Introduction
Multiple myeloma is a neoplastic plasma cell
disorder that is characterized by the clonal proliferation of
malignant plasma cells in the bone marrow microenvironment,
monoclonal protein in the blood or urine and associated organ
dysfunction. Worldwide, it is estimated that approximately 86,000
cases of multiple myeloma occur annually, accounting for
approximately 0.8% of all new cancer cases. Approximately 63,000
individuals are reported to succumb to the disease each year,
accounting for 0.9% of all cancer mortalities and 13% of all
mortalities due to hematological cancers. In recent years, the
introduction of autologous stem-cell transplantation and the
availability of agents such as thalidomide, lenalidomide and
bortezomib have changed the management of myeloma and extended
overall survival. However, the treatment outcome is far from
satisfactory and novel drugs are in urgent demand to more
effectively treat this malignancy (1,2).
It is well known that numerous natural compounds,
especially plant products, have been found to exhibit anticancer
effects and that some play important roles in cancer treatment.
Rotenoids, which are typically insecticidal agents, constitute a
class of compounds from the flavonoid family and have cancer
chemopreventive and anticancer activities (3,4).
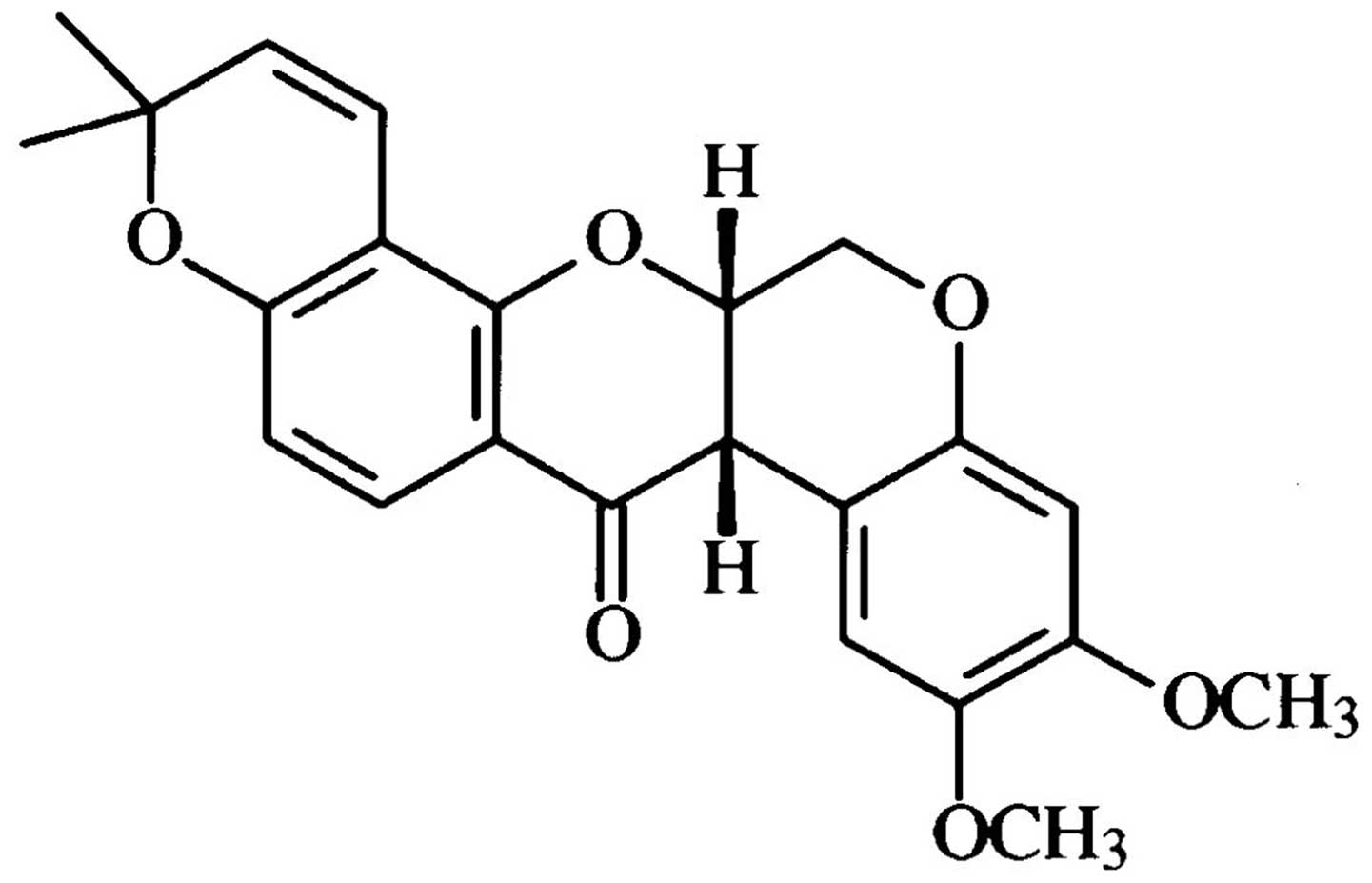
Deguelin is a natural rotenoid which has been isolated from several
plant species, including Mundulea sericea (Leguminosae;
Fig. 1). Recently, deguelin has
been found to exhibit strong cancer chemopreventive and antitumor
activities in vitro and in vivo in various model
systems (2–9). However, the antitumor effects of
deguelin against multiple myeloma have not been reported.
In the present study, we investigated the antitumor
effect of deguelin against multiple myeloma cells and the mechanism
by which it occurs. Using murine myeloma MPC-11 cells, we
demonstrated that deguelin exhibits antitumor activity on the cells
by inhibiting the activity of the Akt pathway and inducing
apoptosis. Our study suggests that deguelin is a potential agent to
combat multiple myeloma.
