Introduction
Endometrial cancer is the most frequent malignancy
of the female genital tract, with an estimated 46,470 cases and
8,120 mortalities expected to be recorded in 2011 in the USA
(1). Despite such high prevalence,
the understanding of its molecular background in terms of genesis,
growth and progression remains insufficient. Furthermore, little is
known concerning factors which would allow for the differentiation
between types I and II endometrial cancer, which differ
substantially in prognosis.
In view of the poor understanding of the molecular
background of endometrial cancer we have attempted to identify new
markers which may: i) correlate with patients’ clinico-pathological
features; ii) further elucidate molecular pathways of endometrial
carcinogenesis; iii) aid the differentiation of types I and II.
One of the key elements to be investigated in this
particular context are cohesins: multisubunit protein complexes
which are highly conserved and play canonical roles in processes
such as chromatin regulation, chromosome segregation and DNA damage
response (2–4). Is has been shown that
cohesin-defective cells possess features known to be crucial
drivers of oncogenesis. These features include genomic instability,
impaired DNA repair and anomalies concerning gene expression
(4–7). The deregulation of cohesin expression
and cohesin-regulated genes is common in numerous types of human
cancer (8–13), including endometrial cancer
(14). Furthermore,
cohesin-defective cells have been discovered to be sensitive to
ionizing radiation and DNA-damaging drugs (8,15).
RAD21 (double-strand-break repair protein rad21
homolog), a mammalian ortholog of Mcd1p, is one of the four core
proteins comprising a cohesin ring in sister chromatid cohesion
(SSC), a physical linkage between sister chromatids. SSC allows for
cell cycle checkpoint control and homologous repair of DNA
double-strand breaks (16).
Experiments performed on zebrafish revealed rad21 to be a
regulator of runx1 (6).
RUNX1/AML1 (runt-related transcription factor 1/ acute myeloid
leukemia 1) belongs to the family of RUNX transcription factors
which, when complexed with other proteins, activates or represses
the transcription of regulators involved in cell differentiation,
growth and survival. The RUNX genes function as tumor
suppressors and dominant oncogenes, depending on the context
(17).
RAD21 and RUNX1 actions are crucial for sustaining
basic functions in healthy cells. These two markers have been found
to be deregulated in different types of tumors, including
endometrioid, prostate, breast and oral squamous carcinoma together
with acute lymphoblastic leukemia (8,9,11,12,14,18–25).
The present study was designed to address the hypothesis of cohesin
deregulation in endometrial cancer. It aimed to investigate
RAD21 and RUNX1 mRNA expression profiles with the use
of reverse transcription quantitative PCR in endometrial cancer
tumors. Additionally, RAD21 mRNA expression was compared
with RAD21 gene dosage measured by quantitative PCR, as it
has been shown that copy number variations (CNVs) are common in
various types of cancer (26) and
that some of them may contribute to aberrant cohesin expression in
cancer (9,16).
Materials and methods
Patients and tissues
The retrospective study encompassed 144 frozen tumor
samples collected from a cohort of endometrial cancer patients
treated at the Department of Gynaecology, Gynaecological Oncology
and Gynaecological Endocrinology (Medical University of Gdańsk,
Gdańsk, Poland) between 2005 and 2011. The inclusion criteria were
operable endometrial cancer confirmed by histological examination
and a signed consent form. The characteristics of the patients are
summarized in Table I. The mean age
was 63.3 years (range, 30–87). The study was accepted by the Ethics
Committee of the Medical University of Gdańsk.
 | Table I.Clinicopathological data (n=144). |
Table I.
