effective antitumor
activity.
Acknowledgements
The authors thank members of the State Key
Laboratory of Biotherapy for their helpful discussions. This study
was supported by the National Natural Science Foundation of China
(30973507) and the National 973 Basic Research Program of China
(2010CB529900 and 2010CB529906).
References
|
1
|
Soengas MS and Lowe SW: Apoptosis and
melanoma chemoresistance. Oncogene. 22:3138–3151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rook G: Tumours and Coley's toxins.
Nature. 357:5451992. View
Article : Google Scholar
|
|
3
|
Dass CR, Tran TM and Choong PF:
Angiogenesis inhibitors and the need for anti-angiogenic
therapeutics. J Dent Res. 86:927–936. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dawson DW, Volpert OV, Gillis P, et al:
Pigment epithelium-derived factor: a potent inhibitor of
angiogenesis. Science. 285:245–248. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Elayappan B, Ravinarayannan H, Pasha SP,
Lee KJ and Gurunathan S: PEDF inhibits VEGF- and EPO- induced
angiogenesis in retinal endothelial cells through interruption of
PI3K/Akt phosphorylation. Angiogenesis. 12:313–324. 2009.
View Article : Google Scholar
|
|
6
|
Doll JA, Stellmach VM, Bouck NP, et al:
Pigment epithelium-derived factor regulates the vasculature and
mass of the prostate and pancreas. Nat Med. 9:774–780. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang LP, Cheng P, Peng XC, et al:
Anti-tumor effect of adenovirus-mediated gene transfer of pigment
epithelium-derived factor on mouse B16-F10 melanoma. J Exp Clin
Cancer Res. 28:752009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fritz V and Jorgensen C: Mesenchymal stem
cells: an emerging tool for cancer targeting and therapy. Curr Stem
Cell Res Ther. 3:32–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rao MS and Mattson MP: Stem cells and
aging: expanding the possibilities. Mech Ageing Dev. 122:713–734.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pasquinelli G, Tazzari P, Ricci F, et al:
Ultrastructural characteristics of human mesenchymal stromal (stem)
cells derived from bone marrow and term placenta. Ultrastruct
Pathol. 31:23–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rus Ciuca D, Soritau O, Susman S, Pop VI
and Mihu CM: Isolation and characterization of chorionic mesenchyal
stem cells from the placenta. Rom J Morphol Embryol. 52:803–808.
2011.PubMed/NCBI
|
|
12
|
In 't Anker PS, Scherjon SA, Kleijburg-van
der Keur C, et al: Isolation of mesenchymal stem cells of fetal or
maternal origin from human placenta. Stem Cells. 22:1338–1345.
2004.
|
|
13
|
Kadam S, Muthyala S, Nair P and Bhonde R:
Human placenta-derived mesenchymal stem cells and islet-like cell
clusters generated from these cells as a novel source for stem cell
therapy in diabetes. Rev Diabet Stud. 7:168–182. 2010.PubMed/NCBI
|
|
14
|
Mihu CM, Mihu D, Costin N, Rus Ciuca D,
Susman S and Ciortea R: Isolation and characterization of stem
cells from the placenta and the umbilical cord. Rom J Morphol
Embryol. 49:441–446. 2008.PubMed/NCBI
|
|
15
|
Chang CJ, Yen ML, Chen YC, et al:
Placenta-derived multipotent cells exhibit immunosuppressive
properties that are enhanced in the presence of interferon-gamma.
Stem Cells. 24:2466–2477. 2006. View Article : Google Scholar
|
|
16
|
Jaffe EA, Nachman RL, Becker CG and Minick
CR: Culture of human endothelial cells derived from umbilical
veins. Identification by morphologic and immunologic criteria. J
Clin Invest. 52:2745–2756. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu JY, Wei YQ, Yang L, et al:
Immunotherapy of tumors with vaccine based on quail homologous
vascular endothelial growth factor receptor-2. Blood.
102:1815–1823. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao G, O'Brien CD, Zhou Z, et al:
Involvement of human PECAM-1 in angiogenesis and in vitro
endothelial cell migration. Am J Physiol Cell Physiol.
282:C1181–C1190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang S, Cao Z, Tian H, et al: SKLB1002, a
novel potent inhibitor of VEGF receptor 2 signaling, inhibits
angiogenesis and tumor growth in vivo. Clin Cancer Res.
17:4439–4450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu M, Yang JL, Teng H, et al:
Anti-angiogenesis therapy based on the bone marrow-derived stromal
cells genetically engineered to express sFlt-1 in mouse tumor
model. BMC Cancer. 8:3062008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Schmitz V, Perez-Mediavilla A,
Izal I, Prieto J and Qian C: Suppression of angiogenesis and tumor
growth by adenoviral-mediated gene transfer of pigment
epithelium-derived factor. Mol Ther. 8:72–79. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng XC, Yang L, Yang LP, et al: Efficient
inhibition of murine breast cancer growth and metastasis by gene
transferred mouse survivin Thr34-->Ala mutant. J Exp Clin Cancer
Res. 27:462008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
North S, Moenner M and Bikfalvi A: Recent
developments in the regulation of the angiogenic switch by cellular
stress factors in tumors. Cancer Lett. 218:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Emmett MS, Dewing D and Pritchard-Jones
RO: Angiogenesis and melanoma - from basic science to clinical
trials. Am J Cancer Res. 1:852–868. 2011.PubMed/NCBI
|
|
25
|
Ek ET, Dass CR and Choong PF: Pigment
epithelium-derived factor: a multimodal tumor inhibitor. Mol Cancer
Ther. 5:1641–1646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garcia M, Fernandez-Garcia NI, Rivas V, et
al: Inhibition of xenografted human melanoma growth and prevention
of metastasis development by dual antiangiogenic/antitumor
activities of pigment epithelium-derived factor. Cancer Res.
64:5632–5642. 2004. View Article : Google Scholar
|
|
27
|
Abe R, Shimizu T, Yamagishi S, et al:
Overexpression of pigment epithelium-derived factor decreases
angiogenesis and inhibits the growth of human malignant melanoma
cells in vivo. Am J Pathol. 164:1225–1232. 2004. View Article : Google Scholar
|
|
28
|
Evangelista M, Soncini M and Parolini O:
Placenta-derived stem cells: new hope for cell therapy?
Cytotechnology. 58:33–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Parolini O, Alviano F, Bagnara GP, et al:
Concise review: isolation and characterization of cells from human
term placenta: outcome of the first international Workshop on
Placenta Derived Stem Cells. Stem Cells. 26:300–311. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wagner JE, Rosenthal J, Sweetman R, et al:
Successful transplantation of HLA-matched and HLA-mismatched
umbilical cord blood from unrelated donors: analysis of engraftment
and acute graft-versus-host disease. Blood. 88:795–802.
1996.PubMed/NCBI
|
|
31
|
Bailo M, Soncini M, Vertua E, et al:
Engraftment potential of human amnion and chorion cells derived
from term placenta. Transplantation. 78:1439–1448. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meng XT, Li C, Dong ZY, et al:
Co-transplantation of bFGF-expressing amniotic epithelial cells and
neural stem cells promotes functional recovery in spinal
cord-injured rats. Cell Biol Int. 32:1546–1558. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sankar V and Muthusamy R: Role of human
amniotic epithelial cell transplantation in spinal cord injury
repair research. Neuroscience. 118:11–17. 2003. View Article : Google Scholar : PubMed/NCBI
|