Contents
Introduction
Overview of AIB1 structure and function
Implication of AIB1 in breast cancer
Conclusion
Introduction
Amplified in breast cancer 1 (AIB1), also known as
steroid receptor coactivator-3 (SRC-3), nuclear receptor
coactivator-3 (NCoA-3), receptor associated coactivator-3 (RAC-3),
activator of thyroid hormone and retinoid receptor (ACTR), thyroid
hormone receptor activating molecule-1 (TRAM-1) and p300/CBP
interacting protein (p/CIP) is a member of the p160 nuclear
receptor coactivator family (1).
Other members of this family include SRC-1 and SRC-2. The
AIB1 gene is located on chromosome 20q12-12, and it was
first identified in human breast cancer cells, where approximately
10% of these cells revealed amplification of the gene and 64%
revealed overexpression of the protein (2). AIB1 was later considered as an
oncogene since overexpression of AIB1 in mice led to the
spontaneous development of malignant mammary tumors (3), whereas AIB1−/− mice were
resistant to chemical carcinogen-induced mammary tumorigenesis
(4,5). However, AIB1 also acts as a tumor
suppressor since deletion of the AIB1 gene in B-cell
lymphoma mice led to the development of B-cell lymphomas (6). Furthermore, results from cell culture
systems and targeted gene disruption experiments in mice have
demonstrated that AIB1 also plays an essential role in the female
reproductive function, puberty, cytokine signaling and
vasoprotection (7). The correlation
between AIB1 and cancer has been widely investigated since it was
shown to be amplified in breast cancer. Initially, AIB1 was thought
to promote cancer development through hormone-dependent pathways
since it acts as a transcriptional coactivator for nuclear
receptors in estrogen receptor (ER)-positive breast cancer.
However, various non-nuclear receptor transcription factors, such
as E2F1, p53 and NF-κB were found to be coactivated by AIB1, which
provides supporting evidence that AIB1 also influences the progress
of cancer cells through hormone-independent pathways (8–10).
Over the past decade, numerous reviews focusing on the function of
AIB1 and its role in cancer have been published (11–19).
The focus of this review is to highlight the important progress
made with recent findings and to present an overview of the current
understanding of the signaling pathways through which the influence
of AIB1 leads to the development and progression of breast
cancer.
Overview of AIB1 structure and function
Structural domains of AIB1 and its
functions
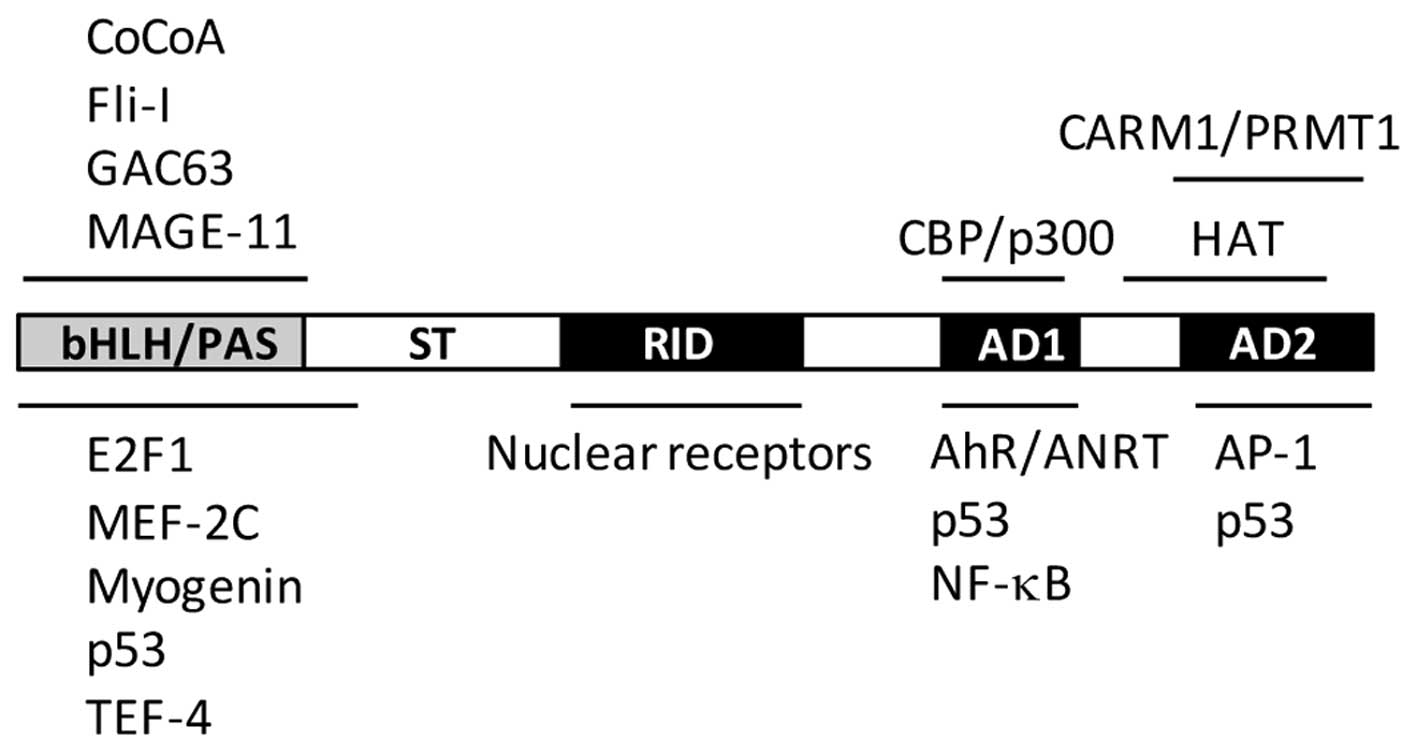
AIB1 is approximately 160 kDa in size and its
structure is conserved across different species. The structure of
AIB1 consists of a central nuclear receptor interaction domain
(NID), an N-terminal basic helix-loop-helix/Per-ARNT-Sim (bHLH/PAS)
domain and two activation domains, known as AD1 and AD2, located in
the C-terminal region (Fig. 1). In
addition, it also contains a serine/threonine-rich domain in the
N-terminus, a glutamine (Q)-rich domain, and a histone
acetyltransferase (HAT) domain in the C-terminus. The relatively
conserved NID domain mainly mediates direct interaction between
AIB1 and nuclear receptors, such as ER and androgen receptor (AR)
through ligand-dependent pathways. Analysis of the NID sequence has
revealed three conserved LXXLL motifs (where L is leucine and X is
any amino acid) that act as a nuclear receptor box (20). These three LXXLL motifs form an
amphipathic α-helix in the secondary structure, which then allows
the conserved leucines to form a hydrophobic surface that mediates
the binding of ligands to the ligand-binding domain of nuclear
receptors (13).
