Introduction
Melanoma is a malignant tumor originating from
melancytes that constitute the human body’s melancytic system. As
one of the most lethal types of cancer in humans, malignant
melanoma accounts for 75% of all mortalities associated with skin
cancer. Although malignant melanoma in the early stage may be cured
by surgical resection, the disease is notoriously difficult to
treat and does not respond to current therapies once it has
progressed to the metastatic stage. For instance, therapies
including immune therapy with systemic high-dose interleukin (IL)-2
or interferon (INF)-α, antigen-specific immunization or
chemotherapy with dacarbazine or temozolomide have been shown to
induce objective tumor responses in only 5–20% of patients. It has
also been reported that patients with pulmonary metastases have an
overall 5-year survival rate of 4% and a median survival of only
6–7 months with palliative treatment. Therefore, a more effective
protocol for the prevention and therapy of malignant melanoma is
urgently needed (1–4).
As an effective, safe, minimally invasive or
noninvasive approach, hyperthermia is capable of directly killing
tumor cells and acts as a sensitizer for radiation therapy or
chemotherapy, as demonstrated in numerous clinical studies
(5,6). As a superficial tumor, melanoma can
easily be administered local hyperthermia from the clinical point
of view. Either as a monotherapy or as an adjuvant therapy with
other treatments, such as chemotherapy or radiotherapy, the results
of hyperthermia treatment from clinical trials and in vivo
studies are encouraging, suggesting a promising protocol for
malignant melanoma (7–9).
As a dominant parameter of hyperthermia, temperature
acts as a sensitive regulator during the treatment. Results from
previous studies have shown that malignant melanoma may be even
more sensitive to temperature (8,9).
Studies of hyperthermia have been focused on two commonly applied
strategies: conventional hyperthermia at mild temperatures
(42–45°C) (6,8,9) and
ablation therapy at high temperatures (>70°C) (10). Stojkovic and Radacic reported that
local hyperthermia of 43.5°C administered alone had good antitumor
activity, as in all experiments tumor growth time was prolonged by
2.3–3.5 times compared with the control (8). Results from the study of Ito et
al suggested that a temperature of 43°C was insufficiently high
to destroy the malignant melanoma and a higher treatment
temperature was proposed (9).
However, the induced antitumor immunity of thermal ablation was
found to be weak and possibly not sufficient to eradicate
established tumors alone (10). A
study by Zhou et al suggested that the thermal ablation of
hepatoma at 50±5°C may promote maturing and homing of immature
dendritic cells (DCs) and stimulate immunity of lymphocytes, but
not at temperatures >60°C. The immunogenicity of tumor cells was
lost when their cellular structures were completely destroyed, and
the local tissue blood flow and local lymph circulation path were
damaged as the heat temperature was too high, which prevented the
homing of the DCs (11). All the
above-mentioned studies highlight the fact that the choice of
treatment protocol is critical in the evaluation of the therapeutic
effect of hyperthermia, and as a result, temperatures between 46
and 55°C were used in this study.
In our study, the effect of local hyperthermia on
the antitumor effect and immune response in B16 melanoma at various
thermal doses was systematically analyzed. Local hyperthermia can
result in higher temperatures compared with whole body
hyperthermia, which heats to milder temperatures of around 41–43°C.
In order to eliminate the effect of energy from hyperthermia, a
specially designed biological hyperthermia platform using hot
humidified vapor was adopted in the current study. The tumor area
was heated at various temperatures by the hot vapor from the
nozzle. A series of nozzles with different diameters were prepared
to fit the actual tumor size of the mice. Evaluation of the tumor
volume and survival period as well as histological analysis was
employed to examine the antitumor effect of local hyperthermia at
different thermal doses. In addition, tumor necrosis factor
(TNF)-α, IL-2 and IFN-γ mRNA expression in the spleen of mice were
evaluated by relative quantitative RT-PCR for a detailed
understanding of the immune response induced by hyperthermia. The
preliminary detection of cytokines IL-2, IFN-γ and TNF-α in
hyperthermia was performed using ELISA and focused mainly on the
cytokine protein in serum and tumor tissues. In this study, the
changes in IFN-γ, IL-2 and TNF-α mRNA levels in the spleen were
investigated from a genetic perspective by relative quantitative
PCR assay. The rechallenge of the surviving mice with B16 tumor
cells confirmed that the mice had developed long-term
tumor-specific immunity against malignant melanoma following
hyperthermia at the optimal thermal dose. Compound 48/80 (C48/80)
is a stimulus of mast cell degranulation (12,13).
It has been reported that the mast cell activator C48/80 was an
effective and safe adjuvant for the induction of anthrax lethal
toxin neutralizing antibody responses when delivered intranasally,
intradermally or via the footpad to mice with anthrax protective
antigen (13,14). However, few studies were found
concerning the use of C48/80 in tumor treatment; therefore, in the
present study, local hyperthermia combined with C48/80 was applied
for melanoma lung metastasis treatment. This study aimed to
establish a local hyperthermia protocol for B16 melanoma. The
results of the experiments may have clinical significance for
melanoma treatment.
Materials and methods
Cell line and cell culture
The murine B16-F10 melanoma cell line was obtained
from Cell Center, Institute of Basic Medical Sciences, Chinese
Academy of Medical Sciences and Peking Union Medical College,
China. The cells were cultured in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 10% heat-inactivated fetal bovine
serum, 100 U/ml penicillin and 100 μg/ml streptomycin. The
cell cultures were maintained at 37°C in a 5% CO2
humidified atmosphere at pH 7.4. When the cells reached 90%
confluence, a cell suspension was obtained by trypsinization.
Animal and tumor models
Male C57BL/6 mice (aged 8 weeks) were purchased from
the Hsing-Long laboratory animal breeding center (Beijing, China).
Throughout the experiment, the animals were housed four or five per
cage in standard cages. The animals were kept in these facilities
for at least 3 days prior to the experiments. All protocols were
approved by the Institutional Animal Care and Use Committee (IACUC)
of Tsinghua University, Beijing, China.
To create a murine model for melanoma skin cancer,
B16 tumor cells (1×106 in 100 μl PBS) were
injected into the flank of mice which were anesthetized by
intraperitoneal injection of sodium pentobarbital (50 mg/kg body
weight). Melanoma nodules that had grown to 5–6 mm in diameter were
used for the experiment.
The murine model for melanmoma pulmonary metastasis
was developed by administration of B16 cell suspension
(1×106 cells) through the tail vein of the mice.
Histological evalution was performed 14 days after the
administration of cell suspension.