Clinicopathological data (n=144).
| Variable | Number of cases
(%) |
|---|
| Menopausal
status |
|
Premenopausal | 9 (6.3) |
|
Postmenopausal | 135 (93.7) |
| Obesity | |
| Absent | 43 (29.9) |
| Present | 54 (37.5) |
| Missing data | 47 (32.6) |
| Ca-125 status |
| Negative | 86 (59.7) |
| Positive | 11 (7.6) |
| Missing data | 47 (32.6) |
| Histology |
| Endometrioid | 135 (93.7) |
|
Nonendometrioid | 9 (6.3) |
| Stage (FIGO) |
| IA–IB | 107 (74.3) |
| II | 19 (13.2) |
| IIIA–IIIC | 14 (9.7) |
| IVA–IVB | 3 (2.1) |
| Missing data | 1 (0.7) |
| Grade |
| I | 53 (36.8) |
| II | 61 (42.4) |
| III | 22 (15.3) |
| Missing data | 8 (5.6) |
| Lymph node
status |
| Negative | 39 (27.1) |
| Positive | 8 (5.6) |
| Missing data | 97 (67.4) |
| Myometrial
infiltration |
| ≤1/2 | 81 (56.3) |
| >1/2 | 62 (43.1) |
| Missing data | 1 (0.7) |
| Cervical
invasion |
| Absent | 104 (72.2) |
| Present | 39 (27.1) |
| Missing data | 1 (0.7) |
| Metastases |
| Absent | 102 (70.8) |
| Cervix | 18 (12.5) |
| Cervix and other
organs | 13 (9) |
| Other organs | 9 (6.3) |
| Missing data | 2 (1.4) |
| ESR1
status |
| Positive | 37 (25.7) |
| Negative | 103 (71.5) |
| Missing data | 4 (2.8) |
Tumor samples were collected by surgical excision
prior to any systemic treatment and were immediately frozen and
stored at −80°C. Tissue samples covered the spectrum of
pathological stages of endometrial carcinoma, from noninvasive IA
to metastatic IVB cancer according to the staging by FIGO in 2009
(International Federation of Gynecology and Obstetrics) (27). A Ca-125 level between 0 and 35 U/ml
was considered normal (28).
Patients with a body mass index >30 were classified as obese
(29).
DNA and RNA isolation
Prior to nucleic acid isolation, tissue specimens
(25 mg per sample) were homogenized (1 min, 6,000 rpm) with the use
of MagNA Lyser (Roche, Basel, Switzerland). DNA and RNA were
isolated with AllPrep DNA/RNA Mini kit (Qiagen, Hilden, Germany)
using the tissue protocol, in accordance with the manufacturer’s
instructions. After the isolation, DNA/RNA concentration and purity
were determined by Spectrophotometer ND-1000 (NanoDrop
Technologies, Wilmington, DE, USA). Good quality DNA was defined as
an A260 nm/280 nm ratio between 1.70 and 1.90. Good
quality RNA was defined as an A260 nm/280 nm ratio of
∼2.
RNA was subsequently reverse transcribed to cDNA
with the Transcriptor First Strand cDNA Synthesis kit (Roche),
according to the manufacturer’s instructions, with the use of
random hexamer primers. There was 1000 ng of total RNA per
reaction.
Quantitative PCR
Control DNA and RNA from five frozen samples of
healthy donors were isolated, pooled and used for qPCR assay
optimization as well as a calibrator. Analysis was performed with
StepOnePlus™ Instrument (Applied Biosystems, Carlsbad, CA, USA).
Each new set of the master mix was verified by a standard curve.
The thermal profiles used were the default settings of the
manufacturer, dedicated to either SYBR-Green or TaqMan probe
assays. Results were analyzed and reported with the use of StepOne
Software v2.1.
Gene dosage analysis
RAD21 and ESR1 (estrogen receptor 1)
gene copy numbers were determined by qPCR with Power SYBR-Green
Master mix (Applied Biosystems), using the APP (amyloid
precursor protein) gene as a reference. APP was selected as
a reference gene upon a search performed in the Atlas of Genetics
and Cytogenetics in Oncology and Haematology (http://www.atlasgeneticsoncology.org/).
Its stability against 3P (RNA, U4 small nuclear pseudogen)
and SOD2 (superoxide dismutase 2) genes was verified using
geNorm software (30). The primer
sequences were as follows: APP F, 5′-AGC CCA GAA GGT GTC AAA CA-3′;
APP R, 5′-CAT CTT CAT GTC CGT TGC AT-3′; RAD21 F, 5′-GGC ACT GTT
ACC ACA AAC CTT TGG-3′; RAD21 R, 5′-GGG GAC ATT TGA ATG CTG ACT
GGC-3′; ESR1 F, 5′-ACA TGG ACA CCT CCC AGT C-3′; ESR1 R, 5′-ACA GAC
TAA CAC AGC CCA TC-3′. The quantity of DNA used per well was 100
ng.