The bHLH/PAS domain of AIB1 is conserved in the SRC
family with a sequence similarity of approximately 60%. This domain
mediates protein-protein interactions that result in the
recruitment of other coactivators. The AD1 domain, also named
CBP-interaction domain (CID), is involved in direct interaction
with the general transcriptional cointegrators, CBP/p300 and PCAF,
without interacting with nuclear receptors (21). The AD1 domain also contains three
LXXLL/LXXLL-like motifs, which are crucial in promoting the
interaction between AIB1 and p300 (22,23).
The AD2 domain is mainly responsible for the interaction with
histone methyltransferases, including coactivator-associated
arginine methyltransferase-1 (CARM1) and protein arginine
methyltransferase-1 (PRMT1) (24–26).
The HAT activity of the C-terminal domain of AIB1 is weaker than
that of CBP/p300 and PCAF, and its importance in AIB1
transcriptional activation has yet to be clarified (27–29).
The structure and function of AIB1 has previously been extensively
reviewed (11,17).
Importance of post-translational
modification
Certain serines and threonines in the
serine/threonine-rich domain are also sites of phosphorylation.
In vitro phosphorylation of AIB1 converts it into a potent
transcriptional activator, thereby modifying its oncogenic
potential and leading to differential gene expression (30). Phosphorylation of tyrosine residue
(Y1357) in the AD2 domain by AbI kinase is required for its
activity in cancer cells (31).
Dephosphorylation of AIB1 by phosphatases has been shown to be
critical for regulating its function and preventing its
proteasome-dependent turnover (32), and phosphorylation by atypical
protein kinase C (aPKC), which is frequently overexpressed in
cancers, specifically stabilizes AIB1 in an ER-dependent manner
through coordinating the inhibition of both ubiquitin-dependent and
ubiquitin-independent degradations (33). The turnover of activated AIB1 during
tumorigenesis has recently been shown to be mediated by
speckle-type POZ protein (SPOP), which is a cullin 3-based
ubiquitin ligase (34). A high
percentage of loss of heterozyogisty at the SPOP locus was found in
breast cancers, and restoration of its expression resulted in the
suppression of AIB1-mediated oncogenic signaling and
tumorigenesis.
Our previous study demonstrated that phosphorylation
of AIB1 is accompanied by a loss in sumoylation and an increase in
its transactivation, while dephosphorylation is accompanied by a
concomitant increase in sumoylation and reduced transactivation
(35). We have recently reported
that sumoylation of AIB1 requires the SUMO E3 PIAS1, which
coprecipitated with AIB1 in extract prepared from MCF-7 cells, and
that overexpression of PIAS1 and AIB1 in MCF-7 cells led to
increased sumoylation of AIB1, resulting in repression of its
transcriptional activity (36).
PIAS1 also increased the stability of AIB1 and attenuated its
interaction with ERα. These findings suggest that PIAS1 may play a
crucial role in the regulation of AIB1 transcriptional activity and
its interaction with accessory proteins through sumoylation.
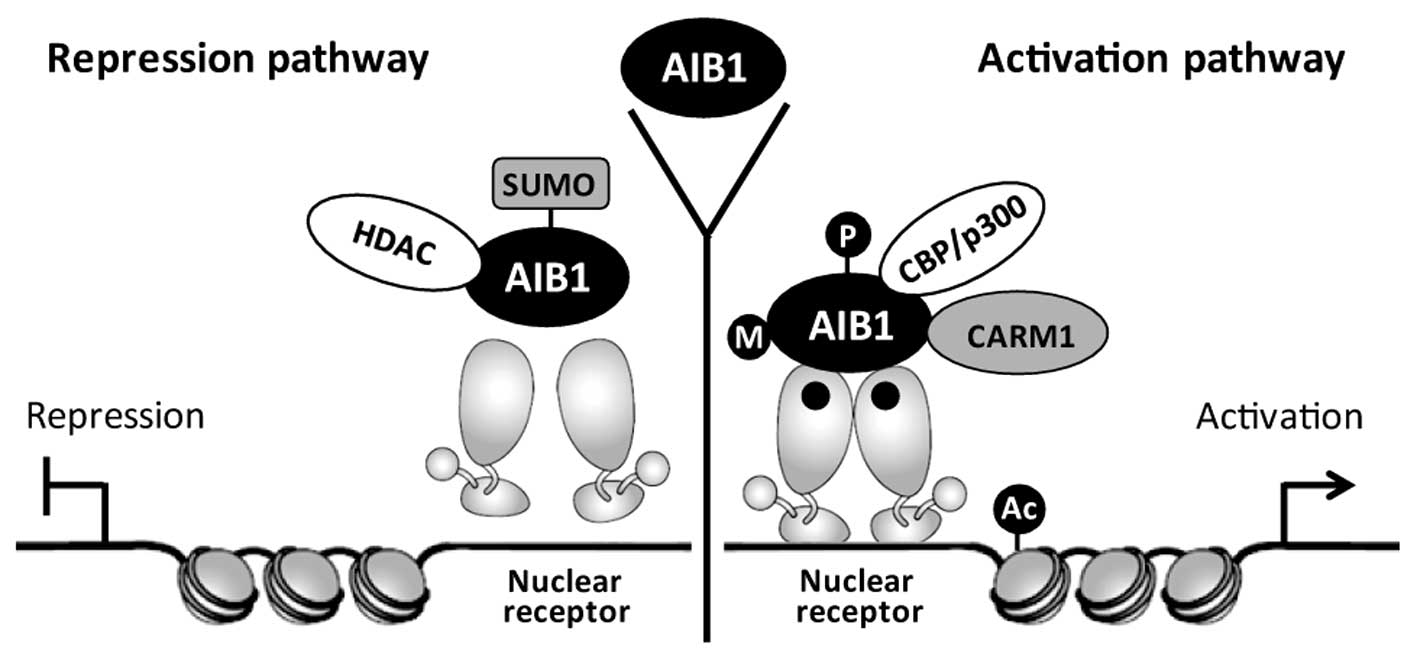
As an ER coactivator, AIB1 regulates ER
transcriptional activity through recruitment of the histone
acetyltransferases CBP/p300 and PCAF. Acetylation of histones could
modify chromatin structure and facilitate ER to bind at the
promoters of downstream target genes, leading to enhanced
expression of cancer genes. Through sequencing and mapping of
genomic DNA fragments obtained by AIB1 ChIP assays, Labhart et
al (37) identified 18 putative
AIB1 target genes based on their strong AIB1-binding sites, and
demonstrated ERα binding with all of these genes. AIB1 also
promotes certain transcription factors to interact with other
transcription cofactors and this process is regulated by
post-translational modifications, including methylation,
sumoylation, phosphorylation and acetylation (38). The transcriptional complex of AIB1
in hormone-induced gene expression mediated by the nuclear receptor
is shown in Fig. 2.
Implication of AIB1 in breast cancer
AIB1 and hormone-dependent breast
cancer
The AIB1 gene is amplified in approximately
5–10% of human breast cancers and is overexpressed at both the mRNA
(as high as 60%) and protein levels in approximately 30% of breast
cancers (2,39–43).
