Introduction
Persistent left superior vena cava (PLSVC) is the
most common thoracic venous anomaly (1). PLSVC is present in 0.3–0.5% of the
general population (2,3). It is usually asymptomatic and
hemodynamically irrelevant or associated with disturbances of
cardiac rhythm. Awareness of this condition may be useful when
placement of left-side transvenous subclavian or internal jugular
catheters is required, in such settings as critical care,
nefrology, onco-hematology and anaesthesiology (4). Ultrasound-guided central vascular
access is an emerging application which offers the advantage of a
shorter access time and a reduced number of attempts compared with
the landmark-guided technique (5),
but is not useful in identifying PLSVC. Therefore, this anomaly may
be detected only by chest X-ray during catheterization or following
placement of a catheter (6). In the
present study, we report the prevalence of PLSVC, its diameters and
the outcome of cancer patients with this anomaly undergoing
placement of a long-term catheter for nutrition and chemotherapy at
our institution.
Materials and methods
Catheter placement
Adult patients with hematological or solid tumors
admitted to our surgery department for implantation of a central
venous catheter (CVC) were considered. The study was approved by
the ethics committee of the Second University of Naples, Naples,
Italy. Informed consent was obtained from each patient prior to the
study. All procedures were performed by the same surgeon with
specific experience in ultrasound guided catheterization. The CVC
was routinely implanted in the left internal jugular vein; however,
if conditions were unsuitable for implantation on this side, such
as in the case of lymphadenopathy or postradiation therapy, or on
patient request, the CVC was placed on the right side. Ultrasound
examinations were performed using ESAOTE (Genova, Italy), equipped
with a 7.5 mHz probe. Patients were placed in the Trendelenburg
position with the head rotated toward the opposite side. All
procedures were performed using standard aseptic techniques and
local anesthesia. Puncture of the internal jugular vein was applied
to the last portion 1 cm above the clavicle and behind the
clavicular head of the sternocleidomastoid muscle (7). Correct venipuncture was always
confirmed by ultrasound guidance and easy aspiration of venous
blood. The Seldinger technique, using a peel-away sheath, was used
to place the catheter into the superior vena cava until insertion
into the right atrium.
In all cases, at the end of the procedure, a chest
radiograph in double projection was carried out to verify the
correct placement of the catheter. In case of suspected PLSVC, a
cine magnetic resonance imaging (MRI) scan was always performed.
The patients’ demographic data, diagnosis, indications for CVC
insertion, type of CVC, side of venipuncture, number of attempts,
time of procedure, malposition of the catheter and early
complications were recorded for all procedures. Systemic
prophylaxis against deep-vein thrombosis was adopted for 20 days
and no antibiotic prophylaxis was prescribed. Follow-up for each
patient with clinical examination and ultrasound exploration of the
vein was scheduled after 30 days.
Data analysis
Demographic data and clinical features were analyzed
using descriptive methods. Quantitative variables were summarized
using the mean and standard deviations (SD). Categorical variables
were summarized as counts and percentages.