RAD21 and ESR1 copy number was
calculated using the ΔΔCt quantification method (31), which relates the gene dosages of a
studied gene and a reference gene in the tumor tissue and a
calibrator. The reactions were performed in duplicate on 96-well
plates; a negative control for each gene and three calibrators were
included on each plate.
We used experimentally determined cut-off values
calculated using the critical difference parameter, as described
previously (32). The amplification
of RAD21 and ESR1 was classified as a relative
quantity >1.36 and 1.14, respectively.
mRNA expression analysis
RAD21 and RUNX1 RNA expression levels
were determined by qPCR with TaqMan® Universal PCR Master mix
(Applied Biosystems), using HPRT1 (hypoxanthine
phosphoribosyltransferase 1) as a reference. HPRT1 gene
expression stability was verified against the expression of
GAPDH (glyceraldehyde 3-phosphate dehydrogenase) and
ACTB (β-actin) genes. TaqMan® Expression Assays
(Applied Biosystems) used were as follows: HPRT1 Endogenous
Control Hs99999909_m1; RAD21 Gene Expression Assay
Hs01085854_mH and RUNX1 Gene Expression Assay Hs01021967_m1.
The quantity of cDNA per well was 75 ng.
RUNX1 and RAD21 expression was also
calculated using the ΔΔCt quantification method. Reactions were
performed in triplicate on 96-well plates; on each plate two
negative controls for each gene and four calibrators were included.
RUNX1 and RAD21 overexpression was classified as a
value 2-fold higher than the value in the calibrator sample.
Statistical analysis
All statistical analyses were performed using the
STATISTICA software, version 10. Logarithmized relative quantities
of RAD21 gene dosage together with RUNX1 and
RAD21 expression levels were assessed by Spearman
correlation and Crosstabs statistics with Pearson’s chi-square
test. Various comparisons of the results and clinicopathological
data were performed with the nonparametric statistics, including
the Mann-Whitney U test (Table II).
P<0.05 was considered to indicate a statistically significant
result.
 | Table II.RAD21 and RUNX1 status
with regard to clinicopathological data. |
Table II.
RAD21 and RUNX1 status
with regard to clinicopathological data.
| Variable | RAD21 gene
dosage
| RAD21
expression
| RUNX1
expression
|
|---|
| n | Average ± SD | P-value | n | Average ± SD | P-value | n | Average ± SD | P-value |