Further study has revealed that overexpression of AIB1 is
correlated with tumor recurrence and survival (44). Increases in AIB1 transcript levels
in human breast tumors may also occur by mechanisms other than gene
amplification, such as overexpression of AIB1 mRNA resulting from a
loss of ER expression in breast tumor samples (45). A recent study investigating the
prognostic significance of AIB1 and its correlation with various
steroid hormone receptors (ER, PR, AR, DAX-1 and HER2) shows that
for patients suffering from ER-negative breast cancers, strong AIB1
protein expression is correlated with poorer overall survival
(46). Besides breast cancer,
amplification of AIB1 has also been detected in many other
hormone-sensitive tumors, including prostate and ovarian cancers
(2,47).
As a member of the steroid receptor coactivator
family, AIB1 is essential for the transcriptional activity of
certain nuclear receptors (including ERα), which control processes
important for development, homeostasis and reproduction (48). AIB1 is considered to play
significant roles in ER-positive breast cancers. The level of ER in
breast cancer is considered to be an important marker for most
breast cancer therapy and prognosis. As a coactivator for ER, AIB1
is thought to influence the growth of hormone-dependent breast
cancer through mediating the effects of estrogen on ERα-dependent
gene expression (2,41), and this serves as a mechanism by
which AIB1 modulates the growth of hormone-dependent breast cancer.
This mechanistic model is supported by a study showing that
depletion of AIB1 may inhibit estrogen-stimulated cell
proliferation and survival in ER-positive MCF-7 human breast cancer
cells, leading to a decrease in growth of MCF-7 xenografts in mice
(49,50). However, not all ERα-positive breast
cancers are associated with higher levels of AIB1 mRNA, as
ERα-negative breast cancer has also been found to be associated
with high levels of AIB1 mRNA (14). Discrepancy among these studies is
thought to be caused by differences in the role and regulation of
AIB1 and the hormone receptors at different stages of breast
cancer. A recent study has uncovered evidence of an association
between silencing mediator of retinoic acid and thyroid hormone
receptor (SMRT) with AIB1 in the regulation of ER-dependent gene
expression, such as the expression of progesterone receptor and
cyclin D1 genes (51). SMRT is able
to bind directly to AIB1 independently of ER, and this complex then
promotes the subsequent E2-dependent binding of AIB1 to ER,
demonstrating that SMRT promotes ER- and AIB1-dependent gene
expression in breast cancer.
The correlation between AIB1 and breast cancer has
also been investigated using several AIB1-depleted mouse models. In
mice harbouring the mouse mammary tumor virus/v-Ha-ras (ras)
transgene, breast tumor incidence was notably reduced in intact
AIB1−/− -ras virgin mice compared to complete inhibition
in ovariectomized AIB1−/− -ras mice (4). Furthermore, the level of IGF-1
expression and insulin receptor substrate (IRS)-1 and -2 proteins
in the mammary glands and tumors of these mice were significantly
reduced, which contributed in part to the suppression of mammary
tumorigenesis and metastasis. In another model, mice lacking AIB1
were shown to be resistant to chemical carcinogen-induced mammary
tumorigenesis (5). In a different
mouse model, deletion of one allele of AIB1 in MMTV-Neu mice was
found to significantly delay Neu-induced mammary tumor development,
demonstrating that AIB1 is required for Neu (ErbB2/HER2)
activation, signaling and mammary tumorigenesis (52). Although these animal models have
provided important data on the involvement of AIB1 in breast cancer
by allowing the disease to be simulated or recreated under
controlled conditions, they still do not represent the real
condition of the disease in the case of humans, and at best, should
only be regarded as a mimic. Thus, what have been learned from
animal models may not necessarily be completely applicable to
humans.
AIB1 also appears to play a significant role in the
resistance of breast cancer to anti-estrogen therapy. Over the past
few decades, tamoxifen has been the standard endocrine therapy for
treating ER-positive breast cancer. Tamoxifen is a non-steroidal
estrogen receptor antagonist (but also exhibits agonist activity)
and functions by competitively blocking the binding of estrogen to
ER, thereby inhibiting estrogen-mediated gene expression and
estrogen-dependent cell growth (53). However, the use of tamoxifen has
gradually led to the emergence of tamoxifen resistance in breast
cancer. The involvement of AIB1 in tamoxifen resistance has been
demonstrated in breast cancer patients, whereby disease-free and
overall survival is correlated with high expression of AIB1. There
is also evidence linking the expression of AIB1 protein and breast
tumor recurrence in ErbB2-positive breast tumors, and knockdown of
AIB1 in tamoxifen-resistant, ErbB2-positive breast cancer cell line
BT474 restored its sensitivity to tamoxifen (54). These results appear to indicate that
the expression of AIB1 is associated with resistance to tamoxifen
for ER-positive breast cancer patients undergoing tamoxifen
treatment. The underlying mechanism for tamoxifen resistance has
been further clarified by data obtained from an in vivo
breast tumor model, which showed that tamoxifen resistance in
ER-positive breast cancer is also mediated by EGFR/HER2, even
though ER genomic function is suppressed by tamoxifen (55). In a recent study, Karmakar et
al (56) evaluated the role of
AIB1 and two other SRC coactivators in the growth of the
estrogen-independent and tamoxifen-resistant breast cancer cell
line LCC2 and found that these coactivators exert a mixture of
ligand-dependent and ligand-independent effects on the regulation
of cell growth and apoptosis. Furthermore, these authors
demonstrated that growth of LCC2 cells is controlled by AIB1 and
SCR-2, largely through the control of basal cell growth, and
suggested that targeting growth inhibition via SRC-2 and AIB1 may
offer a more effective way to inhibit the growth of
tamoxifen-resistant breast cancer. Resistance to tamoxifen is now
being viewed as a result of crosstalk between ER and growth factor
signaling pathways (57,58). AIB1 is considered to play a positive
role in tamoxifen resistance since overexpression of AIB1 alone has
been shown to increase the agonist properties of tamoxifen in
breast cancer cell lines (59).
Taken together, these existing findings support the notion that
tamoxifen resistance is a product of multiple mechanisms, and that
AIB1 appears to play an important role; however, further
investigation is required to provide a more definitive
understanding of its underlying mechanism.
A more recent study concerned with the global
characterization of the transcriptional impact of AIB1 has shed
more light on the scope of AIB1 target genes and provides a
molecular framework for AIB1 in interpreting estrogen signaling
(60). This study, which combines
genome-wide mapping of AIB1 affinity sites in MCF-7 cells with RNA
expression signatures and a proteomic approach, is so far the most
sophisticated study to identify the transcriptional regulatory
network of AIB1. It also opens up new areas for exploring the
hormone-dependent signaling of breast cancer afforded by AIB1.
AIB1 and hormone-independent breast
cancer
Although AIB1 levels have been shown to be a
limiting factor for ER-positive breast cancer growth through
hormone-dependent pathways, substantial evidence has suggested that
AIB1 stimulates the growth of cancer cells through
hormone-independent pathways. For example, in a recent study,
Torres-Arzayus et al (61)
addressed the role of estrogen and ERα in AIB1-mediated tumor
formation and showed that AIB1 transgenic mice that had their
ovaries removed at the prepubertal stage to block estrogen
production did not develop any invasive mammary gland tumors.
