Introduction
Transitional cell carcinoma (TCC), the most common
histopathological type of bladder cancer, is one of the most
prevalent malignancies and a leading cause of genitourinary system
cancer mortality worldwide (1,2).
Although a number of therapeutic strategies are available,
including intravesical chemotherapy, surgery, radiation therapy and
systemic chemotherapy, approximately 75% of patients with
non-muscle invasive bladder cancer face a five-year survival rate
of between 88–98%. Additionally, approximately 50% of all advanced
patients develop subsequent metastatic disease following the first
aggressive treatment (3,4). Despite technological advances, the
survival rates of bladder cancer patients have not changed over the
past 20 years. Therefore, it is essential to identify new
anticancer agents, which may not only aid in the understanding of
the molecular mechanisms of TCC, but may also improve the survival
rates of TCC patients.
FOXO1, a member of the forkhead box O (FOXO)
subfamily of transcription factors, functions as a tumor suppressor
and regulates genes involved in the apoptotic response, cell cycle
checkpoints and cellular metabolism (5). Clinical and experimental data suggests
that FOXO1 is downregulated in various types of cancer, including
TCC (6–8); however, the molecular mechanism
resulting in FOXO1 aberrant expression is poorly understood. Acting
as a master cell regulator, FOXO1 is activated through
phosphorylation, acetylation or the insulin-stimulated PI3K
signaling pathway (9). Recent
evidence suggested that post-transcriptional regulation may be
important for FOXO1 downregulation and the modulation of its
activity (10).
microRNAs (miRNAs), common post-transcriptional
factors, are a class of small non-coding RNAs that negatively
regulate gene expression by facilitating mRNA degradation or
translational inhibition. Emerging evidence suggests that
downregulated miRNAs are involved in the pathogenesis of bladder
cancer (11). However, to date,
there have been no studies on miR-96 regulating FOXO1 expression in
TCC.
In this study, we used bioinformatic technologies to
identify whether miR-96 was a predicted target for the FOXO1 gene.
We also investigated whether miR-96 was upregulated in TCC compared
to normal bladder (NB) tissues using quantitative real-time PCR
(qRT-PCR) and northern blot analysis, and explored the regulation
mechanisms of miR-96 to FOXO1 by transfection and RNA interference.
Our novel findings suggest that the ability of miR-96 to promote
FOXO1 repression may play a key role in TCC tumorigenesis through
bypassing cell apoptosis control.
Materials and methods
miRNA prediction
The prediction of the FOXO1 3′-untranslated region
(3′-UTR) as a miRNA binding target was determined using miRanda
(http://cbio.mskcc.org/cgi-bin/mirnaviewer/mirnaviewer.pl?type=miRanda),
Targetscan (www.targetscan.org) and PicTar
(http://pictar.mdc-berlin.de/). miRNAs
that were simultaneously predicted by all three programs were
selected for this study.
Cell culture
The human T24 TCC cell line was stored at Central
Laboratory, School of Stomatology, China Medical University,
Liaoning, China. The cells were cultured in RPMI-1640 medium
supplemented with 10% (v/v) fetal bovine serum (Sigma, St. Louis,
MO, USA), penicillin (100 U/ml) and streptomycin (100 μg/ml) at
37°C in a humidified atmosphere containing 5% CO2. Only
cells in the log phase were selected for the following
experiments.
Patients and specimens
A total of 40 bladder TCC samples, from patients who
had not undergone previous chemotherapy or radiation therapy, were
collected at The Affiliated Shengjing Hospital, China Medical
University. Additionally, 20 NB tissues were obtained from patients
with benign diseases. Informed consent was obtained prior to tissue
collection and the study was approved by the local ethics
committees. All specimens were frozen in liquid nitrogen
immediately after resection and stored at −80°C until use.
qRT-PCR
Transcripts were measured using a standard
SYBR-Green-based Real-Time PCR assay. miRNAs were isolated using
the mirVana™ miRNA Isolation kit (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer’s instructions,
and cDNAs were synthesized using the QuantiMir RT Kit Small RNA
Quantitation System (System Biosciences, Mountain View, CA, USA).