|---|
| Menopausal
status |
|
Premenopausal | 9 | 1.15±0.27 | 0.569 | 9 | 1.90±1.21 | 0.269 | 9 | 5.10±5.42 | 0.286 |
|
Postmenopausal | 132 | 1.10±0.35 | | 135 | 1.50±0.91 | | 132 | 3.56±9.00 | |
| Obesity |
| Absent | 41 | 1.18±0.30 | 0.025 | 43 | 1.39±0.82 | 0.069 | 40 | 4.81±15.34 | 0.611 |
| Present | 53 | 1.08±0.44 | | 54 | 1.66±1.09 | | 54 | 3.07±3.90 | |
| Ca-125 |
| Negative | 83 | 1.09±0.26 | 0.284 | 86 | 1.57±1.04 | 0.629 | 84 | 3.87±10.90 | |
| Positive | 11 | 1.41±0.86 | | 11 | 1.28±0.35 | | 10 | 3.26±4.73 | 0.718 |
| Histology |
| Endometrioid | 133 | 1.11±0.35 | 0.969 | 135 | 1.51±0.91 | 0.975 | 132 | 3.61±9.05 | 0.073 |
|
Nonendometrioid | 7 | 1.11±0.26 | | 8 | 1.76±1.37 | | 8 | 4.83±3.68 | |
| Stage |
| I, II | 125 | 1.07±0.23 | 0.021 | 126 | 1.55±0.97 | 0.564 | 124 | 3.77±9.28 | 0.527 |
| III, IV | 15 | 1.41±0.77 | | 17 | 1.35±0.53 | | 16 | 2.92±4.00 | |
| Grade |
| I, II | 113 | 1.09±0.35 | 0.021 | 114 | 1.44±0.71 | 0.589 | 112 | 3.72±9.69 | 0.691 |
| III | 20 | 1.22±0.32 | | 22 | 1.92±1.68 | | 21 | 3.57±4.19 | |
| Lymph node
status |
| Negative | 38 | 1.10±0.24 | 0.197 | 39 | 1.61±1.31 | 0.543 | 37 | 2.77±2.72 | 0.083 |
| Positive | 8 | 1.56±1.03 | | 8 | 1.28±0.45 | | 7 | 2.41±4.99 | |
| Myometrial
infiltration |
| ≤1/2 | 79 | 1.07±0.23 | 0.353 | 81 | 1.53±0.79 | 0.432 | 80 | 4.23±11.32 | 0.934 |
| >1/2 | 59 | 1.14±0.46 | | 60 | 1.53±1.12 | | 59 | 2.78±3.23 | |
| Cervical
invasion |
| Absent | 102 | 1.05±0.22 | 0.01 | 104 | 1.60±0.97 | 0.027 | 104 | 3.94±9.99 | 0.306 |
| Present | 38 | 1.24±0.54 | | 39 | 1.32±0.82 | | 36 | 2.65±3.76 | |
| ESR1
status |
| Negative | 103 | 1.09±0.38 | 0.269 | 103 | 1.40±0.64 | 0.085 | 102 | 3.73±10.06 | 0.213 |
| Positive | 37 | 1.13±0.26 | | 37 | 1.87±1.45 | | 35 | 4.59±4.20 | |
Results
An increased level of RAD21 gene dosage was
identified in 18/141 samples. The average gene dosage was
1.103±0.345. RAD21 overexpression was found in 23/144
samples, with an average of 1.524±0.931. Increased RUNX1
expression was observed in 58/141 samples, with an average of
3.656±8.805. RAD21 gene dosage was significantly associated
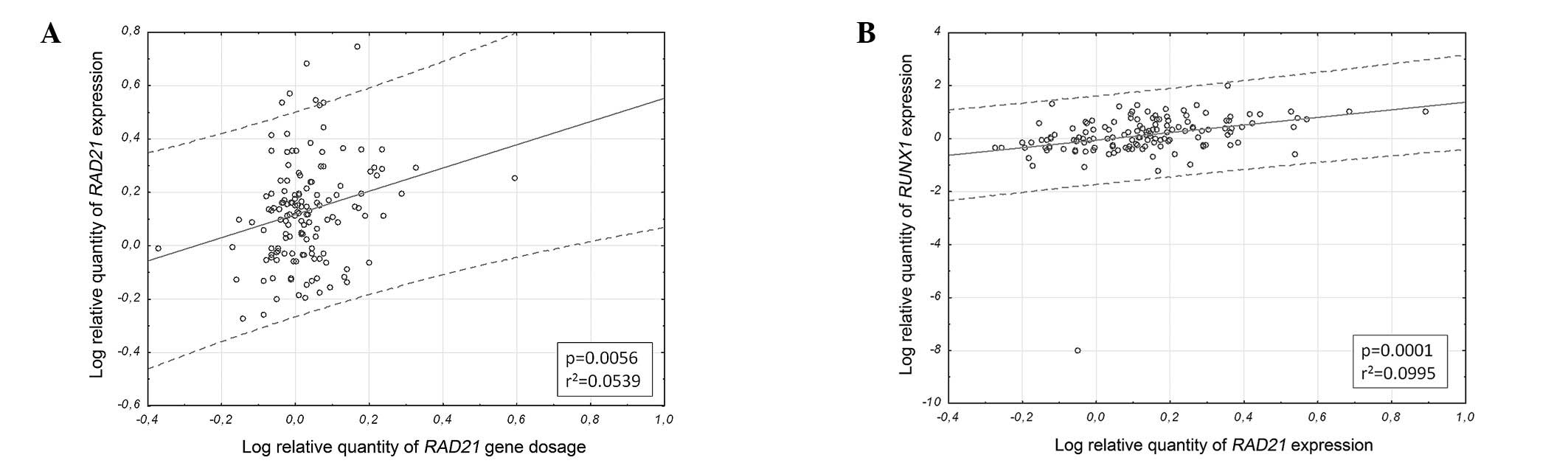
with RAD21 mRNA expression (ϱ=0.22; p=0.009; Fig. 1A). Furthermore, RAD21
expression markedly correlated with RUNX1 expression
(ϱ=0.43; p<0.0000001; Fig.