Results
From July 2005 to December 2010, 600 patients with
hematological or solid tumors underwent central venous
catheterization (CVC). Indications for CVC were chemotherapy
(86.6%), transfusion (0.8%), parenteral nutrition (8.3%) and
palliative care (4.1%). The group comprised of 420 females (80%)
and 120 males (20%), with a mean age of 58 years (range, 15–75)
(Table I). The type of catheter,
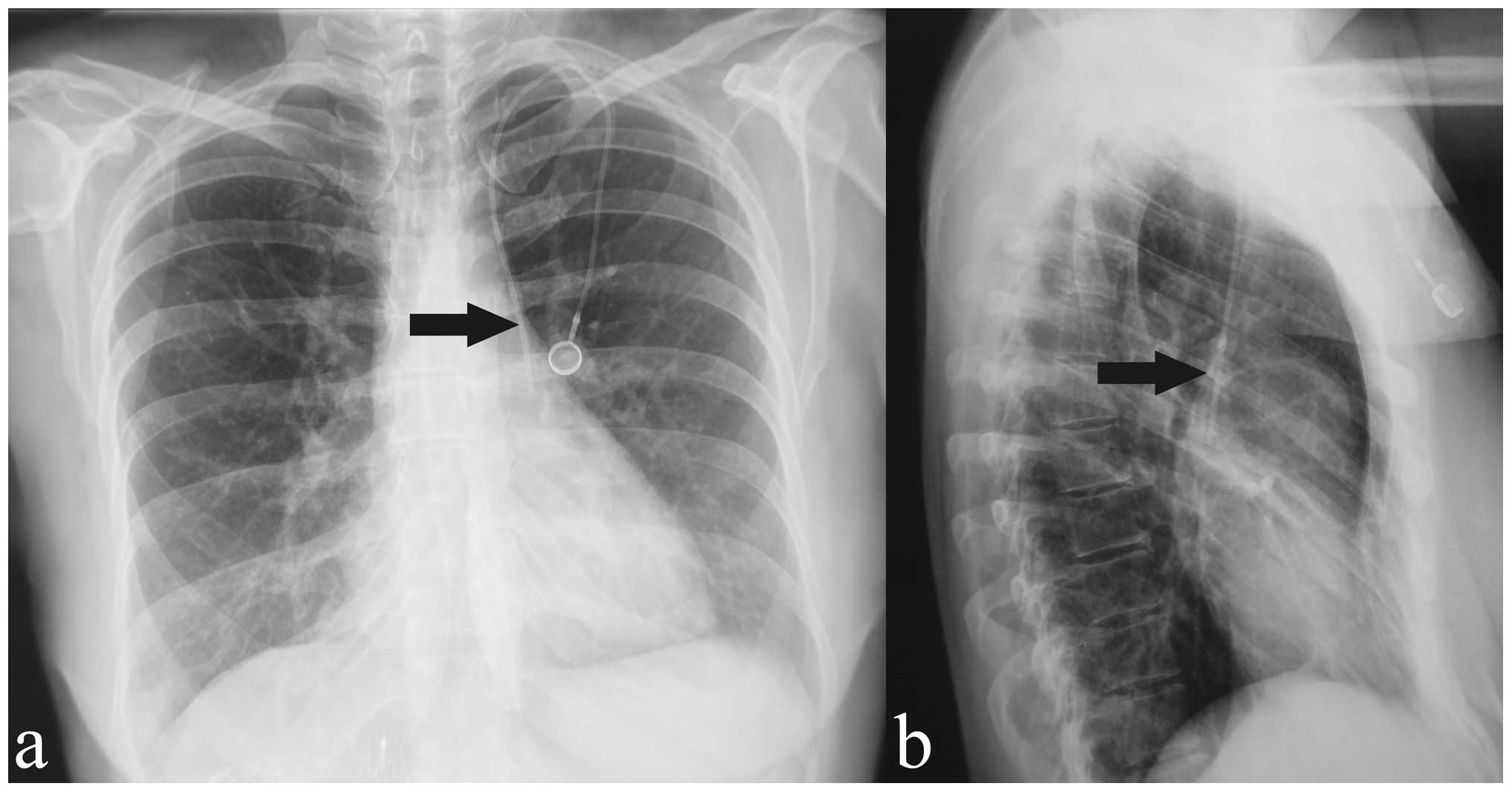