Animal groups and local hyperthermia for
melanoma skin tumor model
A total of 150 mice with melanoma nodules were
randomly divided into five groups: group I formed the control
group, group II received 46°C hyperthermia for 15 min, group III
received 48°C hyperthermia for 15 min, group IV received 50°C
hyperthermia for 15 min and group V received 55°C hyperthermia for
10 min. As we observed in our preliminary experiment, certain mice
died of dehydration under 55°C hyperthermia for 15 min, therefore a
10-min treatment was adopted for group V. Mice were anesthetized
with 1% pentobarbital and subjected to hyperthermia using the
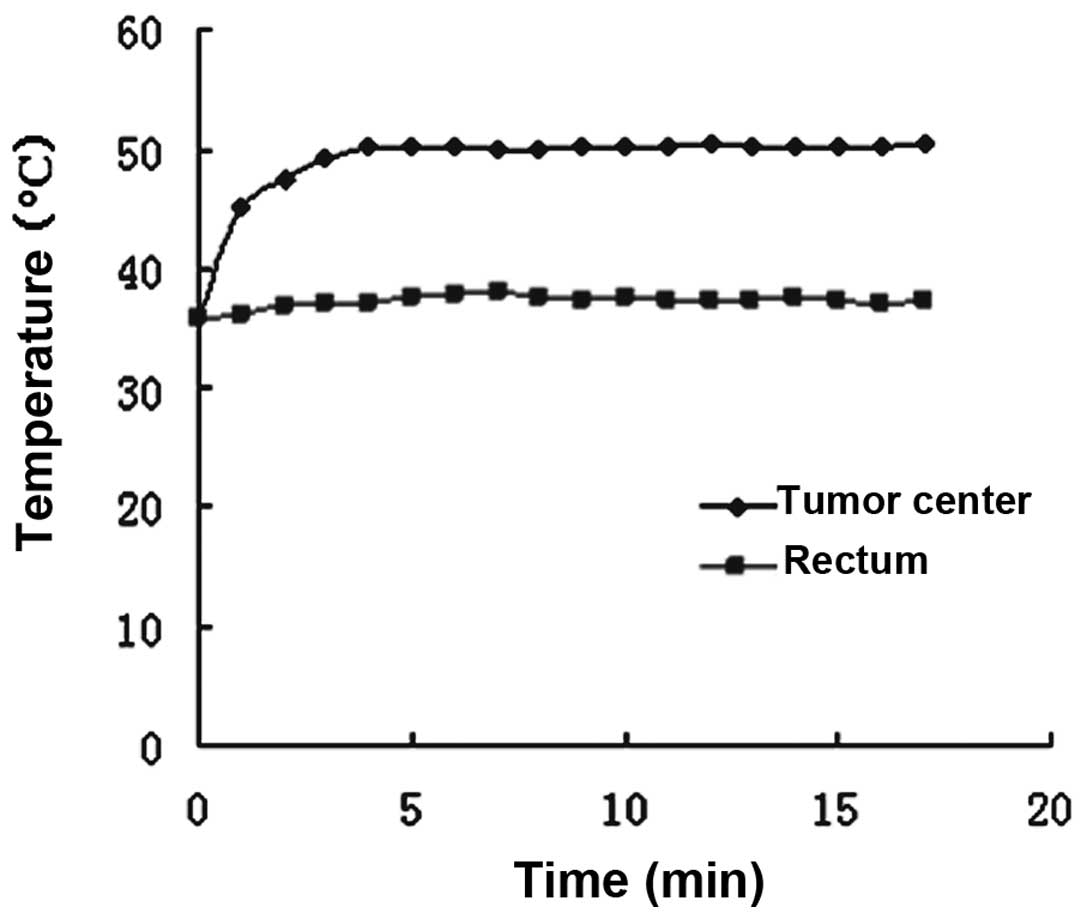
heating and humidification biological platform (Fig. 1). The temperature was measured at
the tumor center and in the rectum by a 0.1-mm thermocouple
temperature probe (Model IT-18, copper-constantan, Physitemp, New
Jersey, USA) inserted into the tumor tissue and rectum,
respectively. The probe fibers were connected to a four-channel
millivoltmeter (model XSOL-4, Beijing Kunlun Tianchen Instrument
Technology, Co., Ltd, Beijing, China) and the data were collected
every 6 sec using a PC with home-written software.
After inducing hyperthermia, the long and short axes
of the tumors were measured with digital calipers at 1–2-day
intervals, and tumor volumes were calculated using the formula:
V = 1/2 × A2 × B, where A is
the length of the short axis and B is the length of the long
axis. Surviving mice were counted until all group II mice had died.
Survival rates were recorded as percentage survivals.
Histopathological analysis
At 16 h and at 7 days after the hyperthermia
treatment was completed, a necropsy was performed after the animals
were sacrificed and organs were placed immediately into 10%
neutral-buffered formalin (NBF). Tissues were processed and
embedded in paraffin and sectioned at 6 μm. Sections were
stained with hematoxylin and eosin (H&E) from routine
histological evaluation. Six randomly selected animals per group
were used in the experiments. Tumor tissues were analyzed in 5
randomly selected fields per section.
Detection of cytokine mRNA in the spleen
using RT-PCR
Spleens were collected from randomly selected mice
in each group at 7 and 14 days after the hyperthermia treatment was
completed. RNA was prepared by homogenization of the tumor with
RNAzol followed by RNA extraction according to the manufacturer’s
instructions. RNA concentrations were measured, and 2 μg
total cellular RNA was reverse transcribed in a 200-μl
volume using oligo(dT)15 as a primer and Moloney murine
leukemia virus reverse transcriptase. cDNA (2 μl) was
amplified by PCR using primers specific to individual murine
cytokines. The reaction mixtures used in the reverse transcription
of cDNA and PCR are shown in Tables
I and II.
 | Table I.Reaction mixture used for reverse
transcription of cDNA. |
Table I.
Reaction mixture used for reverse
transcription of cDNA.
| Amount |
|---|
| Positive control
RNA | 2 μg |
|
Oligo(dT)15 | 2 μl |
| dNTP mixture (10
mM) | 5 μl |
| RNase
inhibitor | 1 μl |
| M-MLV reverse
transcriptase (200 U/μl) | 1 μl |
| 5X M-MLV RT
buffer | 5 μl |
| RNase free
dH2O | Added to make 25
μl |
 | Table II.Reaction mixture used for PCR. |
Table II.
Reaction mixture used for PCR.
| Amount |
|---|
| cDNA templates | 2 μl |
| 2X SYBR-Green PCR
Master mix | 12.5 μl |
| Upstream primers
(0.9 nmol/μl) | 1 μl |
| Downstream primers
(0.9 nmol/μl) | 1 μl |
| Double-distilled
water | 8.5 μl |
| Total | 25 μl |
Rechallenge
Mice with complete tumor regression which were cured
by local hyperthermia were rechallenged with B16 melanoma cells
(approximately 1×106 cells) by subcutaneous inoculation
on the right side abdomen 60 days after hyperthermia treatment. The
tumor growth and survival time of the mice were observed.