1B).
Increased RAD21 gene dosage correlated with
more advanced tumor stage (p=0.021), higher grade (p=0.021),
cervical involvement (p= 0.01) and the absence of obesity (p=
0.025), while RAD21 mRNA expression was correlated with
cervical involvement (p=0.027; Table
II). In the case of RUNX1 mRNA expression only a trend
was observed, with a higher expression level in the nonendometrioid
histological type (p=0.073) and in the tumors with negative lymph
node status (p=0.083; Table II).
Menopausal status, level of Ca-125, myometrial infiltration and
ESR1 status did not correlate with any of the examined
molecular markers.
Discussion
Although endometrial cancer is well characterized at
the level of clinicopathological features, its molecular background
to date has received far less attention. As RAD21 and RUNX1 actions
are crucial for sustaining basic functions in healthy cells and
their expression tends to be deregulated in various types of cancer
(2–4,8,9,11,12,14,18–25),
we analyzed the role of these two markers in the context of
endometrial cancer formation using the qPCR method. The examined
markers correlated with each other, partially unraveling the
network of genes involved in endometrial tumorigenesis. Findings of
previous studies have suggested that the decreased expression of
cohesins results in an inappropriate increase in homologous
recombination which may drive tumorigenesis through the promotion
of genomic instability, such as loss of heterozygosity (15,33,34).
In the present study, a marked correlation between
mRNA expression of RAD21 and RUNX1 was observed.
Furthermore, RAD21 copy number variations were significantly
associated with RAD21 mRNA expression and RAD21 and
RUNX1 status correlated with the clinicopathological
features of the endometrial cancer patients.
Aberrant expression of RAD21 in cancer has been
documented by several authors (8,9,11,18,19).
RAD21 was found to be especially overexpressed in undifferentiated
cancers of the breast, lung, bladder, brain and ovaries (18). Its suppression by siRNA reduced the
proliferation of breast cancer cells (8). RAD21 overexpression has also been
reported to confer poor prognosis in breast cancer patients
(9,19). Notably, RAD21 was downregulated in
oral squamous cells with high metastatic potential (11).
The aberrant expression of RUNX1 in cancer has also
been documented in the literature (14,25),
in particular RUNX1 amplification has been reported to be
implicated in the development of leukemia (22,35,36).
The upregulation of RUNX1, as measured by qPCR, has been
reported in invasive endometrioid carcinoma (14). On the contrary, in breast cancer
RUNX1 may act as a tumor suppressor gene. RUNX1
down-regulation is a component of a 17-gene signature predicting
metastasis (37). It has also been
shown that RUNX1 expression decreases as the breast tumor
grade increases (38). Notably,
experiments performed on neuroblastoma cell lines have shown that
high and low RUNX1 levels disrupt proliferation, inducing cell
death (23).
Our analyses did not reveal any link between
RAD21/RUNX1 gene expression status and the stage of
the tumor, however, RAD21 gene dosage was found to correlate
with more advanced tumor stage, grade and cervical involvement.
This suggests that RAD21-positive status is correlated with
unfavorable clinical characteristics. Therefore, RAD21
amplification may serve as a marker of poor prognosis in
endometrial cancer, as in breast cancer (9,19).
Furthermore, similarly to breast cancer (9), we have observed a significant
association between RAD21 expression and RAD21 gene
dosage. This suggests that in case of endometrial cancer CNVs
contribute to the deregulation of RAD21 expression.
Comparative genomic hybridization revealed RAD21 to be
within the region which is prone to high-level chromosomal gains
(www.progenetix.net/progenetix).