Materials and methods
Materials
3-(4,5)-dimethylthiazol(-z-y1)-3,5-diphenyltetrazolium
bromide (MTT), dimethyl sulfoxide (DMSO), RNase A and propidium
iodide (PI) were purchased from Sigma Chemical Co. (St. Louis, MO,
USA). The total protein extraction kit was purchased from Keygen
Co. (Nanjing, China). All the chemicals employed in this study were
analytically pure and of culture grade. The primary antibodies for
Akt/p-Akt, Bcl-2 and Bax were purchased from Cell Signaling
Technology (Beverly, MA, USA) and cleaved caspase-3 antibody was
purchased from Beyotime Co. (Hangzhou, China). The protein assay
kit was purchased from Bio-Rad (Hercules, CA, USA).
Deguelin was purchased from Sigma Chemical Co.,
dissolved in DMSO as a stock solution, then stored at 4°C. Prior to
experiments, the stock solution was diluted in cell culture medium
at a final DMSO concentration of 0.05% (V/V).
Cell culture
The murine myeloma MPC-11 cell line was obtained
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). The cells were grown in RPMI-1640 medium (Life Technologies,
Bedford, MA, USA) containing 10% heat-inactivated fetal bovine
serum, 100 U/ml penicillin and 100 U/ml streptomycin in a
humidified chamber at 37°C with a 5% CO2 atmosphere.
Cell viability assay
The cell viability of the deguelin-treated cancer
cells was determined using the MTT assay. Briefly, the cells
(4–5×103) were seeded in 96-well plates and cultured for
24 h, followed by deguelin treatment for 24, 48 or 72 h. A volume
of 10 μl of 10 mg/ml MTT was added per well and the cells
were incubated for another 4 h at 37°C. The plates were then
centrifuged at 1,000 × g for 10 min, the supernatant fluid was
removed and DMSO was added, 150 μl/well for 15–20 min. The
light absorptions (OD) were measured at 570 nm with SpectraMAX M5
microplate spectrophotometer (Molecular Devices, Sunnyvale, CA,
USA). All experiments were performed in triplicate. The effect of
deguelin on the proliferation of cells was expressed as the cell
growth inhibition, using the following formula: inhibition rate =
(A570 of control – A570 of treated cells)/A570 of control cells ×
100%.
Agarose gel DNA electrophoresis
In order to clarify whether the inhibitory effect of
the deguelin on MPC-11 cells was due to apoptosis, the pattern of
DNA cleavage was analyzed by agarose gel electrophoresis as
described previously (10,11). Briefly, following treatment with
deguelin, the cells (3×106) were lysed with 0.5 ml lysis
buffer (5 mM Tris-HCL (pH 8.0), 0.25% Nonidet P40 and 1 mM EDTA),
followed by the addition of RNase A (Sigma) at a final
concentration of 200 μg/ml. Following incubation for 1 h at
37°C, the cells were treated with 300 μg/ml proteinase K for
an additional hour at 37°C. A volume of 20 μl of sample in
each lane was subjected to electrophoresis on a 1.5% agarose at 50
V for 3 h. DNA was stained with ethidium bromide.
Morphological analysis
Following culture and drug treatment as described
above, the morphological changes of the cells were observed. The
cells were fixed using 70% ethanol following rinsing with PBS.
After examination for morphological changes with an inverted
microscope, the cells were stained with PI (1 μg/ml in PBS)
and analyzed under a fluorescence microscope (Zeiss, Axiovert 200,
Göttingen, Germany) to identify the apoptotic cells.
Western blot analysis
To identify the mechanisms of proliferation
inhibition and apoptosis induction of deguelin, an immunoblot
analysis was performed. Briefly, 5×106 cells were lysed
in 1 ml lysis buffer and the protein concentration was determined
using the Bio-Rad protein assay reagent. The samples were denatured
in sample buffer and the proteins were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. The gels were
electroblotted onto a polyvinylidene difluoride membrane. The
membrane blots were rinsed with TBS/T (20 mM Tris, 500 mM NaCl,
0.1% Tween-20, pH 7.6) and blocked with 5% non-fat dry milk in
blocking buffer. The membrane was incubated with the desired
primary antibody overnight at 4°C. The membrane was then incubated
with the appropriate peroxidase-conjugated secondary antibody and
the immunoreactive bands were visualized using the enhanced
chemiluminescence method.
Statistical analysis
Data are expressed as the mean ± SD. Statistical
comparisons were performed using the ANOVA test. All data were
analyzed using SPSS software (SPSS for Windows, ver. 13.0; SPSS,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant result.