However, these animals showed higher incidence of pituitary, lung,
skin and bone tumors than their non-ovariectomized counterparts.
They also crossed AIB1 transgenic mice with ERα-null mutant mice
and found that mice lacking ERα were unable to respond to estrogen
through this receptor. At the same time, these animals showed no
signs of mammary tumors but developed tumors in the lung, skin, and
pituitary gland with the same incidence as their ERα-positive AIB1
transgenic counterparts. The authors concluded from these findings
that depending on the organ or tissue affected, AIB1 causes tumor
formation by estrogen-dependent and estrogen-independent
mechanisms, and that AIB1 exerts its oncogenic activities in cell
signaling that are independent of its function as an ER
coactivator. Other studies have also indicated the involvement of
AIB1 in breast cancer through certain hormone-independent pathways,
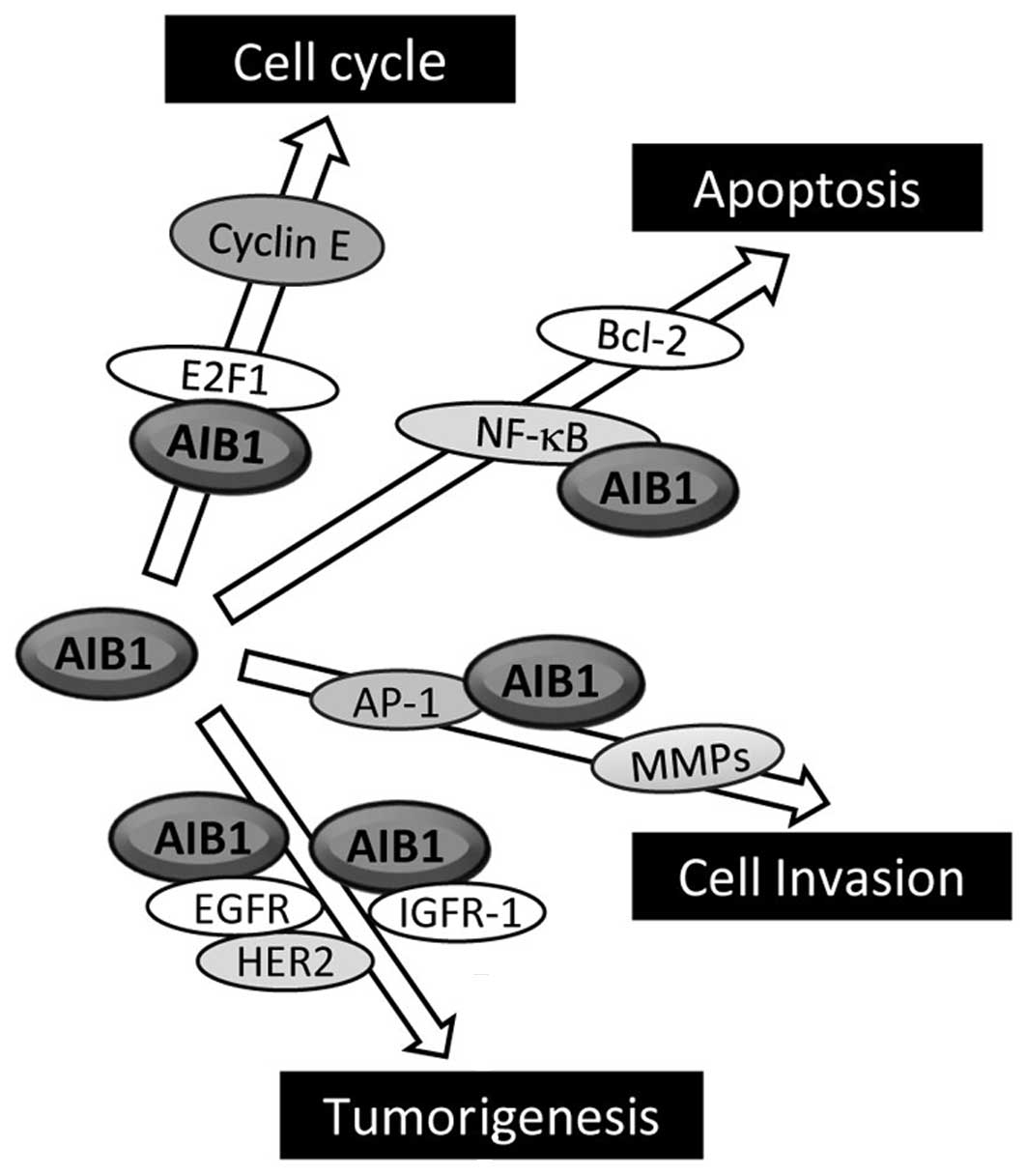
including E2F1, IGF-I and EGF signaling (Fig. 3) (7,62).
Overexpression of AIB1 has been found to increase
cell proliferation even in the presence of nuclear receptor
antagonist (9). More convincingly,
the overexpression of AIB1 promotes the growth of
hormone-independent cancer cells through enhancing the
transcription of E2F target genes, which are mostly G1/S cycle
transition-related proteins, such as E2F1, cyclin E and
cyclin-dependent kinase 2 (Cdk2) (9). AIB1 interacts with the transcriptional
factor E2F1, and this then results in the recruitment of AIB1 to
the E2F-binding sites on the target gene promoters, eventually
leading to activation of these E2F-dependent downstream genes.
Notably, AIB1 promotes its own transcription with E2F1, and this
positive feedback regulatory loop is thought to enhance the
influence AIB1 exerts on cell cycle control. Overexpression of AIB1
has also been found to correlate with high levels of p53 proteins
in invasive breast cancer cells (45).
The association of AIB1 with the regulation of IGF-I
signaling in cancer is supported by the finding that AIB1 knockout
downregulates the expression levels of both IGF-I mRNA and protein,
whereas overexpression of AIB1 has the opposite effect (63). In addition, AIB1 may also regulate
the expression of many IGF-I signaling components, including IGF-I
receptor β (IGF-IRβ), IRS-1 and IRS-2 in vitro and in
vivo. Although the mechanism as to how AIB1 modulates IGF-I
signaling in cancers is not clear, it has been reported that AIB1
binds to the transcription factor AP-1 and promotes AP-1-mediated
transcription of IGF-I and IRS-1 (64). In addition, AIB1 knockout mice have
an impaired ability in their response to IGF-I stimulation and are
unresponsive to IGF-I-induced DNA synthesis. This may in part be
explained by the result from the study conducted by Liao et
al (65), which shows that
AIB1−/− mice have lower levels of circulating IGF-I
compared to wild-type mice, a consequence of rapid IGF-I
degradation rather than lower expression. The rapid IGF-I
degradation is caused by a lack of expression of IGF-binding
protein 3 (IGFBP-3), which is induced by vitamin D through a
vitamin D receptor (VDR). These results point to a role of AIB1 in
maintaining the circulating level of IGF-I through increasing
VDR-regulated IGFBP-3 expression. The PI3K/Akt/mTOR pathway is also
associated with AIB1-mediated tumorigenesis, since it was shown
that mammary hyperplasia and hypertrophy induced in mice via
overexpression of AIB1 can be prevented by inhibition of mTOR with
the rapamycin analog RAD001 (66).