Real-time PCR was then conducted in a 30-μl reaction volume using
the Applied Biosystems 7500HT PCR system with miR-96-specific
primers. Amplifications were analyzed using the comparative
CT method and U6 small nuclear RNA (snRNA) was used as a
reference control.
Northern blot analysis
Total RNA was isolated from each tissue using TRIzol
reagent (Invitrogen Life Technologies) and Northern blotting was
conducted as described by Myatt et al and Várallyay et
al (6,12). The probe sequence of miR-96 was
5′-AGCAAAAATGTGCTAGTGCCAAA-3′. Following Perfect Hyb Plus
hybridization at 68°C, membranes were developed and analyzed.
Northern blots hybridized with an 18S ribosomal RNA (rRNA) cDNA
were used as controls.
Transfection
Appropriate cells were seeded onto 6-well plates 24
h prior to transfection using oligofectamine according to the
manufacturer’s instructions (Invitrogen Life Technologies). Cells
were incubated with 60 nM pre-miR-96, anti-miR-96 or appropriate
scramble controls (Invitrogen Life Technologies) for 4 h in
Opti-MEM media prior to the addition of normal growth media. The
cells were then assayed 48 h after transfection. For small
interfering RNA (siRNA) transfection, 100 nM of a siRNA designed to
target FOXO1 (Cat No. HSS103719) and a matching negative control
oligonucleotide were used. qRT-PCR was conducted to determine the
efficiency of transfection.
Western blot analysis
Transfected cells were washed with ice-cold PBS and
solubilized with lysis buffer. A total of 50 μg of cell lysate was
subjected to 10% SDS-PAGE gel electrophoresis and transferred onto
a polyvinylidene fluoride (PVDF) membrane. The membrane was then
blocked and hybridized with a FOXO1 primary antibody (1:1000) and a
horseradish peroxidase-conjugated secondary antibody. After
washing, proteins were detected using the enhanced
chemiluminescence system (ECL; Santa Cruz Biotechnology Inc., Santa
Cruz, CA, USA). Results were quantified by scanning densitometry
using a thermal imaging system (FTI-500; Pharmacia Biotech,
Sweden). β-actin was used as a housekeeping protein.
Apoptosis assays
Apoptosis was detected using the Annexin V-FITC
Apoptosis Detection kit (Biosea, Beijing, China) (13). Cells incubated with Annexin V-FITC
and propidium iodide (PI) were subjected to flow cytometry (Ex=488
nm; Em=635 nm) within 1 h and analyzed using CellQuest software.
Annexin V-positive cells were regarded as apoptotic.