Results
Effects of deguelin on cell
proliferation
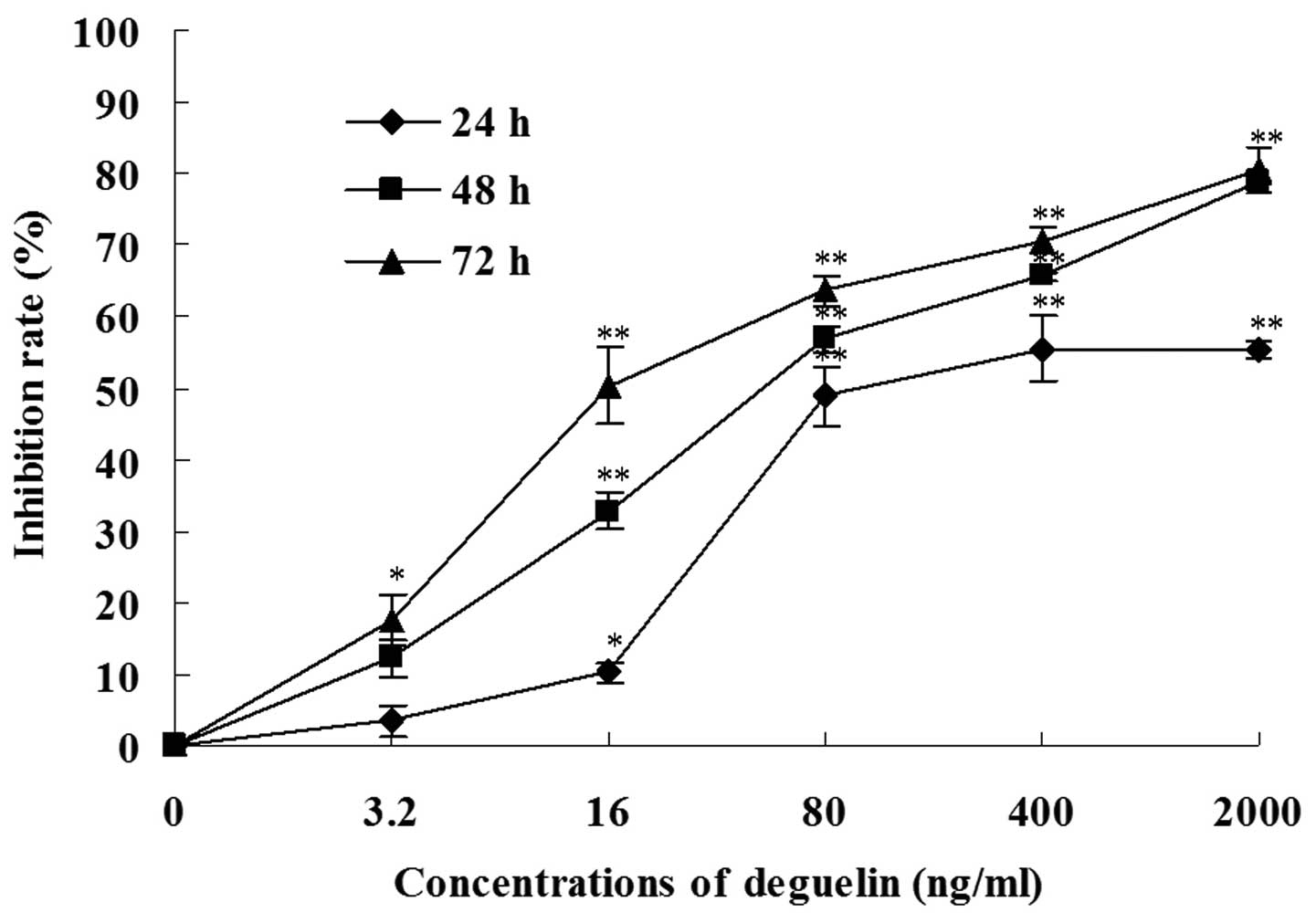
We tested the antiproliferative effect of deguelin
on MPC-11 cells using the MTT assay. The deguelin treatment
resulted in a decrease in the cell viability in vitro and
the effect was dependent on the dose of deguelin and incubation
time (Fig. 2). For example, when
MPC-11 cells were treated for 48 h, the inhibition rates of 3.2 and
400 ng/ml deguelin were 12.2±2.7 and 65.5±0.6%, respectively. When
MPC-11 cells were treated with 16 ng/ml deguelin, the inhibition
rate was 10.2±1.5, 32.8±2.5 and 50.3±5.3% for 24, 48 and 72 h,
respectively.
Effects of deguelin on cell
apoptosis
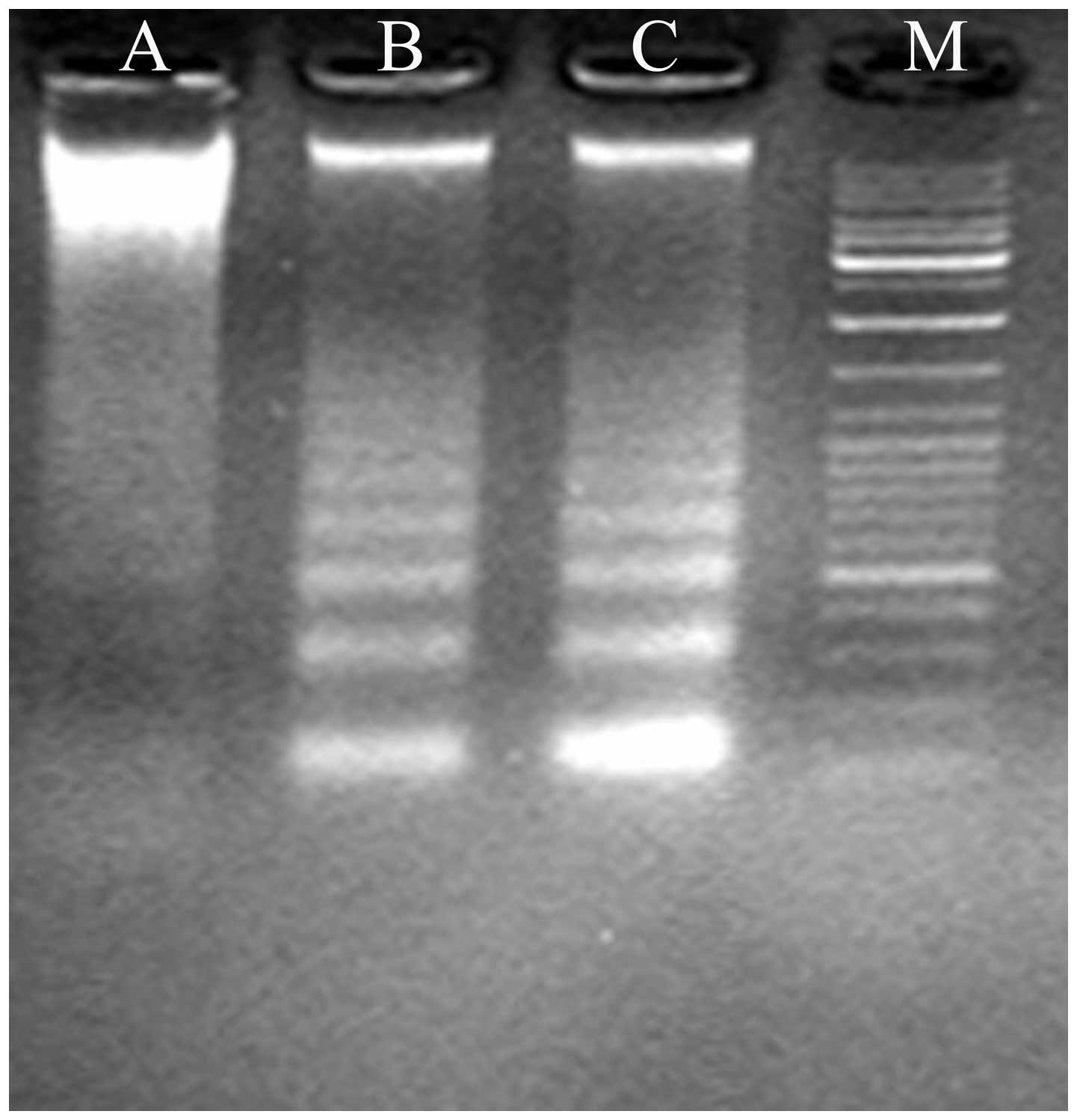
Agarose gel electrophoresis of deguelin-treated