The qPCR analyses of Abal et al revealed
RUNX1 upregulation in endometrial cancer. The authors
postulated that RUNX1 plays a crucial role during early stages of
endometrial carcinogenesis and is responsible for the switch to
myometrial infiltration (39). The
findings of Doll et al indicated that RUNX1 overexpression,
measured by immunohistochemistry and RT-qPCR, is associated with
distant metastasis in an orthotopic endometrial cancer model in
nude mice in which the endometrial cancer cell line HEC1A was used
(21). Our results do not confirm
these two particular hypotheses, showing a correlation only between
RUNX1 mRNA expression and tumor histological type. This,
however, is in agreement with the findings of Planagumà et
al who measured the expression levels of 53 genes, including
RUNX1, with cDNA array hybridization. RUNX1,
additionally verified with RT-qPCR, was reported to be the most
upregulated gene among those studied in endometrioid carcinoma
(14). Unfortunately, the authors
did not perform such analyses for nonendometrioid carcinoma.
Our results clearly demonstrate that the mRNA
expression of RAD21 and RUNX1 is deregulated and
co-dependent in endometrial cancer cells. This is in accordance
with analyses performed by Horsfield in zebrafish, in which
rad21 gene dosage reduction resulted in the decrease of
runx1 transcription, suggesting that rad21 is a
regulator of runx1 (6). This
reveals another gene to be dependent on RAD21 function.
Furthermore, the correlation between RUNX1 and ERM/ETV
(40) as well as p21WAF1/CIP1 has
been reported (41), partially
unraveling the network of molecular interactions occurring in
endometrial cancer. Nevertheless, the exact molecular background of
these changes requires further elucidation in a broader context
which would include a larger number of potential molecular markers
whose role could be additionally investigated at the protein level,
measured by immunohistochemistry and through correlation with
patients’ outcome.
Given the possibility of RAD21 status being a
prognostic factor, we assume that a correlation between the gene
amplification and patients’ outcome is worth investigating. This is
to be performed as soon as we gather necessary information
concerning the patients’ survival. The role of RAD21 should also be
verified in the context of therapy selection as in vitro
experiments have demonstrated that cohesin depletion leads to
higher sensitivity to DNA-damaging agents and ionizing radiation
(5,8,42,43).
As RAD21 downregulation increases the sensitivity to certain drugs
used in breast cancer therapy, the inhibition of this gene may
facilitate the more effective eradication of cancer cells (8,9). This
may allow prognostic and predictive analyses of endometrial tumor
response to radiation and drugs.
Acknowledgements
This study was supported by a grant
from the National Science Centre (5715/B/P01/2010/38) and a grant
from the Foundation for Polish Science Parent-Bridge Programme
co-financed by the European Union within the European Regional
Development Fund (DPS-424-5053/11).
References
|
1.
|
American Cancer SocietyCancer Facts &
Figures 2011AtlantaAmerican Cancer Society2011
|
|
2.
|
C MichaelisR CioskK NasmythCohesins:
chromosomal proteins that prevent premature separation of sister
chromatidsCell913545199710.1016/S0092-8674(01)80007-69335333
|
|
3.
|
V GuacciD KoshlandA StrunnikovA direct
link between sister chromatid cohesion and chromosome condensation
revealed through the analysis of MCD1 in S.
cerevisiaeCell914757199710.1016/S0092-8674(01)80008-89335334
|
|
4.
|
E WatrinJM PetersCohesin and DNA damage
repairExp Cell
Res31226872693200610.1016/j.yexcr.2006.06.02416876157
|
|
5.
|
E SonodaT MatsusakaC
MorrisonScc1/Rad21/Mcd1 is required for sister chromatid cohesion
and kinetochore function in vertebrate cellsDev
Cell1759770200110.1016/S1534-5807(01)00088-011740938
|
|
6.
|
JA HorsfieldSH AnagnostouJK
HuCohesin-dependent regulation of Runx
genesDevelopment13426392649200710.1242/dev.00248517567667
|
|
7.
|
C BauschS NooneJM HenryTranscription
alters chromosomal locations of cohesin in Saccharomyces
cerevisiaeMol Cell
Biol2785228532200710.1128/MCB.01007-0717923700
|
|
8.