Statistical analysis
Each experiment was conducted in triplicate. Data
are expressed as the mean ± standard deviation (SD) and were
analyzed with SPSS version 13.0 software. Statistical significance
was analyzed using the one-way analysis of variance (ANOVA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
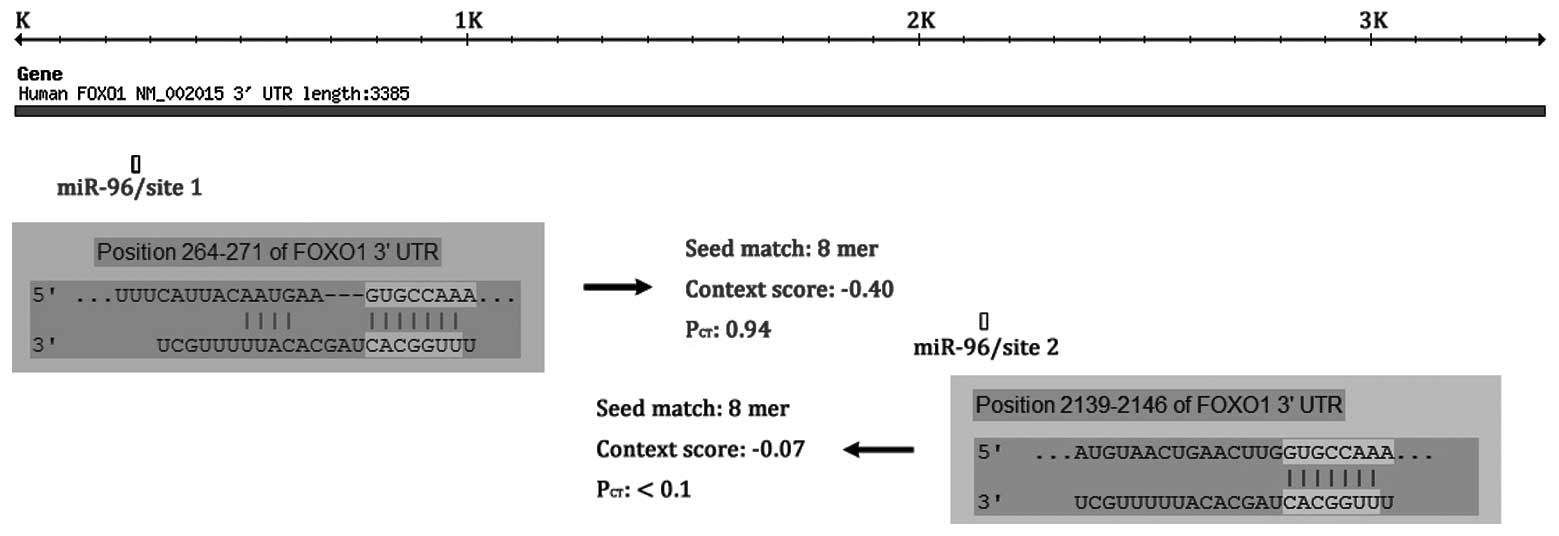
miR-96 as a putative target for
FOXO1
By combining the results of miRanda, TargetScan and
PicTar, we identified a panel of conserved miRNAs with the
potential to target the 3′-UTR of FOXO1 transcripts. Only
miR-96/site 1 (probability of conserved targeting,
PCT=0.94) (miR-96) was predicted in all three databases
and was highly conserved among vertebrates; although, miR-96 has
another predictive target site in the FOXO1 3′-UTR, miR-96/site 2
(PCT<0.1; Fig.
1).
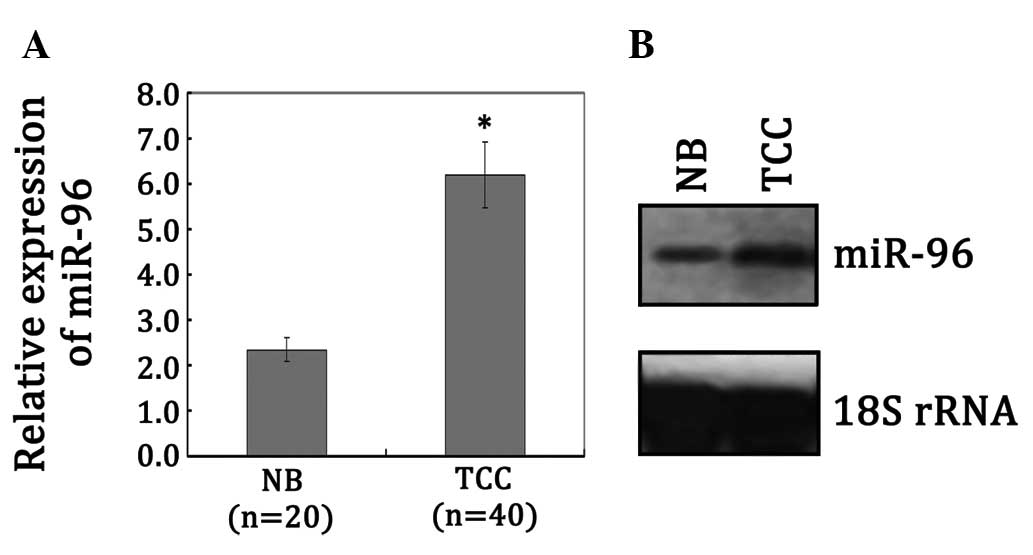
miR-96 is upregulated in TCC
In order to explore the regulation mechanisms of
miR-96 to FOXO1, we first examined miR-96 expression levels in 40
TCC and 20 NB samples by qRT-PCR analysis. As shown in Fig. 2A, the levels of miR-96 were
significantly upregulated in TCC compared with NB samples
(P<0.05). We also conducted northern blot analysis to further
confirm the expression difference of miR-96 in TCC and NB samples.