cells revealed a ladder-like pattern of DNA fragments consisting of
multiples of ∼180–200 base pairs, consistent with internucleosomal
DNA fragmentation (Fig. 3).
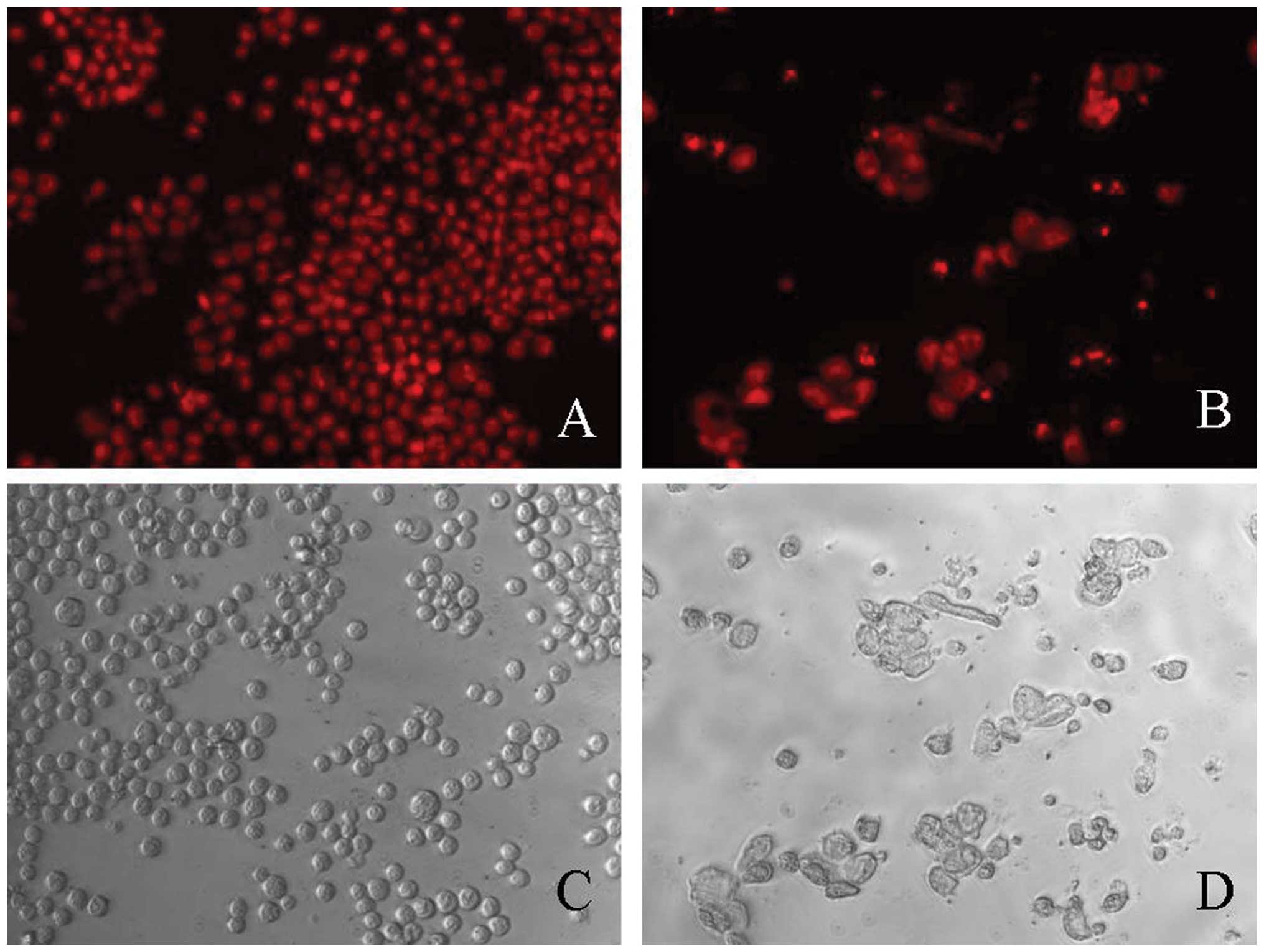
Furthermore, fluorescence microscopic examination of
PI-stained cells was performed to confirm the apoptosis-inducing
effect of deguelin. Treatment with deguelin resulted in
morphological changes characteristic of apoptosis, including bright
red fluorescent condensed nuclei (intact or fragmented) by
fluorescence microscopy of PI-stained nuclei, blebbing, expansion
of cell volume, condensation of nuclear chromatin, nuclear
fragmentation and apoptotic bodies, and the change was
concentration-dependent (Fig.