RAD001 also inhibits the growth of AIB1-induced tumor xenografts in
mice.
EGF signaling is known to play a crucial role in the
initiation and progression of breast cancer (67). The influence of AIB1 on breast
cancer via EGF signaling is supported by a study showing that AIB1
knockdown by siRNA reduced EGF-mediated phosphorylation of EGFR and
HER-2, leading to inhibition of the activation of EGF signaling in
lung, pancreatic and breast cancer cells and confirming that AIB1
regulates EGF signaling to promote the proliferation of cancer
cells (68). Taken together, these
studies indicate that the role of AIB1 extends beyond the actions
of steroid hormone receptors in cancer cells.
The role of AIB1 in cancer progression
and metastasis
AIB1 plays a significant role in mammary tumor
progression and metastasis through mediating tumor cell motility
and invasion (18,43,69).
AIB1−/− mice harboring the mouse mammary tumor
virus-polomavirus middle T (PyMT) transgene or
AIB1−/−/PyMT human tumor cells were found to have
reduced expression of MMP2 and MMP9, resulting in lower
cell-invasive and metastatic capabilities (43). AIB1 acts as a PEA3 coactivator by
forming complexes with PEA3 on MMP2 and MMP9 promoters to enhance
their expression in both mouse and human breast cancer cells.
Furthermore, a AIB1 splice isoform lacking the N-terminal bHLH
domain (due to the deletion of exon 4) that is overexpressed in
breast cancer cells and tumors has also been shown to play critical
roles in promoting cancer cell proliferation, invasion and
metastasis through acting as a missing adaptor protein that bridges
the interaction between EGFR and FAK (a non-receptor tyrosine
kinase) following EGF stimulation (69).
Influence of AIB1 on tumor suppressor
gene and cancer
A hallmark of cancer is often an improper balance
between cell proliferation and apoptosis. Oncogene activation
coupled with loss of tumor suppressor function would enable the
cell to escape senescence and apoptosis, thereby providing an
advantage for tumorigenesis. A number of studies have demonstrated
the involvement of AIB1 in apoptosis. For example, knockdown of
AIB1 by siRNA in human chronic myeloid leukemia K562 cells reduces
activation of NF-κB signaling, ultimately leading to apoptosis
(70), while overexpression of AIB1
in human embryonic kidney 293 (HEK293) cells has the reverse effect
(71). A study by Ferragud et
al (72) revealed that AIB1
acts as a negative regulator for DROI, a tumor suppressor gene that
was first identified as upregulated in brown adipose tissue of mice
deficient in bombesin receptor subtype-3 (73). The function of DROI as a tumor
suppressor gene was later demonstrated when its expression was
shown to be highly reduced in colon and pancreatic cancers
(74). Further investigation
revealed that DROI plays an important role in adipogenesis through
downregulating Wnt/β-catenin signaling and inducing C/EBPα and
PPARγ (75). The expression of DROI
was significantly reduced in mouse mammary epithelial cells or
human primary cultures overexpressing AIB1 (72). Furthermore, DROI expression levels
decreased in MCF-7 cells treated with estrogen but increased when
treated with tamoxifen. Another study identified the tumor
suppressor 53BP1 (a DNA-damaging response protein) as a novel
AIB1-interacting protein, and through chromatin immunoprecipitation
(ChIP) and siRNA knockdown experiments, AIB1 and 53BP1 were shown
to co-occupy the same region of the breast cancer type 1
susceptibility protein (BRCA1) promoter, and both proteins were
required for BRCA1 expression in HeLa cells (76). There was no evidence to indicate
that AIB1 plays a direct role in DNA damage response; however,
these authors concluded that the association between 53BP1 and AIB1
may modulate the transcriptional response of the BRCA1 gene
and possibly regulate the activity of a subset of target genes
involved in DNA repair. Taken together, these results demonstrate
yet another mechanistic feature of AIB1 in cancer development, one
that occurs via its suppression of tumor suppressor genes.
Conclusion
For the past few decades, endocrine therapy has been
the most effective treatment for women with ER-positive breast
cancer. However, the emergence of resistance to endocrine therapy
has prompted the search for a different strategy, which involves
targeting both ER and growth factor receptor signaling (77). Therapeutic approaches that
simultaneously target ER and components of growth factor signaling,
such as EGFR, HER2 and PI3K, have had some success in the
preclinical setting. However, extensive further study is required
to take this approach beyond the preclinical setting. As far as the
role of AIB1 in breast cancer is concerned, given the pleiotropic
effect of this coactivator and the complexity of the signaling
network that it participates in and regulates, it becomes a great
challenge to conceive of a long-term therapeutic strategy that
effectively eliminates the cancer and prevents recurrence of the
disease. Post-translational modification of AIB1 in the form of
phosphorylation and its relevance to cancer is still not fully
elucidated, and there has been suggestion that if the phosphoforms
of AIB1 are important in human malignant disease then targeting
these phosphoforms of AIB1 may be a useful therapeutic approach
(17). Interrupting nuclear
receptor coactivator interactions has been suggested to be
beneficial in treating nuclear receptor-mediated diseases,
including breast cancer (19).
However, specifically targeting AIB1 or any of its associated
transcription factors, coactivators or accessory proteins is
unlikely to be effective, since such a measure is likely to upset
the overall cellular reaction network in the long term, resulting
in potential side effects. Nevertheless, future research should
focus on gaining more insight into the mechanistic functions and
regulations of AIB1, particularly those concerned with its
dysfunctions that ultimately trigger the onset of cancers. Studies
on the post-translational modifications (including sumoylation,
which still requires extensive research) that govern the regulation
and turnover of the AIB1 protein with respect to its modulation of
cancer development and progression are also important and relevant
to the overall therapeutic strategy for combating breast cancers
mediated by this transcriptional coactivator.
Acknowledgements
This study was supported by a grant
(30771221 to H.W.) from the National Natural Science Foundation of
China and a grant (973 Program 2011CB504201 to H.W.) from the
Ministry of Science and Technology of China.