Combined therapy for lung metastasis
treatment
Mice were randomly divided into three groups. Group
I formed the control group, which received no treatment. In group
II (PBS + hyperthermia group), local subcutaneous tumors were
heated to 50°C and maintained for 15 min, then PBS was injected
into the tumors locally following hyperthermia treatment. In group
III (C48/80 + hyperthermia group), local subcutaneous tumors were
heated to 50°C and maintained for 15 min, then C48/80 was injected
into the tumors locally following hyperthermia. The heating method
was the same as that used in the thermal dose filtering
experiment.
Statistical analysis
All experiments were repeated at least twice. SPSS
10.0 statistical software was applied for analysis. Variances were
homogeneous in each group and a q-test was used to analyze the
variance between the means in groups. Differences between the means
were considered statistically significant at P<0.05, and were
considered highly significant at P<0.01. MxPro QPCR 3.20
software was used for analysis of RT-PCR data to obtain Ct
values.
Results
Temperature monitoring in local
hyperthermia
Target temperatures at the center of the tumors were
attained within 2–4 min and maintained by controling the thermal
current intensity. The temperature in the rectum increased only
slightly, and was maintained within the normothermic range
(approximately 37°C) during heat treatment. All experimental
animals tolerated the hypothermic treatment well (Fig. 2).
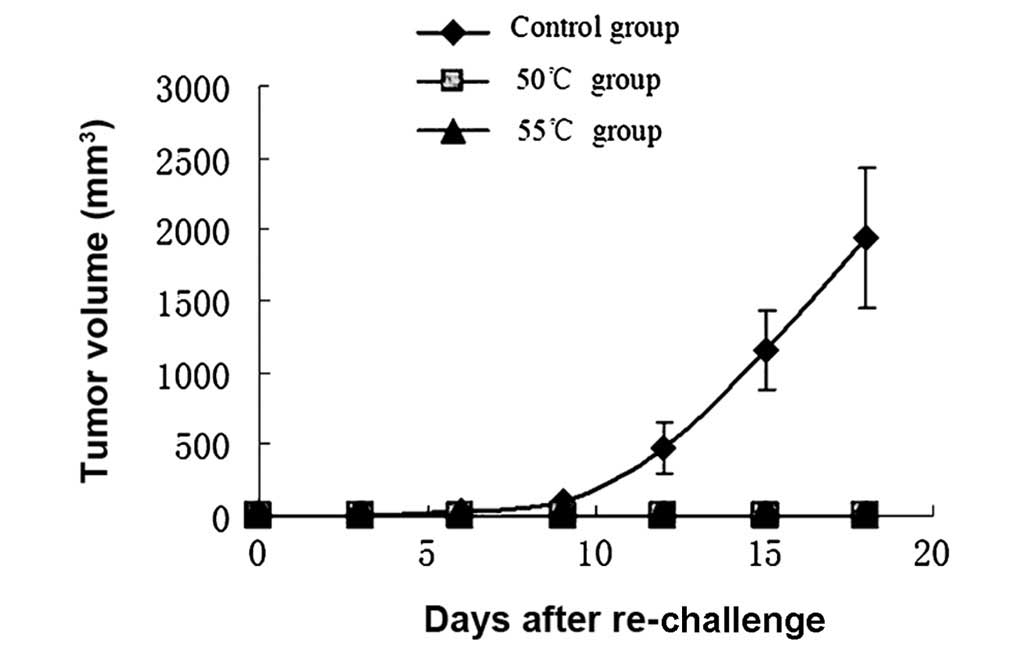
Tumor growth following hyperthermia
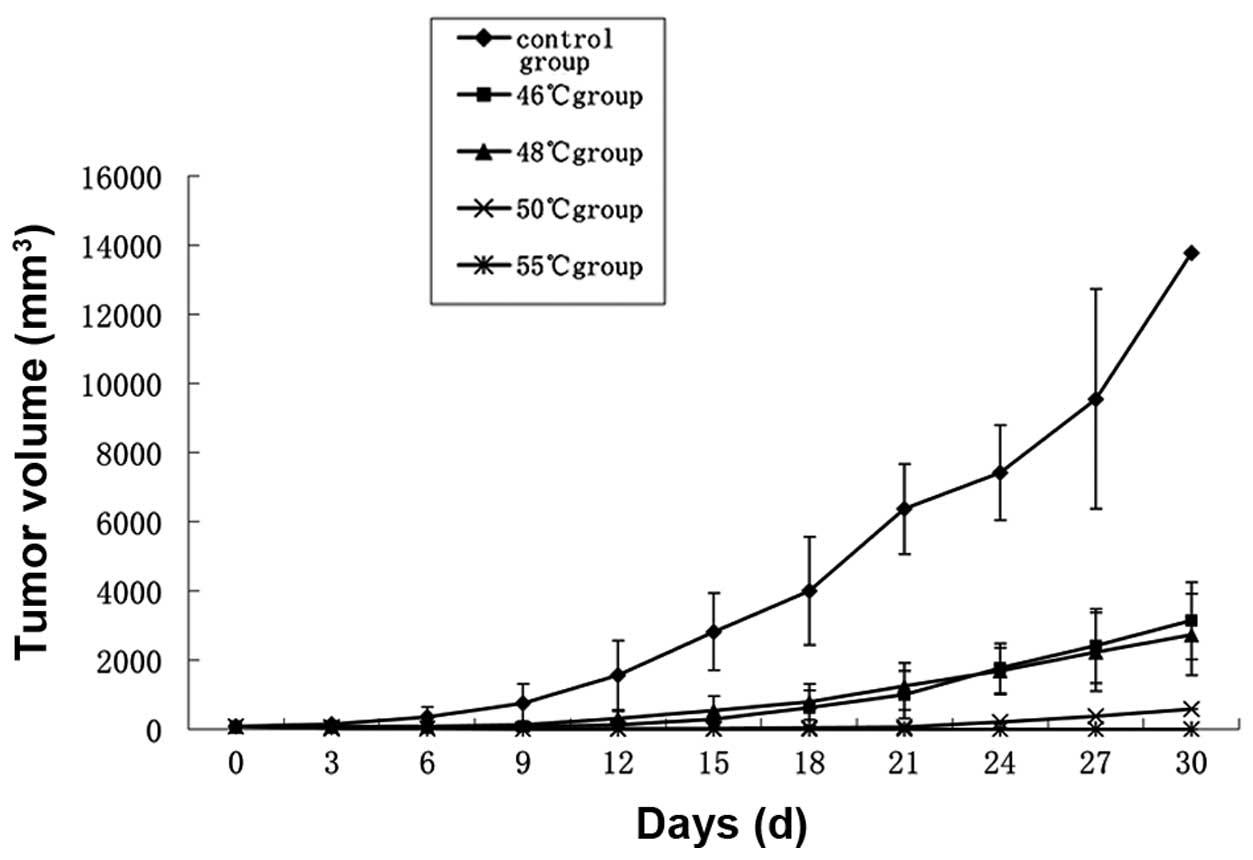
Tumor volumes in the 46 and 48°C groups were
significantly reduced compared with those in the control group
(P<0.05). Inhibition of tumor growth by hyperthermia at 50 and
55°C was particularly effective (P<0.01; Fig. 3). One of the mice in the 50°C group
exhibited tumor growth 15 days after treatment, the remainder of
the group and all mice in the 55°C group remained tumor-free. Two
mice in the 55°C group suffered burns to the skin in the process of
heating.