4).
Mechanistic studies of deguelin
effect
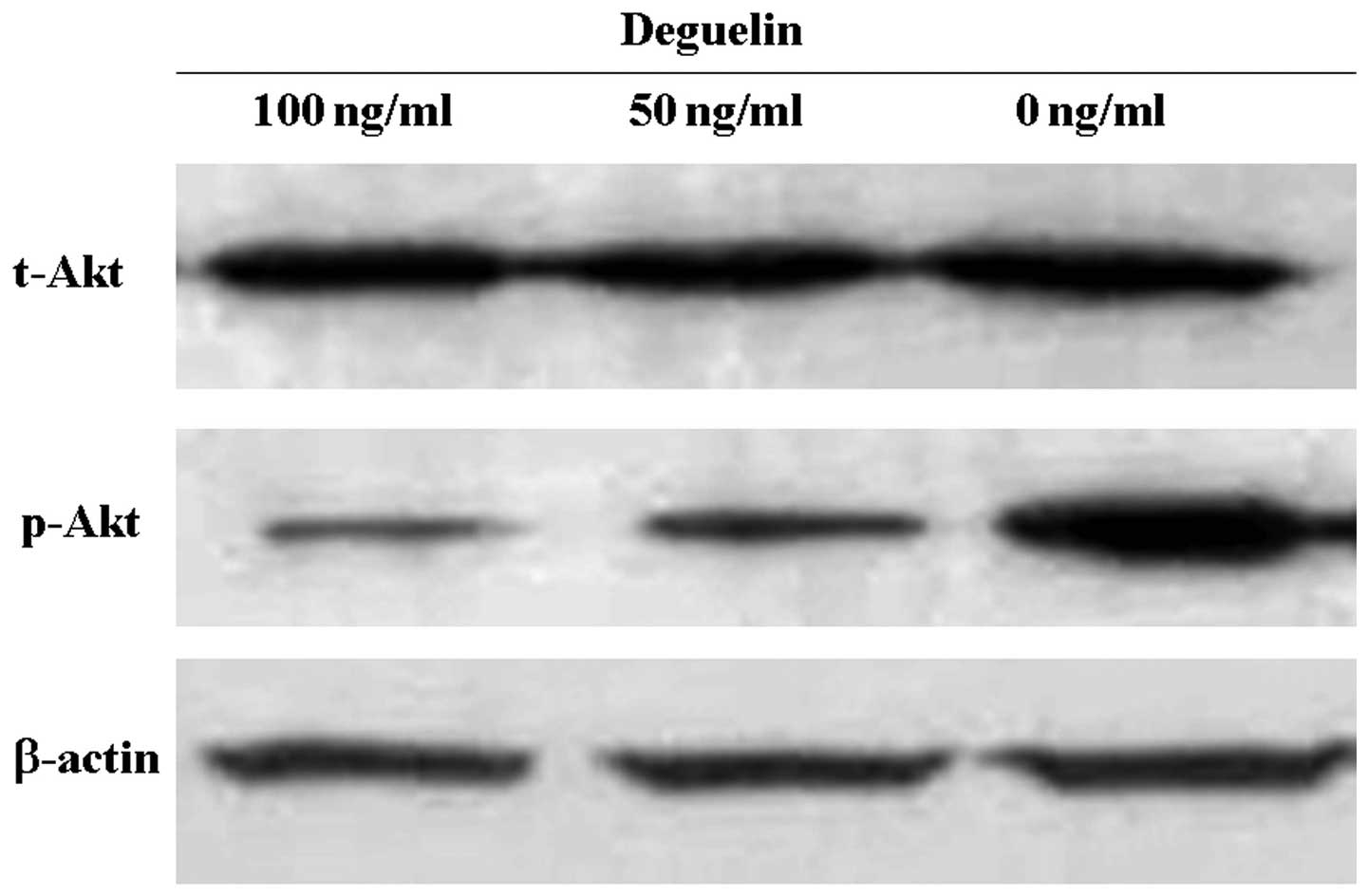
To determine whether deguelin was able to modulate
the activities of Akt, MPC-11 cells were exposed to increasing
concentrations of deguelin in vitro and analyzed for changes
in protein levels of Akt. The results revealed that deguelin
significantly reduced the level of phosphorylated Akt (p-Akt) in a
concentration-dependent manner, but the level of total Akt (t-Akt)
was not visibly changed (Fig. 5).
It was suggested that deguelin inhibits Akt activity through the
inhibition of Akt phosphorylation.