In accordance with the qRT-PCR data, miR-96 demonstrated a higher
level of expression in the TCC samples (P<0.05; Fig. 2B). In conjunction with the results
from Kim et al (8) and our
prediction, we speculated that the loss of FOXO1 expression upon
malignant transformation is correlated with miR-96 aberrant
expression in human TCC.
miR-96 represses FOXO1 expression in T24
cells
To examine the repressive potential of miR-96 on
FOXO1, pre-miR-96 was transfected into T24 cells and endogenous
FOXO1 expression levels were monitored using western blot analysis.
Cells were also transfected with a scramble control, which had no
effect on FOXO1 expression. The results revealed that
overexpression of miR-96 effectively downregulated FOXO1 expression
(P<0.05; Fig. 3A). In the
reverse experiment, we used anti-miR-96 to silence the activity of
miR-96 in T24 cells and monitored the expression of FOXO1. The
results demonstrated that the transfection of miR-96 inhibitors
elicited a reproducible induction of FOXO1 levels (P<0.05;
Fig. 3B). In contrast, FOXO1 levels
were effectively unchanged upon transfection of the anti-miR
scramble control. These observations indicate that miR-96 may
repress FOXO1 expression, but transfection of miR-96 inhibitors was
insufficient to completely restore FOXO1 expression in T24 TCC
cells.
miR-96 inhibits T24 cells apoptosis in a
FOXO1-dependent manner
Based on the hypothesis that the repression of FOXO1
by miR-96 may cause human TCC cells to escape apoptosis, we next
determined the effect of miR-96 repression in T24 cells. First, we
conducted a cell apoptosis analysis using flow cytometry, and the
results demonstrated that transfection of the anti-miR-96 was
effective in inducing T24 cell apoptosis (Fig. 4A). Subsequently, in order to
demonstrate the requirement of FOXO1 in anti-miR-96-induced cell
apoptosis, we co-transfected anti-miR-96 together with a functional
siRNA targeting FOXO1, which repressed endogenous FOXO1 levels
(Fig. 4B). Under these conditions,
a significant reduction in anti-miR-96-induced cell apoptosis was
observed (P<0.05), which is consistent with FOXO1 induction
being critical for this effect (Fig.
4C). Together, our results suggest that miR-96 may repress
FOXO1 expression, thereby promoting TCC progression.
Discussion
miRNAs function as important regulators of target
genes that are involved in normal development and development of
diseases, including cancer (14).
Various cancer types, stages or differentiation states have unique
miRNA expression profiles (15–17),
and in recent years, researchers have made great efforts to
discover miRNAs that function as novel biomarkers for cancer
diagnosis. Han et al (17)
discovered a great number of miRNAs involved in bladder cancer and
identified the miRNAs and miRNA*s that were
significantly upregulated or downregulated in bladder urothelial
carcinoma compared to the matched normal urothelium. hsa-miR-96
(log2 ratio=4.664328) was revealed as the most significantly
upregulated miRNA. The expression of miR-96 lacks real-time PCR
validation; however, in our study we used qRT-PCR, northern blot
analysis and a serial assay to demonstrate that miR-96 was
upregulated in human TCC and that miR-96 promoted FOXO1 repression
through the bypassing of cell apoptosis control. Our results
revealed that miR-96 is critical for the development of human TCC,
and that miR-96 upregulation is one of the mechanisms of FOXO1
repression in TCC tumorigenesis.