References
|
1.
|
J XuQ LiReview of the in vivo functions of
the p160 steroid receptor coactivator familyMol
Endocrinol1716811692200310.1210/me.2003-011612805412
|
|
2.
|
SL AnzickJ KononenRL WalkerDO AzorsaMM
TannerXY GuanG SauterOP KallioniemiJM TrentPS MeltzerAIB1, a
steroid receptor coactivator amplified in breast and ovarian
cancerScience277965968199710.1126/science.277.5328.9659252329
|
|
3.
|
MI Torres-ArzayusJ Font de MoraJ YuanF
VazquezR BronsonM RueWR SellersM BrownHigh tumor incidence and
activation of the PI3K/AKT pathway in transgenic mice define AIB1
as an oncogeneCancer
Cell6263274200410.1016/j.ccr.2004.06.02715380517
|
|
4.
|
SQ KuangL LiaoH ZhangAV LeeBW O'MalleyJ
XuAIB1/SRC-3 deficiency affects insulin-like growth factor 1
signaling pathway and suppresses v-Ha-ras-induced breast cancer
initiation and progression in miceCancer
Res6418751885200410.1158/0008-5472.CAN-03-374514996752
|
|
5.
|
SQ KuangL LiaoS WangD MedinaBW O'MalleyJ
XuMice lacking the amplified in breast cancer 1/steroid receptor
coactivator-3 are resistant to chemical carcinogen-induced mammary
tumorigenesisCancer Res6579938002200516140972
|
|
6.
|
A CosteMC AntalS ChanP KastnerM MarkBW
O'MalleyJ AuwerxAbsence of the steroid receptor coactivator-3
induces B-cell lymphomaEMBO
J2524532464200610.1038/sj.emboj.760110616675958
|
|
7.
|
G MaY RenK WangJ HeSRC-3 has a role in
cancer other than as a nuclear receptor coactivatorInt J Biol
Sci7664672201110.7150/ijbs.7.66421647249
|
|
8.
|
S WerbajhI NojekR LanzMA CostasRAC-3 is a
NF-kappa B coactivatorFEBS
Lett485195199200010.1016/S0014-5793(00)02223-711094166
|
|
9.
|
MC LouieJX ZouA RabinovichHW ChenACTR/AIB1
functions as an E2F1 coactivator to promote breast cancer cell
proliferation and antiestrogen resistanceMol Cell
Biol2451575171200410.1128/MCB.24.12.5157-5171.200415169882
|
|
10.
|
H YamashitaS TakahashiY ItoT YamashitaY
AndoT ToyamaH SugiuraN YoshimotoS KobayashiY FujiiH IwasePredictors
of response to exemestane as primary endocrine therapy in estrogen
receptor-positive breast cancerCancer
Sci10020282033200910.1111/j.1349-7006.2009.01274.x19659610
|
|
11.
|
L LiaoSQ KuangY YuanSM GonzalezBW
O'MalleyJ XuMolecular structure and biological function of the
cancer-amplified nuclear receptor coactivator SRC-3/AIB1J Steroid
Biochem Mol Bio83314200210.1016/S0960-0760(02)00254-612650696
|
|
12.
|
J YanSY TsaiMJ TsaiSRC-3/AIB1:
transcriptional coactivator in oncogenesisActa Pharmacol
Sin27387394200610.1111/j.1745-7254.2006.00315.x16539836
|
|
13.
|
L AmazitL PasiniAT SzafranV BernoRC WuM
MielkeED JonesMG ManciniCA HinojosBW O'MalleyMA ManciniRegulation
of SRC-3 intercompartmental dynamics by estrogen receptor and
phosphorylationMol Cell
Biol2769136932200710.1128/MCB.01695-0617646391
|
|
14.
|
T LahusenRT HenkeBL KaganA WellsteinAT
RiegelThe role and regulation of the nuclear receptor co-activator
AIB1 in breast cancerBreast Cancer Res
Treat116225237200910.1007/s10549-009-0405-219418218
|
|
15.
|
J XuRC WuBW O'MalleyNormal and
cancer-related functions of the p160 steroid receptor co-activator
(SRC) familyNat Rev Cancer9615630200910.1038/nrc269519701241
|
|
16.
|
O GojisB RudrarajuC AlifrangisJ KrellP
LibalovaC PalmieriThe role of steroid receptor coactivator-3
(SRC-3) in human malignant diseaseEur J Surg
Oncol36224229201010.1016/j.ejso.2009.08.00219716257
|
|
17.
|
O GojisB RudrarajuM GudiK HogbenS SoushaRC
CoombesS CleatorC PalmieriThe role of SRC-3 in human breast
cancerNat Rev Clin
Oncol78389201010.1038/nrclinonc.2009.21920027190
|
|
18.
|
JP LydonBW O'MalleyMinireview: steroid
receptor coactivator-3: a multifarious coregulator in mammary gland
metastasisEndocrinology1521925201110.1210/en.2010-101221047941
|
|
19.
|
AB JohnsonBW O'MalleySteroid receptor
coactivators 1, 2, and 3: critical regulators of nuclear receptor
activity and steroid receptor modulator (SRM)-based cancer
therapyMol Cel
Endocrinol348430439201210.1016/j.mce.2011.04.02121664237
|
|
20.
|
RS SavkurTP BurrisThe coactivator LXXLL
nuclear receptor recognition motifJ Pept
Res63207212200410.1111/j.1399-3011.2004.00126.x15049832
|
|
21.
|
H ChenRJ LinRL SchilzD ChakravartiA NashL
NagyML PrivalskyY NakataniRM EvansNuclear receptor coactivator ACTR
is a novel histone acetyltransferase and forms a multimeric
activation complex with p/CAF and
CBP/300Cell90569580199710.1016/S0092-8674(00)80516-49267036
|
|
22.
|
JJ VoegelMJ HeineM TiniV VivatP ChambonH
GronemeyerThe coactivator TIF2 contains three nuclear
receptor-binding motifs and mediates transactivation through CBP
binding-dependent and -independent pathwaysEMBO
J17507519199810.1093/emboj/17.2.507
|
|
23.
|
J LiBW O'MalleyJ Wongp300 requires its
histone acetyltransferase activity and SRC-1 interaction domain to
facilitate thyroid hormone receptor activation in chromatinMol Cell
Biol2020312042200010.1128/MCB.20.6.2031-2042.2000
|
|
24.
|
C QiJ ChangY ZhuAV YeldandiSM RaoYJ
ZhuIdentification of protein arginine methyltransferase 2 as a
coactivator for estrogen receptor alphaJ Biol
Chem2772862428630200210.1074/jbc.M20105320012039952
|
|
25.
|
C TeyssierD ChenMR StallcupRequirement for
multiple domains of the protein arginine methyltransferase CARM1 in
its transcriptional coactivator functionJ Biol
Chem2774606646072200210.1074/jbc.M20762320012351636
|
|
26.
|
DY LeeC TeyssierBD StrahlMR StallcupRole
of protein methylation in regulation of transcriptionEndocr
Rev26147170200510.1210/er.2004-000815479858
|
|
27.
|
TE SpencerG JensterMM BurcinCD AllisJ
ZhouCA MizzenNJ McKennaSA OnateSY TsaiMJ TsaiBW O'MalleySteroid
receptor coactivator-1 is a histone acetyl
transferaseNature389194198199710.1038/383049296499
|
|
28.
|
D ChenH MaH HongSS KohSM HuangBT
SchurterDW AswadMR StallcupRegulation of transcription by a protein
methyltransferaseScience28421742177199910.1126/science.284.5423.217410381882
|
|
29.