|
JM AtienzaRB RothC RosetteSuppression of
RAD21 gene expression decreases cell growth and enhances
cytotoxicity of etoposide and bleomycin in human breast cancer
cellsMol Cancer Ther4361368200515767545
|
|
9.
|
H XuM YanJ PatraEnhanced RAD21 cohesin
expression confers poor prognosis and resistance to chemotherapy in
high grade luminal, basal and HER2 breast cancersBreast Cancer
Res13R9201110.1186/bcr281421255398
|
|
10.
|
K OikawaT OhbayashiT KiyonoExpression of a
novel human gene, human wings apart-like (hWAPL), is associated
with cervical carcinogenesis and tumor progressionCancer
Res6435453549200410.1158/0008-5472.CAN-03-382215150110
|
|
11.
|
G YamamotoT IrieT AidaY NagoshiR TsuchiyaT
TachikawaCorrelation of invasion and metastasis of cancer cells,
and expression of the RAD21 gene in oral squamous cell
carcinomaVirchows
Arch448435441200610.1007/s00428-005-0132-y16416296
|
|
12.
|
KP PorkkaTL TammelaRL VessellaT
VisakorpiRAD21 and KIAA0196 at 8q24 are amplified and overexpressed
in prostate cancerGenes Chromosomes
Cancer39110200410.1002/gcc.1028914603436
|
|
13.
|
B RyuDS KimAM DelucaRM AlaniComprehensive
expression profiling of tumor cell lines identifies molecular
signatures of melanoma progressionPLoS
One2e594200710.1371/journal.pone.000059417611626
|
|
14.
|
J PlanagumàM Díaz-FuertesA Gil-MorenoA
differential gene expression profile reveals overexpression of
RUNX1/AML1 in invasive endometrioid carcinomaCancer
Res6488468853200415604243
|
|
15.
|
H XuK BalakrishnanJ MalaterreRad21-cohesin
haploinsufficiency impedes DNA repair and enhances gastrointestinal
radiosensitivity in micePLoS
One5e12112201010.1371/journal.pone.001211220711430
|
|
16.
|
H XuJM TomaszewskiMJ McKayCan corruption
of chromosome cohesion create a conduit to cancer?Nat Rev
Cancer11199210201110.1038/nrc301821326324
|
|
17.
|
K BlythER CameronJC NeilThe RUNX genes:
gain or loss of function in cancerNat Rev
Cancer5376387200510.1038/nrc160715864279
|
|
18.
|
DR RhodesJ YuK ShankerLarge-scale
meta-analysis of cancer microarray data identifies common
transcriptional profiles of neoplastic transformation and
progressionProc Natl Acad Sci
USA10193099314200410.1073/pnas.040199410115184677
|
|
19.
|
LJ van ‘t VeerH DaiMJ van de VijverGene
expression profiling predicts clinical outcome of breast
cancerNature415530536200211823860
|
|
20.
|
OD RøeE AnderssenE HelgeGenome-wide
profile of pleural mesothelioma versus parietal and visceral
pleura: the emerging gene portrait of the mesothelioma
phenotypePLoS One4e6554200919662092
|
|
21.
|
A DollM GonzalezM AbalAn orthotopic
endometrial cancer mouse model demonstrates a role for RUNX1 in
distant metastasisInt J
Cancer125257263200910.1002/ijc.2433019384951
|
|
22.
|
PV SpirinF BaskaranNN OrlovaDownregulation
of activated leukemic oncogenes AML1-ETO and RUNX1(K83N) expression
with RNA-interferenceMol Biol (Mosk)448768882010(In Russian)
|
|
23.
|
K InoueY ItoNeuroblastoma cell
proliferation is sensitive to changes in levels of RUNX1 and RUNX3
proteinGene487151155201110.1016/j.gene.2011.05.01621640801
|
|
24.
|
T NiiniJ KanervaK VettenrantaUM
Saarinen-PihkalaS KnuutilaAML1 gene amplification: a novel finding
in childhood acute lymphoblastic
leukemiaHaematologica85362366200010756360
|
|
25.
|
KA JanesRUNX1 and its understudied role in
breast cancerCell
Cycle1034613465201110.4161/cc.10.20.1802922024923
|
|
26.