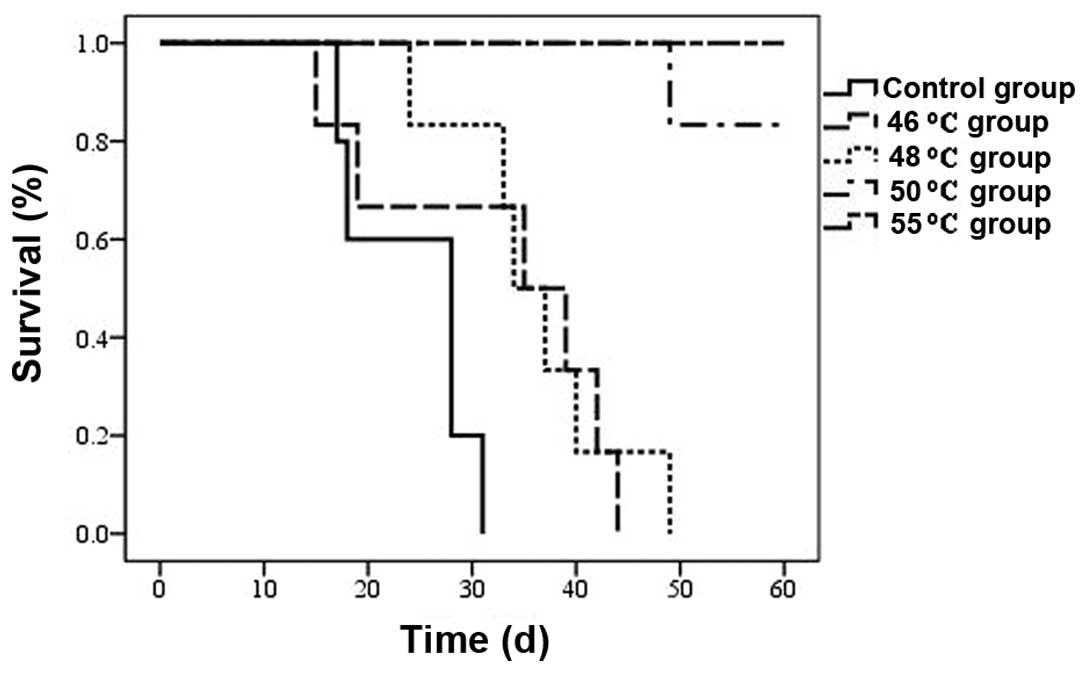
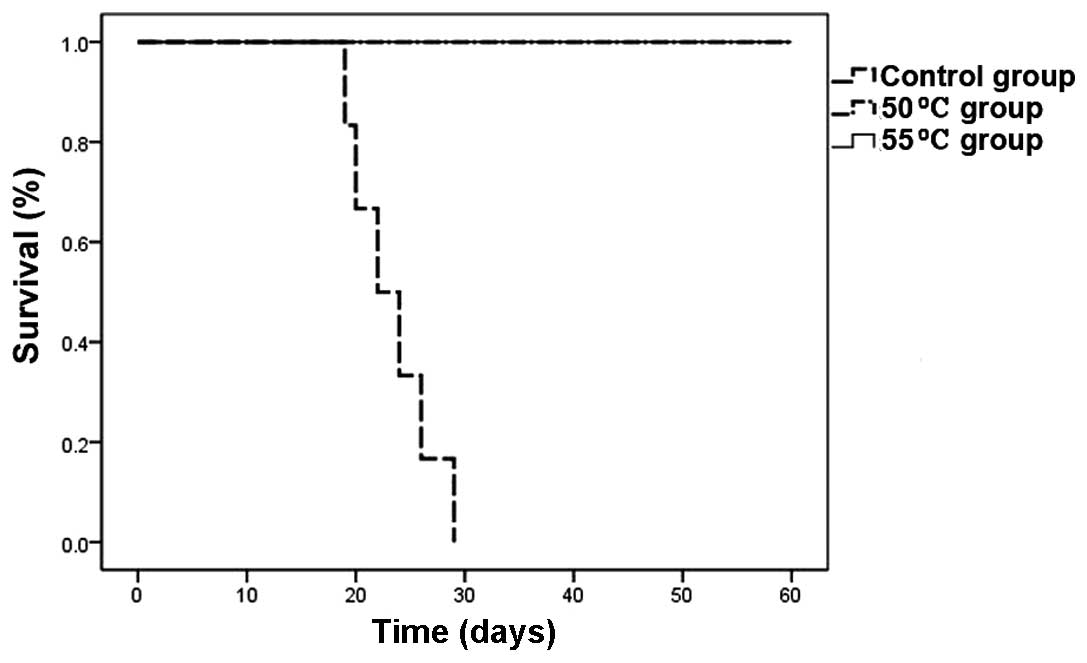
Survival of tumor-bearing mice
As shown in Fig. 4,
the survival of tumor-bearing mice was prolonged by hyperthermic
treatment in all four treatment groups (P<0.05), and this was
most evident in the 48, 50 and 55°C groups (P<0.01). One of the
mice in the 50°C group died on the 48th day due to tumor growth.
The remaining five mice in the 50°C group and all mice in the 55°C
group survived for a period of 60 days following hyperthermia
treatment.