|
SS KohD ChenYH LeeMR StallcupSynergistic
enhancement of nuclear receptor function by p160 coactivators and
two coactivators with protein methyltransferase activitiesJ Biol
Chem27610891098200110.1074/jbc.M00422820011050077
|
|
30.
|
RC WuCL SmithBW O'MalleyTranscriptional
regulation by steroid receptor coactivator phosphorylationEndocr
Rev26393399200510.1210/er.2004-001815814849
|
|
31.
|
AS OhJT LahusenCD ChienMP FereshtehX
ZhangS DakshanamurthyJ XuBL KaganA WellsteinAT RiegelTyrosine
phosphorylation of the nuclear receptor coactivator AIB1/SRC-3 is
enhanced by Abl kinase and is required for its activity in cancer
cellsMol Cell Biol2865806593200810.1128/MCB.00118-0818765637
|
|
32.
|
C LiYY LiangXH FengSY TsaiMJ TsaiBW
O'MalleyEssential phosphatases and a phospho-degron are critical
for regulation of SRC-3/AIB1 coactivator function and turnoverMol
Cell31835849200810.1016/j.molcel.2008.07.01918922467
|
|
33.
|
P YiQ FengL AmazitDM LonardSY TsaiMJ
TsaiBW O'MalleyAtypical protein kinase C regulates dual pathways
for degradation of the oncogenic coactivator SRC-3/AIB1Mol
Cell29465476200810.1016/j.molcel.2007.12.03018313384
|
|
34.
|
C LiJ AoJ FuDF LeeJ XuD LonardBW
O'MalleyTumor-suppressor role for the SPOP ubiquitin ligase in
signal-dependent proteolysis of the oncogenic co-activator
SRC-3/AIB1Oncogene3043504364201110.1038/onc.2011.15121577200
|
|
35.
|
H WuL SunY ZhangY ChenB ShiR LiY WangJ
LiangD FanG WuCoordinated regulation of AIB1 transcriptional
activity by sumolylation and phosphorylationJ Biol
Chem2812184821856200610.1074/jbc.M60377220016760465
|
|
36.
|
S LiC YangY HongH BiF ZhaoY LiuX AoP PangX
XingAK ChangThe transcriptional activity of co-activator AIB1 is
regulated by the SUMO E3 Ligase PIAS1Biol
Cell104287296201210.1111/boc.20110011622283414
|
|
37.
|
P LabhartS KarmakarEM SalicruBS EganV
AlexiadisBW O'MalleyCL SmithIdentification of target genes in
breast cancer cells directly regulated by the SRC-3/AIB1
coactivatorProc Natl Acad Sci
USA10213391344200510.1073/pnas.040957810215677324
|
|
38.
|
S LiY ShangRegulation of SRC family
coactivators by post-translational modificationsCell
Signal1911011112200710.1016/j.cellsig.2007.02.00217368849
|
|
39.
|
S BautistaH VallèsRL WalkerS AnzickR
ZeillingerP MeltzerC TheilletIn breast cancer, amplification of the
steroid receptor coactivator gene AIB1 is correlated with estrogen
and progesterone receptor positivityClin Cancer
Res42925292919989865902
|
|
40.
|
M GlaeserT FloetottoB HansteinMW BeckmannD
NiederacherGene amplification and expression of the steroid
receptor coactivator SRC3 (AIB1) in sporadic breast and endometrial
carcinomasHorm Metab
Res33121126200110.1055/s-2001-1493811355743
|
|
41.
|
HJ ListR ReiterB SinghA WellsteinAT
RiegelExpression of the nuclear coactivator AIB1 in normal and
malignant breast tissueBreast Cancer Res
Treat682128200110.1023/A:101791092439011678305
|
|
42.
|
C ZhaoK YasuiCJ LeeH KuriokaY HosokawaT
OkaJ InazawaElevated expression levels of NCOA3, TOP1, and TFAP2C
in breast tumors as predictors of poor
prognosisCancer981823200310.1002/cncr.1148212833450
|
|
43.
|
L QinL LiaoA RedmondL YoungY YuanH ChenBW
O'MalleyJ XuThe AIB1 oncogene promotes breast cancer metastasis by
activation of PEA3-mediated matrix metallopteinase 2 (MMP2) and
MMP9 expressionMol Cell
Biol2859375950200810.1128/MCB.00579-0818644862
|
|
44.
|
T KirkegaardLM McGlynnFM CampbellS
MüllerSM ToveyB DunneKV NielsenTG CookeJM BartlettAmplified in
breast cancer 1 in human epidermal growth factor receptor-positive
tumors of tamoxifen-treated breast cancer patientsClin Cancer
Res1314051411200710.1158/1078-0432.CCR-06-1933
|
|
45.
|
T BourasMC SoutheyDJ VenterOverexpression
of the steroid receptor coactivator AIB1 in breast cancer
correlates with the absence of estrogen and progesterone receptors
and positivity for p53 and HER2/neuCancer
Res61903907200111221879
|
|
46.
|
K LeeA LeeBJ SongCS KangExpression of AIB1
protein as a prognostic factor in breast cancerWorld J Sur
Oncol9139201110.1186/1477-7819-9-13922035181
|
|
47.
|
VJ GnanapragasamHY LeungAS PulimoodDE
NealCN RobsonExpression of RAC 3, a steroid hormone receptor
co-activator in prostate cancerBr J
Cancer8519281936200110.1054/bjoc.2001.217911747336
|
|
48.
|
T BarkhemS NilssonJA GustafssonMolecular
mechanisms, physiological consequences and pharmacological
implications of estrogen receptor actionAm J
Pharmacogenomics41928200410.2165/00129785-200404010-00003
|
|
49.
|
HJ ListKJ LauritsenR ReiterC PowersA
WellsteinAT RiegelRibozyme targeting demonstrates that the nuclear
receptor coactivator AIB1 is a rate-limiting factor for
estrogen-dependent growth of human MCF-7 breast cancer cellsJ Biol
Chem2762376323768200110.1074/jbc.M102397200
|
|
50.
|
S KarmakarEA FosterCL SmithUnique roles of
p160 coactivators for regulation of breast cancer cell
proliferation and estrogen receptor-alpha transcriptional
activityEndocrinology15015881596200910.1210/en.2008-1001
|
|
51.
|
S KarmakarT GaoMC PaceS OesterreichCL
SmithCooperative activation of cyclin D1 and progesterone receptor
gene expression by the SRC-3 coactivator and SMRT corepressorMol
Endocrinol2411871202201010.1210/me.2009-048020392877
|
|
52.
|
MP FereshtehMT TilliSE KimJ XuBW O'MalleyA
WellsteinPA FurthAT RiegelThe nuclear receptor coactivator
amplified in breast cancer-1 is required for Neu (ErbB2/HER2)
activation, signaling, and mammary tumorigenesis in miceCancer
Res6836973706200810.1158/0008-5472.CAN-07-670218483252
|
|
53.
|
S AlknerPO BendahlD GrabauK LövgrenO StålL
RydénM FernöSwedish and South-East Swedish Breast Cancer GroupsAIB1
is a predictive factor for tamoxifen response in premenopausal
womenAnn Oncol21238244201010.1093/annonc/mdp29319628566
|
|
54.