miR-96, miR-183 and miR-182, located proximally in
the genome, belong to the same miR-183 family. Moreover, miR-96,
miR-183 and miR-182 share the same transcription start site (chr7:
129207158), suggesting that these miRNAs may be coordinately
expressed and function together during tumorigenesis (18). A recent study identified that miR-96
and miR-182, which are highly expressed in Michigan Cancer
Foundation 7 (MCF-7) breast cancer cells, repress the endogenous
expression of FOXO1 gene and cause the oncogenic transformation of
breast cells (7). It was also
revealed that miR-96, miR-182 and miR-183 are overexpressed in
endometrial cancer and function as an oncogene through the
repression of FOXO1 expression. Subsequently, aberrant miR-183
family expression resulted in deregulated cell cycle control and
impaired apoptotic responses (6).
In this study, we predicted miR-182 and miR-183 binding sites in
the 3′-UTR of FOXO1 transcripts using three miRNA prediction
programs (miRanda, Targetscan and PicTar). In conjunction with our
present findings that transfection of miR-96 inhibitors was
insufficient to completely restore FOXO1 expression in T24 TCC
cells, we speculated that as miR-183 family members, miR-182 and/or
miR-183 may regulate FOXO1 expression, directly or indirectly, in a
miR-96-dependent manner. However, this requires further study.
In addition to the miR-96 mediated repression, the
FOXO1 gene may be regulated by a number of other mechanisms,
including methylation and various pathways. Studies demonstrate
that promoter methylation may downregulate FOXO1 gene expression in
certain endometrial cancers (10).
Additionally, the upregulation of Skp2, an oncogenic subunit of the
Skp1/Cul1/F-box protein ubiquitin complex, may downregulate FOXO1
protein levels by promoting ubiquitination and degradation of
phosphorylated FOXO1 (19). FOXO1
activity is associated with the chemosensitization of a number of
cancers and numerous studies support the notion that apoptosis
escape plays an important role in the resistance of carcinomas to
chemotherapy and radiotherapy (6,20).
Using RNA interference and transfection technologies, our study
revealed that FOXO1 downregulation may significantly reduce miR-96
mediated TCC cell apoptosis, which provided a theoretical basic for
further investigation of miR-96/FOXO1 as a potential therapeutic
target for TCC.
In conclusion, we identified that the miR-96
targeting of FOXO1 was upregulated in TCC; in addition, TCC
tumorigenesis may be partly due to the ability of miR-96 to promote
FOXO1 repression, thereby bypassing cell apoptosis controls.
Acknowledgements
This study was supported by the National Nature
Science Foundation of China (81172577) and the Education Science
and Technology Research Project of Liaoning Province, China
(L2011130).
References
|
1
|
Pollard C, Smith SC and Theodorescu D:
Molecular genesis of non-muscle-invasive urothelial carcinoma
(NMIUC). Expert Rev Mol Med. 12:e102010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murta-Nascimento C, Schmitz-Dräger BJ,
Zeegers MP, Steineck G, Kogevinas M, Real FX and Malats N:
Epidemiology of urinary bladder cancer: from tumor development to
patient’s death. World J Urol. 25:285–295. 2007.
|
|
3
|
Hassen W and Droller MJ: Current concepts
in assessment and treatment of bladder cancer. Curr Opin Urol.
10:291–299. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirata H, Hinoda Y, Ueno K, Shahryari V,
Tabatabai ZL and Dahiya R: MicroRNA-1826 targets VEGFC,
beta-catenin (CTNNB1) and MEK1 (MAP2K1) in human bladder cancer.