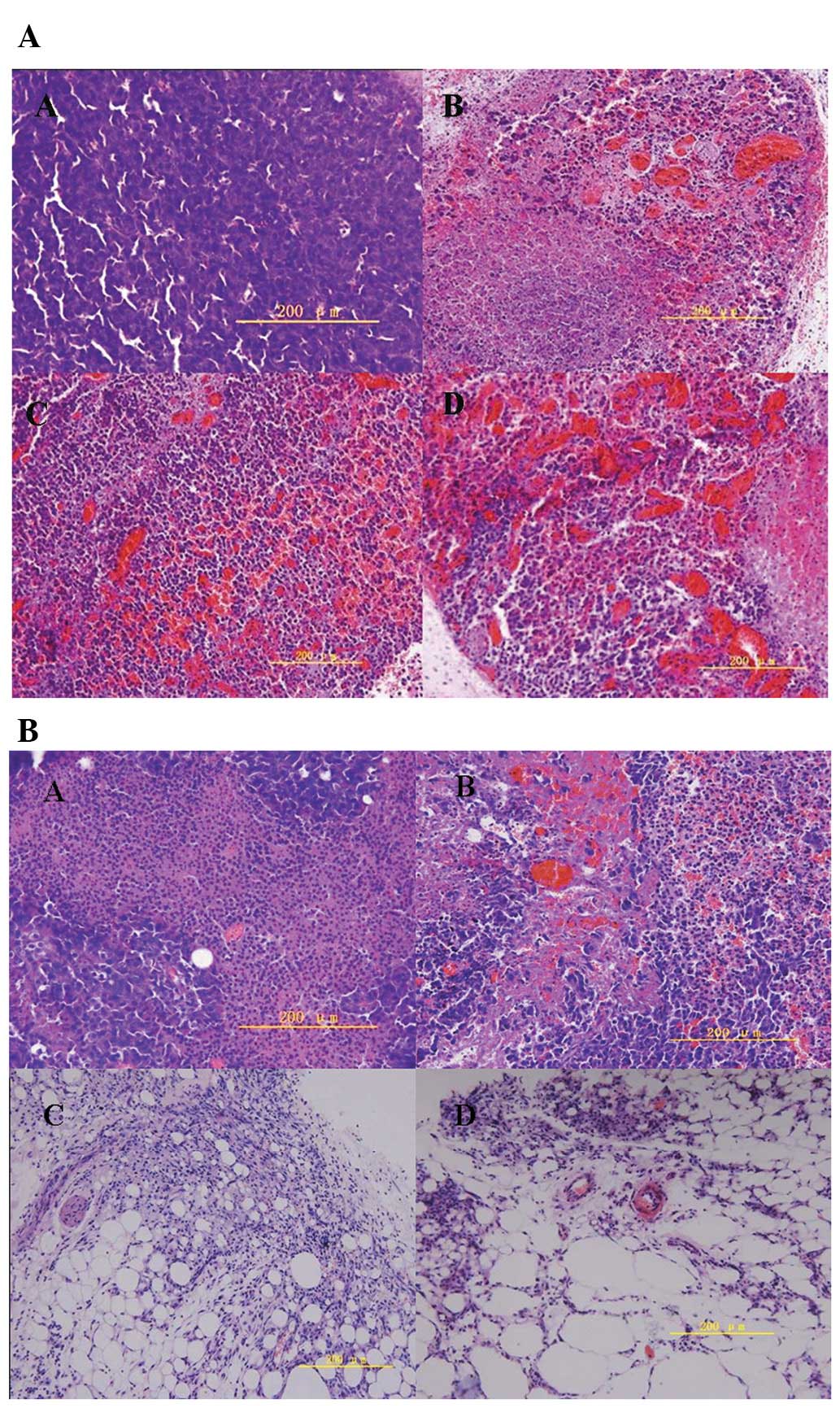
Histopathological observation of
tumors
As shown in Fig. 5A,
large numbers of actively proliferating tumor cells were observed
in the center of the tumors in the untreated control group (top
left) 16 h after the start of the experiments. There was obvious
chromatorrhexis and dissolution of tumor cells in the hyperthermia
groups, as well as blood vessel destruction within tumors. Tumors
showed increased signs of necrosis, congestion and hemorrhage as
the temperature increased. Seven days after hyperthermia treatment,
there was an obvious decrease in the quantity of tumor cells and
increased tumor tissue hemorrhage in the treated groups as the
temperature increased, compared with those in the control group
(Fig. 5B). Tumors in the 50 and
55°C groups disappeared completely 14 days after hyperthermia
treatment, and white knots were formed hypodermically, which on
macroscopic pathological observation were found to contain many fat
vacuoles.
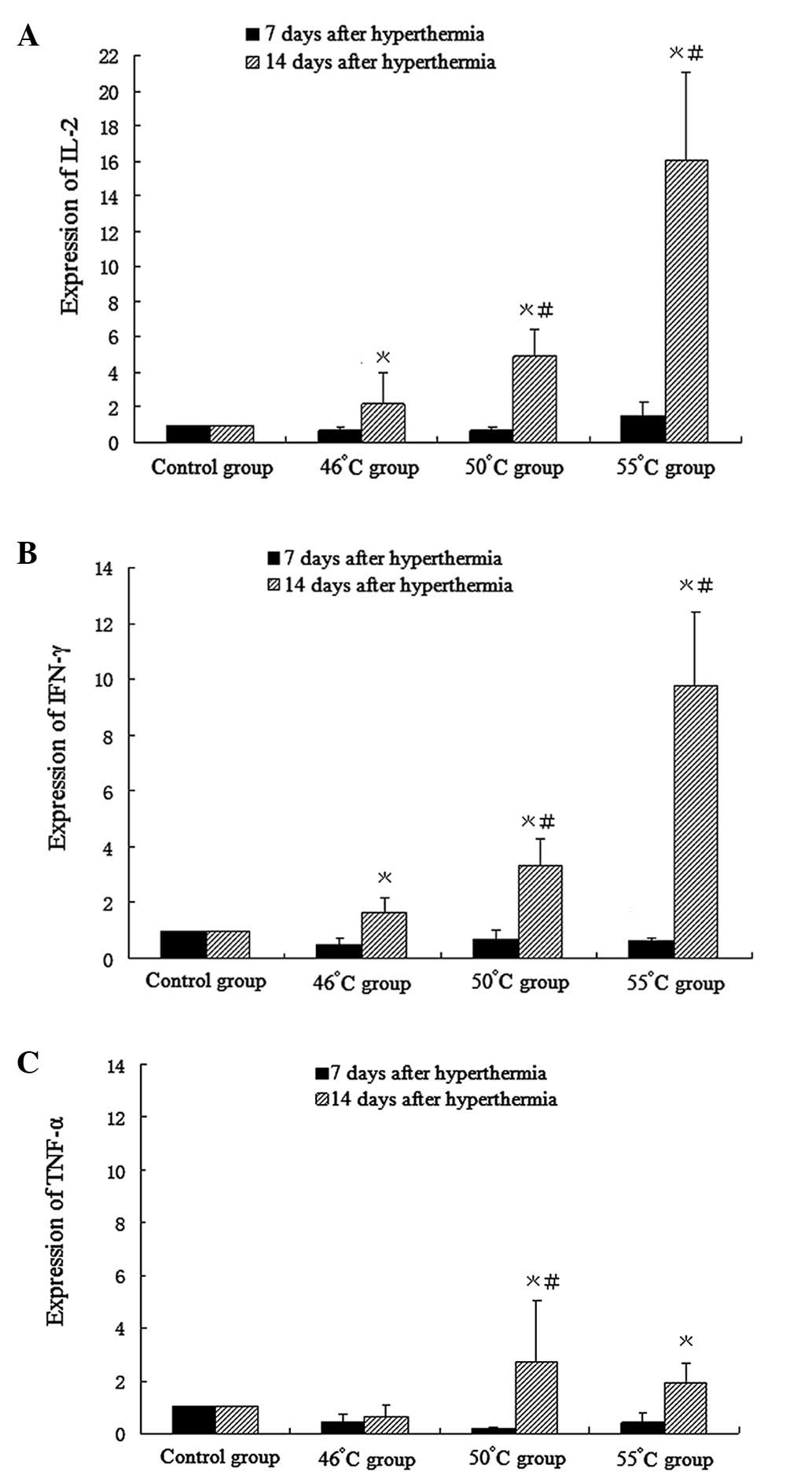
Expression of IL-2, IFN-γ and TNF-α mRNA
in mouse spleens
Compared with the control group, except for a slight
increase in IL-2 mRNA expression in the 55°C group, there was a
slight decrease in the expression levels of IL-2, IFN-γ and TNF-α
mRNA in all groups 7 days after hyperthermic treatment. After 14
days, compared with the control group, IL-2 and IFN-γ mRNA
expression increased significantly in all hyperthermia groups
(1.64-fold in the 46°C group, 3.32-fold in the 50°C group and
9.77-fold in the 55°C group). TNF-α mRNA expression increased in
the 50°C group (2.79-fold) and 55°C group (1.92-fold), and
decreased in the 46°C group (0.65-fold), compared with that in the
control group (Fig. 6).