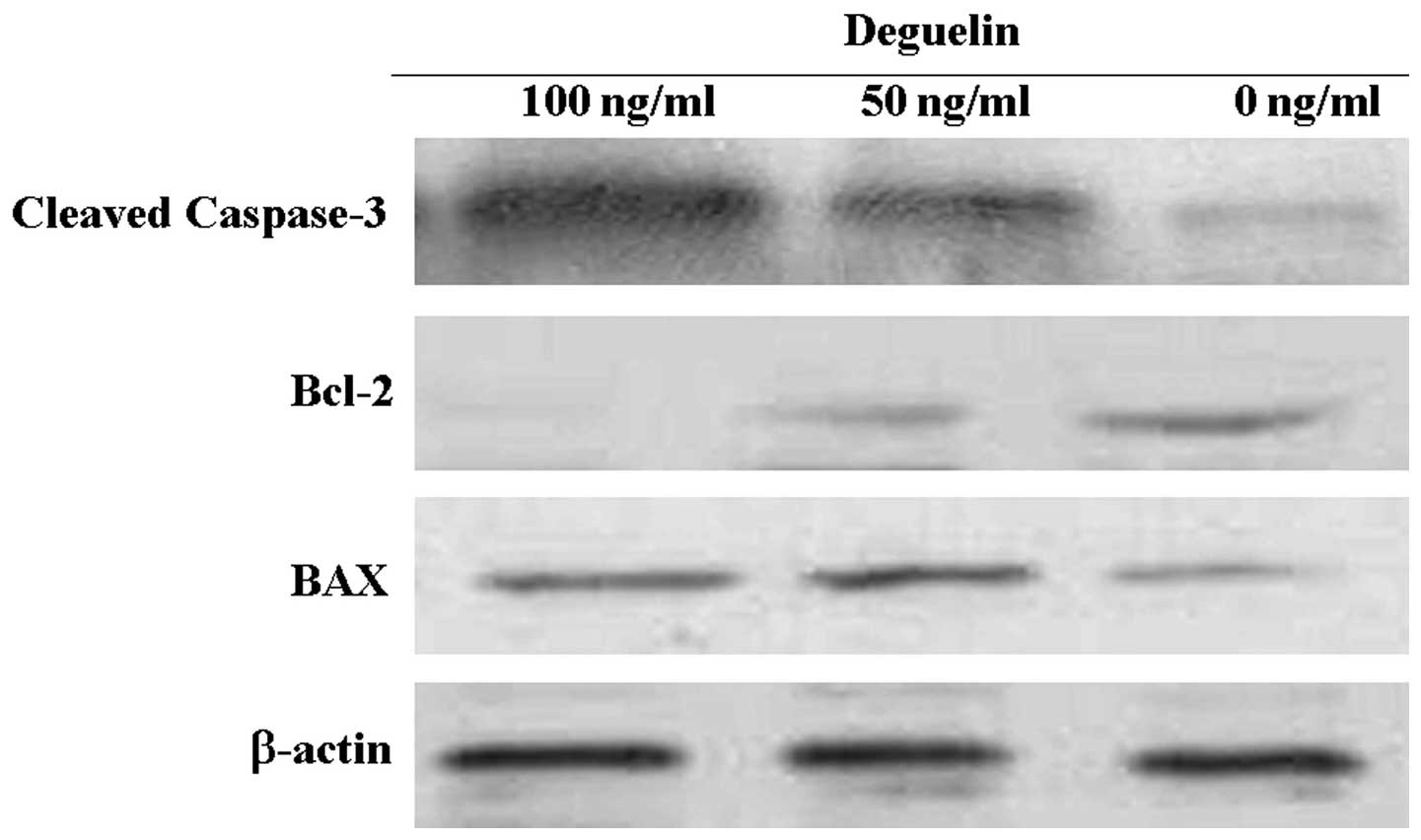
To determine the effects of deguelin on the
expression of the Bcl-2 protein group, we examined Bcl-2 and Bax
expression in the MPC-11 cells following treatment with deguelin at
various concentrations for 48 h. As shown in Fig. 6, inhibition of the expression of
Bcl-2 and increase in Bax were confirmed in response to treatment
with deguelin. The increase in the Bax/Bcl-2 ratio has been
described in association with the activation of the mitochondrial
apoptotic route. The result of the inhibition of Bcl-2 and
concomitant increase in Bax expression was in agreement with the
result of the apoptosis assay.
Caspase-3 is an effector caspase that plays a
central role in cell apoptosis. Therefore, we investigated the
effect of deguelin on the activation of caspase-3. Treatment MPC-11
cells with deguelin for 48 h resulted in a concentration-dependent
increase of cleaved caspase-3 (Fig.
6).
Discussion
Multiple myeloma accounts for more than 10% of all
hematological cancers (12) and the
average 5-year survival rate is 15–20%, with survival ranging
between a few and 10 or more years (1). Novel agents or strategies are needed
due to the poor outcome of treatment.
Deguelin is a natural product isolated from plants,
which exhibits strong cancer chemopreventive and antitumor
activities. However, to the best of our knowledge there are no
published data with regard to the effect of deguelin on multiple
myeloma.
In the present study, we investigated the antitumor
effect of deguelin on the murine myeloma cell line MPC-11 and its
possible mechanism in vitro. Our results revealed that
deguelin inhibits the proliferation and induces the apoptosis of
myeloma cells. Moreover, the cell growth inhibitory effect was
associated with a decrease in the phosphorylated levels of Akt, as
revealed by immunoblotting.