Carcinogenesis. 33:41–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paik JH, Kollipara R, Chu G, Ji H, Xiao Y,
Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S,
Gilliland DG, Chin L, Wong WH, Castrillon DH and DePinho RA: FoxOs
are lineage-restricted redundant tumor suppressors and regulate
endothelial cell homeostasis. Cell. 128:309–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Myatt SS, Wang J, Monteiro LJ, Christian
M, Ho KK, Fusi L, Dina RE, Brosens JJ, Ghaem-Maghami S and Lam EW:
Definition of microRNAs that repress expression of the tumor
suppressor gene FOXO1 in endometrial cancer. Cancer Res.
70:367–377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guttilla IK and White BA: Coordinate
regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast
cancer cells. J Biol Chem. 284:23204–23216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim TH, Jo SW, Lee YS, Kim YJ, Lee SC, Kim
WJ and Yun SJ: Forkhead box O-class 1 and forkhead box G1 as
prognostic markers for bladder cancer. J Korean Med Sci.
24:468–473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bansal N, Yendluri V and Wenham RM: The
molecular biology of endometrial cancers and the implications for
pathogenesis, classification, and targeted therapies. Cancer
Control. 16:8–13. 2009.PubMed/NCBI
|
|
10
|
Goto T, Takano M, Albergaria A, Briese J,
Pomeranz KM, Cloke B, Fusi L, Feroze-Zaidi F, Maywald N, Sajin M,
et al: Mechanism and functional consequences of loss of FOXO1
expression in endometrioid endometrial cancer cells. Oncogene.
27:9–19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fei X, Qi M, Wu B, Song Y, Wang Y and Li
T: MicroRNA-195-5p suppresses glucose uptake and proliferation of
human bladder cancer T24 cells by regulating GLUT3 expression. FEBS
Lett. 586:392–397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Várallyay E, Burgyán J and Havelda Z:
MicroRNA detection by northern blotting using locked nucleic acid
probes. Nat Protoc. 3:190–196. 2008.PubMed/NCBI
|
|
13
|
Guo Y, Liu J, Xu Z, Sun K and Fu W: HLA-B
gene participates in the NF-kappaB signal pathway partly by
regulating S100A8 in the laryngeal carcinoma cell line Hep2. Oncol
Rep. 19:1453–1459. 2008.PubMed/NCBI
|
|
14
|
Engels BM and Hutvagner G: Principles and
effects of microRNA-mediated post-transcriptional gene regulation.
Oncogene. 25:6163–6169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stenvang J, Silahtaroglu AN, Lindow M,
Elmen J and Kauppinen S: The utility of LNA in microRNA-based
cancer diagnostics and therapeutics. Semin Cancer Biol. 18:89–102.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
Downing JR, Jacks T, Horvitz HR and Golub TR: MicroRNA expression
profiles classify human cancers. Nature. 435:834–838. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han Y, Chen J, Zhao X, Liang C, Wang Y,
Sun L, Jiang Z, Zhang Z, Yang R, Chen J, et al: MicroRNA expression
signatures of bladder cancer revealed by deep sequencing. PLoS One.
6:e182862011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozsolak F, Poling LL, Wang Z, Liu H, Liu
XS, Roeder RG, Zhang X, Song JS and Fisher DE: Chromatin structure
analyses identify miRNA promoters. Genes Dev. 22:3172–3183. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang H, Regan KM, Wang F, Wang D, Smith
DI, van Deursen JM and Tindall DJ: Skp2 inhibits FOXO1 in tumor
suppression through ubiquitin-mediated degradation. Proc Natl Acad
Sci USA. 102:1649–1654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoekstra AV, Ward EC, Hardt JL, Lurain JR,
Singh DK, Buttin BM, Schink JC and Kim JJ: Chemosensitization of
endometrial cancer cells through AKT inhibition involves FOXO1.
Gynecol Oncol. 108:609–618. 2008. View Article : Google Scholar : PubMed/NCBI
|