Rechallenge in tumor-free mice
Tumor nodules appeared gradually in control mice
implanted with melanoma cells, and evolved into rapid growth 6 days
after implantation. Five tumor-free mice from the 50°C group and
six from the 55°C group were implanted with melanoma cells. Mice in
both hyperthermic groups survived and remained tumor-free for 60
days after implantation, indicating that they were protected
completely from rechallenge with B16 cells. The tumor-free mice
demonstrated an antitumor memory response to B16 cells (Figs. 7 and 8).
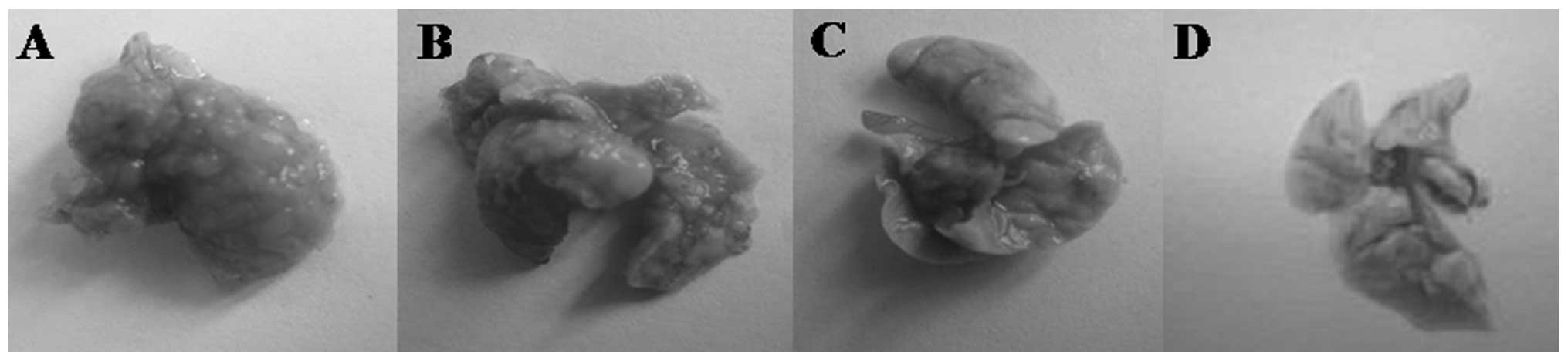
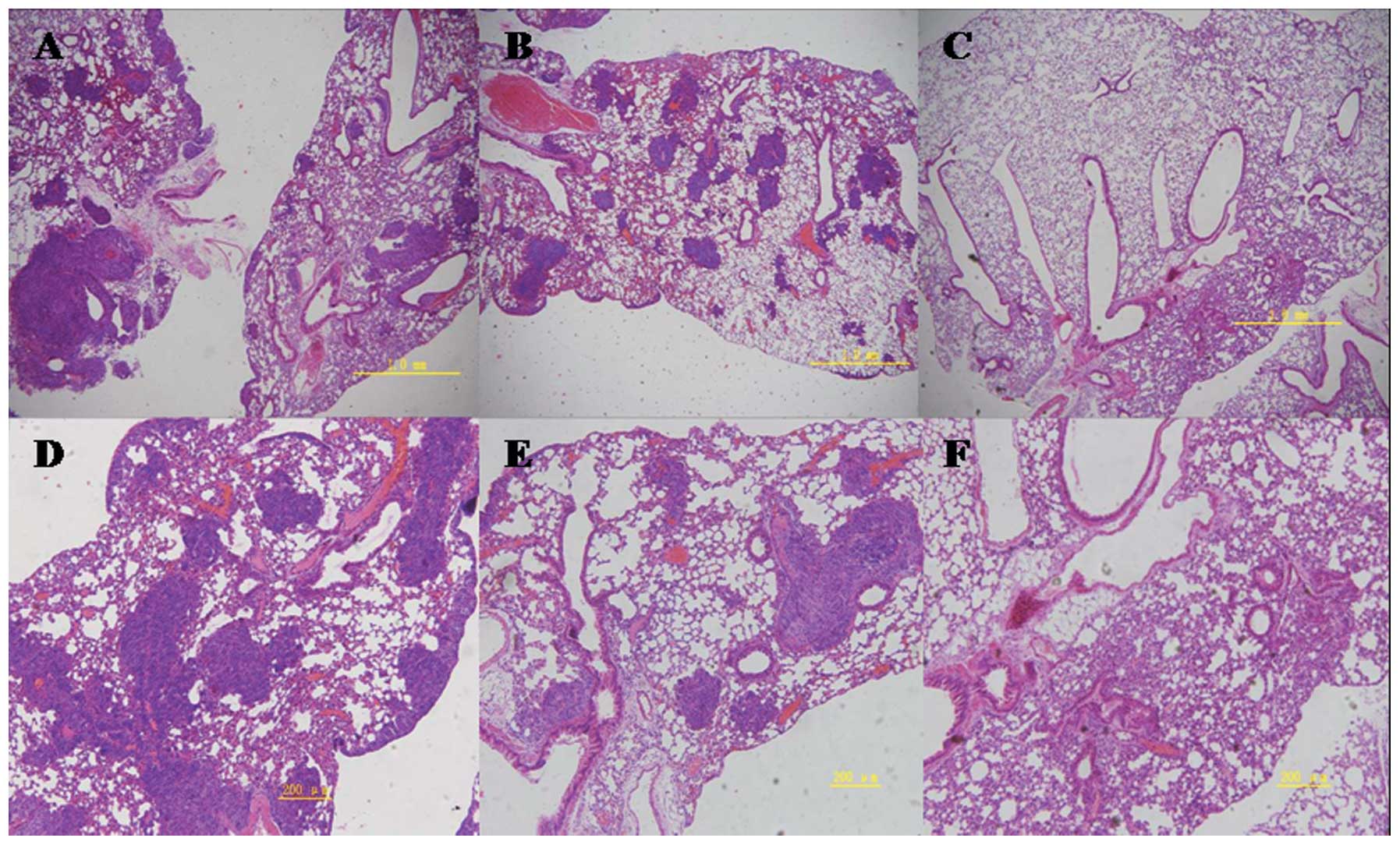
Pulmonary metastasis assay
On day 14, H&E staining indicated areas of tumor
in the lungs. Macroscopic observations were further confirmed by
microscopic examination of lung samples, which revealed an obvious
reduction of tumor cells in the lungs of group II mice administered
hyperthermia alone. In group III mice (receiving C48/80 +
hyperthermia), lungs were similar to normal lungs on visual
inspection, with dotted or absent tumor cells noted on pathological
observation. Traces of inflammation were observed following tumor
regression in the lungs of hyperthermically treated mice. In the
control group, tumor cells were observed in a large area in the
lungs of mice, with strong acidophilic staining, large nuclei, and
with good reproductive potential (Figs.