Firstly, we investigated the cell proliferation
inhibition effect of deguelin using the MTT assay. The results
revealed that the proliferation of MPC-11 cells was inhibited
following exposure to deguelin and that the effect was dose- and
time-dependent.
It is well known that the susceptibility of tumor
cells to apoptosis is an important determinant of chemotherapy
efficacy and that the induction of apoptosis is an important
mechanism of antitumor agents, especially natural products
(13–16). Therefore, the apoptosis-inducing
effect of deguelin was examined. In the present study, DNA
fragmentation analysis demonstrated that deguelin induced the
apoptosis of MPC-11 cells. The results of fluorescence microscopic
examination also revealed features characteristic of apoptosis,
confirming the ability of deguelin to induce apoptosis in MPC-11
cells. Consistent with our results, other groups reported that
deguelin induced apoptosis in various types of cells, including
colon cancer, gastric cancer, leukemia, breast cancer and hepatic
cancer cells (7,8,17–19).
The evolutionarily conserved serine/threonine
protein kinase Akt is one of the most versatile kinases in the
human kinome. Akt is activated by phosphatidylinositol 3-kinase
(PI3K), which transmits signals from cytokines, growth factors and
oncoproteins to multiple targets, including Akt. Once activated,
Akt regulates multiple cellular functions, including survival,
proliferation, growth and various aspects of intermediary
metabolism. Activated Akt is detectable in numerous types of cancer
and has been associated with poor prognosis of cancers, including
skin, pancreas, liver, prostate, breast and blood cancers (20–25).
Based on these clinical observations, targeting Akt may be a
promising strategy against cancer.
In multiple myeloma, Hsu et al reported that
the over-expression and activation of Akt played a significant role
in malignant cell survival and that the growth of multiple myeloma
cells was inhibited if the Akt pathway was paralyzed. The same
conclusion was reported independently by Alkan and Izban, further
confirming the significant role of Akt in multiple myeloma
(26,27).
According to previous studies, deguelin exhibits
cancer chemopreventive and anticancer effects through inhibiting
the activity of Akt (5,6). In the present study, consistent with
others, our data demonstrated that deguelin inhibited the activity
of Akt to decrease the survival and growth of MPC-11 cells.
The induction of apoptosis is a common mechanism of
numerous anticancer agents and our data showed that deguelin also
exhibited apoptosis-inducing activity in MPC-11 cells. To determine
the mechanism of the apoptosis induced by deguelin, the proteins
involved in apoptosis were analyzed by western blotting. Caspase-3
is the key molecule in cellular apoptosis and the activation of
caspase-3 is often considered to be the point of no return in the
apoptotic signaling cascade. In the present study, we found that
caspase-3 was activated following exposure to deguelin. To further
determine the pathway of apoptosis induced by deguelin, we analyzed
the upstream regulators of caspase-3. Bcl-2 family proteins are
central regulators of the apoptosis pathway, which either suppress
or promote changes in mitochondrial membrane permeability required
for the release of cytochrome c (28,29).
Of the Bcl-2 family, Bcl-2 and Bax have been identified as major
regulators in controling the release of mitochondrial cytochrome c
(30). Bcl-2 blocks cytochrome c
efflux, whereas Bax enhances the release of cytochrome c and
induces apoptosis. The overexpression of antiapoptotic Bcl-2
probably occurs in more than half of all cancers (31). Our results revealed that treatment
with deguelin induced the downregulation of Bcl-2 and upregulation
of Bax in MPC-11 cells in a dose-dependent manner. It is suggested
that deguelin induced the apoptosis of MPC-11 cells via an
intrinsic mechanism, which is regulated via the inhibition of Bcl-2
and a concomitant stimulation of Bax protein expression.
We conclude that deguelin is able to inhibit cell
proliferation and induce apoptosis in MPC-11 cells in vitro.
We demonstrated that at least two mechanisms are involved:
inhibition of the activity of Akt/ERK and modulation of the
Bcl-2/Bax ratio to activate caspase-3. The ability of deguelin to
mediate these responses in myeloma cells makes it a potentially
effective therapeutic agent against multiple myeloma and warrants
further investigation.
Acknowledgements
This study was financially supported
by the Technology Project of Changzhou Social Development
(CS20102016) and the Natural Science Funds for Young Teacher of
Soochow University (Q3124943).
References
|
1.
|
N BeckerEpidemiology of multiple
myelomaRecent Results Cancer
Res1832535201110.1007/978-3-540-85772-3_2
|
|
2.
|
A PalumboK AndersonMultiple myelomaN Engl
J Med36410461060201110.1056/NEJMra1011442
|
|
3.
|
N FangJE CasidaAnticancer action of cubé
insecticide: correlation for rotenoid constituents between
inhibition of NADH: ubiquinone oxidoreductase and induced ornithine
decarboxylase activitiesProc Natl Acad Sci
USA9533803384199817000091
|
|
4.
|
C GerhäuserW MarSK LeeRotenoids mediate
potent cancer chemopreventive activity through transcriptional
regulation of ornithine decarboxylaseNat Med12602661995
|
|
5.
|
KH ChunJW Kosmeder IIS SunEffects of
deguelin on the phosphatidylinositol 3-kinase/Akt pathway and
apoptosis in premalignant human bronchial epithelial cellsJ Natl
Cancer Inst95291302200310.1093/jnci/95.4.29112591985
|
|
6.
|
HY LeeSH OhJK WooChemopreventive effects
of deguelin, a novel Akt inhibitor, on tobacco-induced lung
tumorigenesisJ Natl Cancer
Inst9716951699200510.1093/jnci/dji37716288123
|
|
7.