|
R BeroukhimCH MermelD PorterThe landscape
of somatic copy-number alteration across human
cancersNature463899905201010.1038/nature0882220164920
|
|
27.
|
S PecorelliRevised FIGO staging for
carcinoma of the vulva, cervix, and endometriumInt J Gynaecol
Obstet105103104200910.1016/j.ijgo.2009.02.01219367689
|
|
28.
|
AT BaronN MaihleNadir CA125 concentration
as a prognostic indicator in ovarian cancerNat Clin Pract
Oncol2288289200510.1038/ncponc017816264982
|
|
29.
|
WE ConsultationAppropriate body-mass index
for Asian populations and its implications for policy and
intervention
strategiesLancet363157163200410.1016/S0140-6736(03)15268-314726171
|
|
30.
|
J VandesompeleK De PreterF PattynAccurate
normalization of real-time quantitative RT-PCR data by geometric
averaging of multiple internal control genesGenome
Biol3RESEARCH0034200210.1186/gb-2002-3-7-research003412184808
|
|
31.
|
MW PfafflA new mathematical model for
relative quantification in real-time RT-PCRNucleic Acids
Res29e45200110.1093/nar/29.9.e4511328886
|
|
32.
|
A ZaczekA MarkiewiczJ JaskiewiczClinical
evaluation of developed PCR-based method with hydrolysis probes for
TOP2A copy number evaluation in breast cancer samplesClin
Biochem43891898201010.1016/j.clinbiochem.2010.04.06020441774
|
|
33.
|
S CovoJW WestmorelandDA GordeninMA
ResnickCohesin is limiting for the suppression of DNA
damage-induced recombination between homologous chromosomesPLoS
Genet6e1001006201010.1371/journal.pgen.100100620617204
|
|
34.
|
PR PottsMH PorteusH YuHuman SMC5/6 complex
promotes sister chromatid homologous recombination by recruiting
the SMC1/3 cohesin complex to double-strand breaksEMBO
J2533773388200610.1038/sj.emboj.760121816810316
|
|
35.
|
AV RulinaPV SpirinVS PrassolovActivated
leukemic oncogenes AML1-ETO and c-kit: role in development of acute
myeloid leukemia and current approaches for their
inhibitionBiochemistry
(Mosc)7516501666201010.1134/S000629791013009221417999
|
|
36.
|
VI GaidzikL BullingerRF SchlenkRUNX1
mutations in acute myeloid leukemia: results from a comprehensive
genetic and clinical analysis from the AML study groupJ Clin
Oncol2913641372201110.1200/JCO.2010.30.792621343560
|
|
37.
|
S RamaswamyKN RossES LanderTR GolubA
molecular signature of metastasis in primary solid tumorsNat
Genet334954200310.1038/ng106012469122
|
|
38.
|
M KadotaHH YangB GomezDelineating genetic
alterations for tumor progression in the MCF10A series of breast
cancer cell linesPLoS
One5e9201201010.1371/journal.pone.000920120169162
|
|
39.
|
M AbalJ PlanagumaA Gil-MorenoMolecular
pathology of endometrial carcinoma: transcriptional signature in
endometrioid tumorsHistol Histopathol21197204200616329044
|
|
40.
|
J PlanagumàM AbalA Gil-MorenoUp-regulation
of ERM/ETV5 correlates with the degree of myometrial infiltration
in endometrioid endometrial carcinomaJ
Pathol207422429200516175655
|
|
41.
|
J PlanagumàM GonzalezA DollThe
up-regulation profiles of p21WAF1/CIP1 and RUNX1/AML1 correlate
with myometrial infiltration in endometrioid endometrial
carcinomaHum Pathol3710501057200616867868
|
|
42.
|
RP BirkenbihlS SubramaniCloning and
characterization of rad21 an essential gene of
Schizosaccharomyces pombe involved in DNA
double-strand-break repairNucleic Acids
Res2066056611199210.1093/nar/20.24.66051480481
|
|
43.
|
C SjögrenK NasmythSister chromatid
cohesion is required for postreplicative double-strand break repair
in Saccharomyces cerevisiaeCurr Biol11991995200111448778
|