9 and 10).
Discussion
In the present study, local high-temperature
hyperthermia at 46 and 48°C for 15 min inhibited the growth, but
did not induce regression, of subcutaneous B16 melanoma cells.
Hyperthermia at 50°C for 15 min and 55°C for 10 min induced
complete tumor regression. However, in the 55°C group certain mice
suffered skin burns and several suffered skin wound infection as a
consequence. Mice treated with hyperthermia in the 50 and 55°C
groups remained tumor-free and in healthy condition after being
rechallenged with melanoma cells in situ. This suggests that
local high-temperature hyperthermia at 50 and 55°C effectively
stimulates an immune response in mice and creates an antitumor
immune memory, as shown by the cytokine data. Overall, our data
suggest that 50 and 55°C local hyperthermia is effective in the
treatment of melanoma and may have potential clinical value.
The use of hyperthermia to treat cancer in the
clinical practice is based on its direct cytotoxic effect and the
resulting enhanced sensitivity to radiotherapy and chemotherapy,
and heat-induced antitumor immune responses (16). In our study, pathological
observations 16 h after hyperthermia treatment at 46–55°C revealed
temperature-dependent tumor tissue changes, with varying degrees of
necrosis, congestion and hemorrhage. Our specially designed
biological hyperthermia platform was able to achieve a temperature
between 50 and 55°C, which was high enough to induce tumor cell
necrosis. The necrotic tumor cells, destroyed by hyperthermia,
released their intracellular contents including tumor antigenic
peptides. Antigenic peptides are taken up by immature DCs and
presented to T-cells via MHC class I and/or II antigens (17–19).
We speculate that 50 and 55°C hyperthermia resulted in sufficient
antigen exposure, due to tumor cell necrosis, to activate an immune
response, which successfully defended the rechallenge with B16
melanoma cells and destroyed the ectopically transplanted
tumors.
The antitumor immune effect is dominated by Thl
CD4+ T lymphocytes, which produce the cytokines IL-2,
IFN-γ and TNF-α. Humoral immunity is associated with Th2
CD4+ T lymphocytes, which produce the cytokines IL-4,
IL-6 and IL-10 (20). The shift in
Thl/Th2 balance towards Th2 has been shown to be associated with
tumorigenesis and recurrence in numerous malignancies including
melanoma (21). Shifting the
Th1/Th2 balance towards Th1 responses results in tumor rejection,
since Th1 pathways typically produce activation of cytotoxic T-cell
lymphocytes (CTL), natural killer (NK) cells and macrophages and
monocytes, all of which attack cancer cells and defend against
tumor growth (22). Conversely,
shifting the Th1/Th2 balance toward a Th2-mediated immunity
prevents tumor rejection (23). The
expression levels of IL-2, IFN-γ and TNF-α are indices of the Thl
immune response. Previously, it has been reported that hyperthermia
induces a Th1-dominant immune response. The protein concentration
of cytokines IL-2, IFN-γ and TNF-α was shown to increase in the
peripheral blood following whole body or local hyperthermia
(10,24–26).
The spleen is the largest immune organ in the body, and contains a
large number of lymphocytes. Cytokines secreted from cells in the
spleen activate and enhance phagocytosis, migration and cytotoxic
effects of polymorphonuclear leukocytes, monocytes and macrophages,
contributing to a significant antitumor effect. In this study,
IFN-γ, IL-2 and TNF-α mRNA expression increased significantly in
the spleens of mice 14 days after hyperthermia in the 50 and 55°C
groups. The findings in the spleen experiments were identical to
those in the antitumor effect experiments, in that hyperthermia
treatment at 50°C for 15 min and 55°C for 10 min was superior to
other temperatures used. The results may be explained by an
analysis of the mechanisms involved in the antitumor effect. We
hypothesize that enhanced Th1-type cytokine expression in the
spleen is associated with the enhancement of the antitumor effects
found in this study. As mentioned previously, the high expression
of cytokines in the spleen activates CTLs, NK cells, macrophages
and monocytes, and results in increased death of tumor cells in
situ. However, the expression of TNF-α mRNA in the spleen
decreased slightly in the 46°C group compared with those in the
control group, while TNF-α protein was found to be either increased
or unchanged in serum following early ablation (10) or mild hyperthermia (26). Further investigation is needed to
explain these divergent results. At day 7 following hyperthermia
treatment, there was no significant increase in IL-2, IFN-γ and
TNF-α mRNA levels in any of the treatment groups compared with
those in the control group. This is similar to and can be explained
by the study of Zhou et al, who reported that one to two
weeks were needed following ablation treatment (85–95°C for 3 min)
for necrotic tumor tissues to be absorbed, so that the
immunosuppression induced by the burning of mice skin during
heating decreased and the antitumor immune response would occur
(11).
Overall, hyperthermia at 50°C for 15 min and 55°C
for 10 min were the most effective treatment regimens used to
control tumor growth and stimulate an immune response. Although
55°C for 10 min was the most effective treatment regime, it
involved a high risk of skin burn injury and dehydration in mice.
Thus, 50°C for 15 min was considered the optimal treatment regime
for melanoma. Hyperthermia at 50°C for 15 min for the treatment of
lung metastases was not fully effective. However, the lungs of mice
treated with 50°C hyperthermia and C48/80 showed no tumor nodes on
macroscopic analysis, and no obvious tumor cells on microscopic
analysis. Local hyperthermia at the optimal thermal dose in
combination with immunoadjuvant C48/80 is an effective approach for
treating B16 melanoma lung metastasis. Local hyperthermia increased
the infiltration of leukocytes, including mast cells at the site of
tumors (27). The adjuvant activity
of C48/80 was associated with its ability to induce DC migration
via a mechanism that required mast cells and mast cell-derived TNF
(14). Furthermore, McGowen et
al reported that C48/80 produced much greater levels of IFN
than it did IL-4, IL-5, IL-6 or IL-17, and reduced the IgG1/IgG2a
ratio. Therefore they considered it possible that C48/80 acts
through the connective tissue mast cells to stimulate an
environment favorable for the development of Th1 immune responses
(15). Based on the above, synergy
may be created by the combined treatment of C48/80 and local
hyperthermia, which form an effective resistance to lung metastasis
in the therapy for lung metastasis treatment. Research on the
mechanisms of this combined treatment requires further
development.