|
Q SuS HuH GaoR MaQ YangZ PanT WangF LiRole
of AIB1 for tamoxifen resistance in estrogen receptor-positive
breast cancer
cellsOncology75159168200810.1159/00015926718827493
|
|
55.
|
S MassarwehCK OsborneCJ CreightonL QinA
TsimelzonS HuangH WeissM RimawiR SchiffTamoxifen resistance in
breast tumors is driven by growth factor receptor signaling with
repression of classic estrogen receptor genomic functionCancer
Res68826833200810.1158/0008-5472.CAN-07-270718245484
|
|
56.
|
S KarmakarEA FosterJK BlackmoreCL
SmithDistinctive functions of p160 steroid receptor coactivators in
proliferation of an estrogen-independent, tamoxifen-resistant
breast cancer cell lineEndocr Relat
Cancer18113127201110.1677/ERC-09-028521059860
|
|
57.
|
CK OsborneJ ShouS MassarwehR
SchiffCrosstalk between estrogen receptor and growth factor
receptor pathways as a cause for endocrine therapy resistance in
breast cancerClin Cancer Res11865s870s200515701879
|
|
58.
|
G ArpinoL WiechmannCK OsborneR
SchiffCrosstalk between the estrogen receptor and the HER tyrosine
kinase receptor family: molecular mechanism and clinical
implications for endocrine therapy resistanceEndocr
Rev29217233200810.1210/er.2006-0045
|
|
59.
|
R ReiterAS OhA WellsteinAT RiegelImpact of
the nuclear receptor coactivator AIB1 isoform AIB1-Delta3 on
estrogenic ligands with different intrinsic
activityOncogene23403409200410.1038/sj.onc.120720214691461
|
|
60.
|
RB LanzY BulynkoA MalovannayaP LabhartL
WangW LiJ QinM HarperBW O'MalleyGlobal characterization of
transcriptional impact of the SRC-3 coregulatorMol
Endocrinol24859872201010.1210/me.2009-049920181721
|
|
61.
|
MI Torres-ArzayusJ ZhaoR BronsonM
BrownEstrogen-dependent and estrogen-independent mechanisms
contribute to AIB1-mediated tumor formationCancer
Res7041024111201010.1158/0008-5472.CAN-09-408020442283
|
|
62.
|
CM SilvaMA ShupnikIntegration of steroid
and growth factor pathways in breast cancer: focus on signal
transducers and activators of transcription and their potential
role in resistanceMol
Endocrinol2114991512200710.1210/me.2007-010917456797
|
|
63.
|
A OhHJ ListR ReiterA ManiY ZhangE GehanA
WellsteinAT RiegelThe nuclear receptor coactivator AIB1 mediates
insulin-like growth factor I-induced phenotypic changes in human
breast cancer cellsCancer
Res6482998308200410.1158/0008-5472.CAN-04-035415548698
|
|
64.
|
J YanCT YuM OzenM IttmannSY TsaiMJ
TsaiSteroid receptor coactivator-3 and activator protein-1
coordinately regulate the transcription of components of the
insulin-like growth factor/AKT signaling pathwayCancer
Res661103911046200610.1158/0008-5472.CAN-06-244217108143
|
|
65.
|
L LiaoX ChenS WangAF ParlowJ XuSteroid
receptor coactivator 3 maintains circulating insulin-like growth
factor (IGF-I) by controlling IGF-binding protein 3 expressionMol
Cell Biol2824602469200810.1128/MCB.01163-0718212051
|
|
66.
|
MI Torres-ArzayusJ YuanJL DellaGattaH
LaneAL KungM BrownTargeting the AIB1 oncogene through mammalian
target of rapamycin inhibition in the mammary glandCancer
Res661138111388200610.1158/0008-5472.CAN-06-231617145884
|
|
67.
|
KM HardyBW BoothMJ HendrixDS SalomonL
StrizziErbB/EGF signaling and EMT in mammary development and breast
cancerJ Mammary Gland Biol
Neoplasia15191199201010.1007/s10911-010-9172-220369376
|
|
68.
|
T LahusenM FereshtehA OhA WellsteinAT
RiegelEpidermal growth factor receptor tyrosine phosphorylation and
signaling controlled by a nuclear receptor coactivator, amplified
in breast cancer 1Cancer
Res6772567265200710.1158/0008-5472.CAN-07-1013
|
|
69.
|
W LongP YiL AmazitHL LaMarcaF AshcroftR
KumarMA ManciniSY TsaiMJ TsaiBW O'MalleySRC-3Delta4 mediates the
interaction of EGFR with FAK to promote cell migrationMol
Cell37321332201010.1016/j.molcel.2010.01.00420159552
|
|
70.
|
GP ColoRR RosatoS GrantMA CostasRAC3
down-regulation sensitizes human chronic myeloid leukemia cells to
TRAIL-induced apoptosisFEBS
Lett58150755081200710.1016/j.febslet.2007.09.05217927986
|
|
71.
|
GP ColoMF RubioIM NojekSE WerbajhPC
EcheverríaCV AlvaradoVE NahmodMD GalignianaMA CostasThe p160
nuclear receptor co-activator RAC3 exerts an anti-apoptotic role
through a cytoplasmatic
actionOncogene2724302444200810.1038/sj.onc.121090017968310
|
|
72.
|
J FerragudA Avivar-ValderasA PlaJ De Las
RivasJF de MoraTranscriptional repression of the tumor suppressor
DRO1 by AIB1FEBS
Lett58530413046201110.1016/j.febslet.2011.08.02521871888
|
|
73.
|
K AokiYJ SunS AokiK WadaE WadaCloning,
expression, and mapping of a gene that is upregulated in adipose
tissue of mice deficient in bombesin receptor subtype-3Biochem
Biophys Res Commun29012821288200210.1006/bbrc.2002.633711812002
|
|
74.
|
GT BommerC JägerEM DürrS BaehsST
EichhorstT BrabletzG HuT FröhlichG ArnoldDC KressDRO1, a gene
down-regulated by oncogenes, mediates growth inhibition in colon
and pancreatic cancer cellsJ Biol
Chem28079627975200510.1074/jbc.M41259320015563452
|
|
75.
|
F TremblayT RevettC HuardY ZhangJF TobinRV
MartinezRE GimenoBidirectional modulation of adipogenesis by the
secreted protein Ccdc80/DRO1/URBJ Biol
Chem28481368147200910.1074/jbc.M80953520019141617
|
|
76.
|
D CorkeryG ThillainadesanN CoughlanRD
MohanM IsovicM TiniJ TorchiaRegulation of the BRCA1 gene by an
SRC3/53BP1 complexBMC
Biochem1250201110.1186/1471-2091-12-5021914189
|
|
77.
|
CK OsborneR SchiffMechanisms of endocrine
resistance in breast cancerAnnu Rev
Med62233247201110.1146/annurev-med-070909-18291720887199
|