|
JH LeeDH LeeHS LeeJS ChoiKW KimSS
HongDeguelin inhibits human hepatocellular carcinoma by
antiangiogenesis and apoptosisOncol Rep20129134200818575727
|
|
8.
|
G MurilloGI SaltiJW Kosmeder IIJM
PezzutoRG MehtaDeguelin inhibits the growth of colon cancer cells
through the induction of apoptosis and cell cycle arrestEur J
Cancer3824462454200210.1016/S0959-8049(02)00192-212460790
|
|
9.
|
GO UdeaniC GerhauserCF ThomasCancer
chemopreventive activity mediated by deguelin, a naturally
occurring rotenoidCancer Res573424342819979270008
|
|
10.
|
YH LingW PriebeR Perez-SolerApoptosis
induced by anthracycline antibiotics in P388 parent and
multidrug-resistant cellsCancer Res531845185219938467504
|
|
11.
|
YQ WeiX ZhaoY KariyaH FukataK TeshigawaraA
UchidaInduction of apoptosis by quercetin: involvement of heat
shock proteinCancer Res544952495719948069862
|
|
12.
|
RA KyleSV RajkumarMultiple myelomaN Engl J
Med35118601873200410.1056/NEJMra041875
|
|
13.
|
SH KaufmannWC EarnshawInduction of
apoptosis by cancer chemotherapyExp Cell
Res2564249200010.1006/excr.2000.483810739650
|
|
14.
|
A CiucciP GianferrettiR PivaInduction of
apoptosis in estrogen receptor-negative breast cancer cells by
natural and synthetic cyclopentenones: role of the IkappaB
kinase/nuclear factor-kappaB pathwayMol
Pharmacol7018121821200610.1124/mol.106.025759
|
|
15.
|
E FanS JiangL ZhangY BaiMolecular
mechanism of apoptosis induction by resveratrol, a natural cancer
chemopreventive agentInt J Vitam Nutr
Res7838200810.1024/0300-9831.78.1.318654947
|
|
16.
|
I WolfJ O’KellyN WakimotoHonokiol, a
natural biphenyl, inhibits in vitro and in vivo growth of breast
cancer through induction of apoptosis and cell cycle arrestInt J
Oncol3015291537200717487375
|
|
17.
|
B GeeraertsB VanhoeckeW Vanden BergheJ
PhilippéF OffnerD DeforceDeguelin inhibits expression of
IkappaBalpha protein and induces apoptosis of B-CLL cells in
vitroLeukemia2116101618200710.1038/sj.leu.240478817568818
|
|
18.
|
H LeeJH LeeKH JungSS HongDeguelin promotes
apoptosis and inhibits angiogenesis of gastric cancerOncol
Rep24957963201020811676
|
|
19.
|
XH PengP KarnaRM O’ReganDown-regulation of
inhibitor of apoptosis proteins by deguelin selectively induces
apoptosis in breast cancer cellsMol
Pharmacol71101111200710.1124/mol.106.02736717035597
|
|
20.
|
DL DaiM MartinkaG LiPrognostic
significance of activated Akt expression in melanoma: a
clinicopathologic study of 292 casesJ Clin
Oncol2314731482200510.1200/JCO.2005.07.16815735123
|
|
21.
|
JI KreisbergSN MalikTJ
PrihodaPhosphorylation of Akt (Ser473) is an excellent predictor of
poor clinical outcome in prostate cancerCancer
Res6452325236200410.1158/0008-5472.CAN-04-027215289328
|
|
22.
|
J LoPiccoloCA GranvilleJJ GillsPA
DennisTargeting Akt in cancer therapyAnticancer
Drugs188618742007
|
|
23.
|
G Perez-TenorioO StalActivation of AKT/PKB
in breast cancer predicts a worse outcome among endocrine treated
patientsBr J Cancer86540545200210.1038/sj.bjc.660012611870534
|
|
24.
|
MG SchliemanBN FahyR RamsamoojL BeckettRJ
BoldIncidence, mechanism and prognostic value of activated AKT in
pancreas cancerBr J
Cancer8921102115200310.1038/sj.bjc.660139614647146
|
|
25.
|
N TerakawaY KanamoriS YoshidaLoss of PTEN
expression followed by Akt phosphorylation is a poor prognostic
factor for patients with endometrial cancerEndocr Relat
Cancer10203208200310.1677/erc.0.010020312790783
|
|
26.
|
S AlkanKF IzbanImmunohistochemical
localization of phosphorylated AKT in multiple
myelomaBlood9922782279200210.1182/blood-2001-01-031711902142
|
|
27.
|
J HsuY ShiS KrajewskiThe AKT kinase is
activated in multiple myeloma tumor
cellsBlood9828532855200110.1182/blood.V98.9.285311675360
|
|
28.
|
DR GreenJC ReedMitochondria and
apoptosisScience28113091312199810.1126/science.281.5381.13099721092
|
|
29.
|
A GrossJM McDonnellSJ KorsmeyerBCL-2
family members and the mitochondria in apoptosisGenes
Dev1318991911199910.1101/gad.13.15.189910444588
|
|
30.
|
O GhribiMM HermanNK SpauldingJ
SavoryLithium inhibits aluminum-induced apoptosis in rabbit
hippocampus, by preventing cytochrome c translocation, Bcl-2
decrease, Bax elevation and caspase-3 activationJ
Neurochem82137145200210.1046/j.1471-4159.2002.00957.x
|
|
31.
|
SA AmundsonTG MyersD ScudieroS KitadaJC
ReedAJ Fornace JrAn informatics approach identifying markers of
chemosensitivity in human cancer cell linesCancer
Res6061016110200011085534
|