In conclusion, we have shown that local
high-temperature hyperthermia at 50°C for 15 min and 55°C for 10
min is effective in treating melanoma in mice, with 55°C for 10 min
being particularly effective. Additionally, local hyperthermia at
50°C for 15 min in combination with the immune adjuvant compound
48/80 was effective in the treatment of B16 murine lung melanoma
metastases. The significant inhibition of tumor growth by
hyperthermia is likely due to a synergistic effect of direct
cytotoxicity by heating, and induction of an antitumor immune
response elicited by antigen release from necrotic tumor cells. The
increased secretion of Th1-type cytokines in the spleen following
hyperthermic treatment is also likely to participate in the
antitumor immune response. Additional research on the application
of high-temperature hyperthermia and C48/80 for in situ
melanoma and treatment of lung metastases is warranted in order to
translate the treatment to a clinical setting.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China under grant 30571779.
The authors alone are responsible for the content and writing of
this paper.
References
|
1.
|
JF ThompsonRA ScolyerRF KeffordCutaneous
melanomaLancet365687701200510.1016/S0140-6736(05)70937-515721476
|
|
2.
|
VK SondakRJ GonzalezR KudchadkarAdjuvant
therapy for melanoma: a surgical perspectiveSurg Oncol Clin N
Am20105114201110.1016/j.soc.2010.09.00121111961
|
|
3.
|
M HongAL PuauxC HuangL LoumagneC TowJ
AbastadoChemotherapy induces intratumoral expression of chemokines
in cutaneous melanoma, favoring T-cell infiltration and tumor
controlCancer
Res7169977009201110.1158/0008-5472.CAN-11-146621948969
|
|
4.
|
JM McLoughlinJS ZagerVK SondakLB
BerkTreatment options for limited or symptomatic metastatic
melanomaCancer Control15239247200818596676
|
|
5.
|
A ChichełJ SkowronekM KubaszewskaM
KanikowskiHyperthermia - description of a method and a review of
clinical applicationsRep Pract Oncol Radiother122672752007
|
|
6.
|
PI SoaresIM FerreiraRA IgrejaCM NovoJP
BorgesApplication of hyperthermia for cancer treatment: recent
patents reviewRecent Pat Anticancer Drug
Discov76473201210.2174/15748921279835803821854362
|
|
7.
|
M PaceR GattaiEM MascitelliL
MillantaResults of isolated lower limb perfusion for loco-regional
advanced/recurrent melanoma using borderline true hyperthermia plus
additional bolus of melphalan. A critical analysis of homogeneous
casesJ Surg Oncol104718723201110.1002/jso.21949
|
|
8.
|
R StojkovicM RadacicCell killing of
melanoma B16 in vivo by hyperthermia and cytotoxinsInt J
Hyperther186271200211820469
|
|
9.
|
A ItoM FujiokaT
Yoshida4-S-Cysteaminylphenol-loaded magnetite cationic liposomes
for combination therapy of hyperthermia with chemotherapy against
malignant melanomaCancer Sci98424430200717270032
|
|
10.
|
SP HaenPL PereiraHR SalihHG RammenseeC
GouttefangeasMore than just tumor destruction: immunomodulation by
thermal ablation of cancerClin Dev Immunol105574201122242035
|
|
11.
|
ZX ZhouXY YinMD LvHoming of dendritic
cells injected into the mouse hepatoma after microwave ablation
under different temperatureChinese J Pathophys223553592006
|
|
12.
|
WDM PatonCompound 48/80: a potent
histamine liberatorBrit J Pharmacol64995081951
|
|
13.
|
AM RothschildMechanisms of histamine
release by compound 48/80Brit J
Pharmacol38253262197010.1111/j.1476-5381.1970.tb10354.x4189829
|
|
14.
|
JB McLachlanCP ShelburneJP HartMast cell
activators: a new class of highly effective vaccine adjuvantsNat
Med14536541200810.1038/nm175718425129
|
|
15.
|
AL McGowenLP HaleCP ShelburneSN AbrahamHF
StaatsThe mast cell activator compound 48/80 is safe and effective
when used as an adjuvant for intradermal immunization with
Bacillus anthracis protective
antigenVaccine2735443552200910.1016/j.vaccine.2009.03.06919464533
|
|
16.
|
BE DayancSH BeachyJR OstbergEA
RepaskyDissecting the role of hyperthermia in natural killer cell
mediated anti-tumor responsesInt J
Hyperther244156200810.1080/0265673070185829718214768
|
|
17.
|
WC DeweyArrhenius relationships from the
molecule and cell to the clinicInt J
Hyperthermia105783199410.3109/026567394090093517963805
|
|
18.
|
E KayihanBiological rationale and clinical
experience with hyperthermiaClin
Trials17316342199610.1016/0197-2456(95)00078-X
|
|
19.
|
K TanakaA ItoT KobayashiHeat immunotherapy
using magnetic nanoparticles and dendritic cells for T-lymphomaJ
Biosci Bioeng100112115200510.1263/jbb.100.11216233860
|
|
20.
|
P KiddTh1/Th2 balance: the hypothesis, its
limitations, and implications for health and diseaseAltern Med
Rev82346200312946237
|
|
21.
|
L LauerovalL DusekM SimickovaMalignant
melanoma associates with Th1/Th2 imbalance that coincides with
disease progression and immunotherapy
responseNeoplasma49159166200212098001
|
|
22.
|
M TerabeJM ParkJA BerzofskyRole of IL-13
in regulation of anti-tumor immunity and tumor growthCancer Immunol
Immunother537985200410.1007/s00262-003-0445-014610620
|
|
23.
|
S RomagnaniThe Th1/Th2 paradigmImmunol
Today1836199710.1016/S0167-5699(97)80019-9
|
|
24.
|
K SheejaG KuttanEffect of Andrographis
paniculata as an adjuvant in combined chemo-radio and whole
body hyperthermia treatment - a preliminary studyImmunopharmacol
Immunotoxicol301811942008
|
|
25.
|
MH Den BrokRPM SutmullerR Van der VoortIn
situ tumor ablation creats an antigen source for the generation of
antitumor immunityCancer Res6440244029200415173017
|
|
26.
|
D JiaJ LiuW RaoInhibition of B16 murine
melanoma metastasis and enhancement of immunity by fever-range
whole body hyperthermiaInt J
Hyperther27275285201110.3109/02656736.2011.55961321501029
|
|
27.
|
M MoritaH KuwanoK ArakiA EgashiraH
KawaguchiH SaekiPrognostic significance of lymphocyte infiltration
following preoperative chemoradiotherapy and hyperthermia for
esophageal cancerInt J Radiat Oncol Biol
Phys4912591266200110.1016/S0360-3016(00)01465-6
|