side and site of venipuncture are shown in Table II. Four cases of PLSVC (0.6%) were
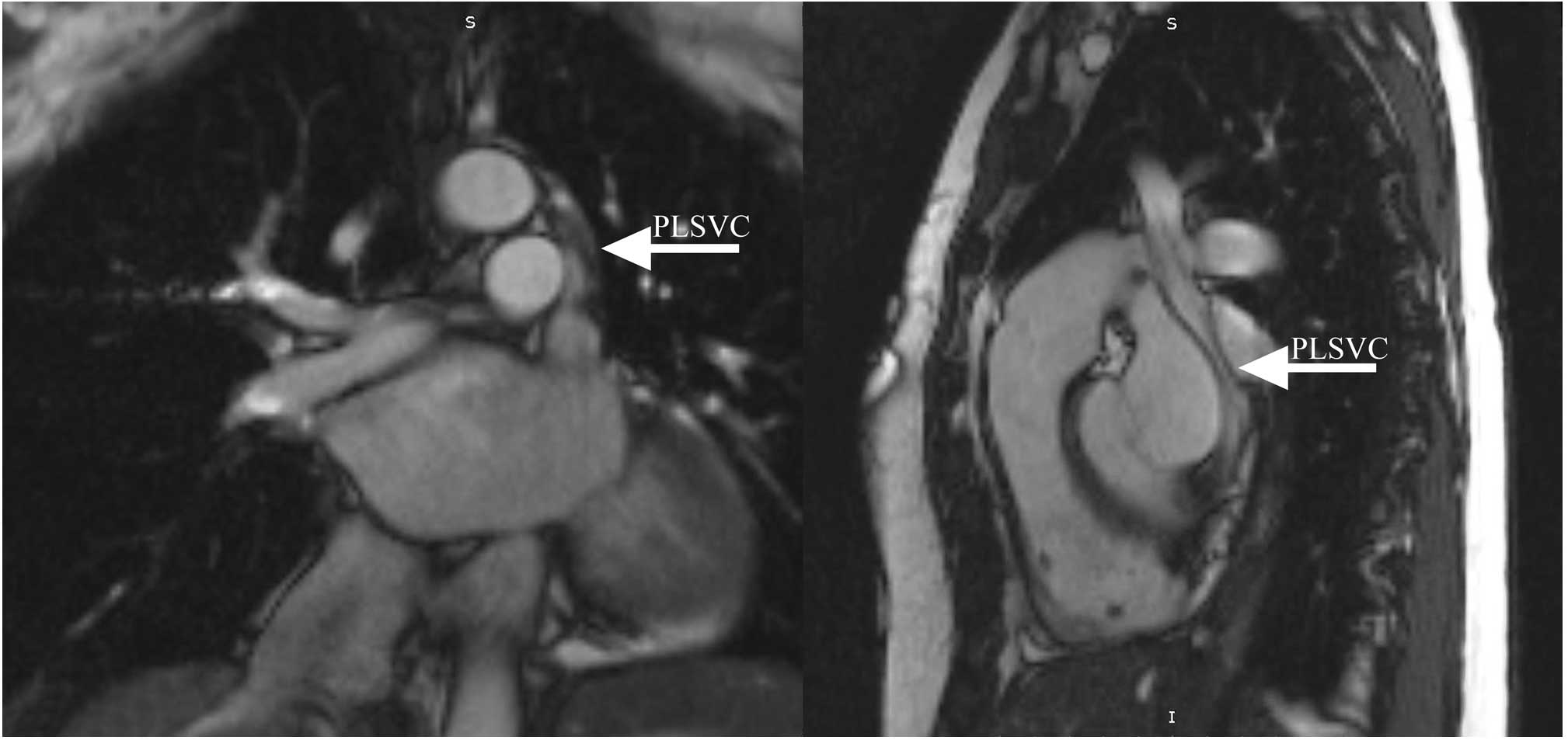
suspected based on chest radiography findings (Fig. 1). Cine MRI confirmed an isolated
PLSVC in one patient and a PLSVC associated with a right superior
vena cava (RSVC) in other three cases (Fig. 2). The mean diameter ± SD of the
PLSVC was 14.77±1.15 mm. In all cases, the CVC was not removed.
Three patients underwent chemotherapy and one patient was subjected
to total parenteral nutrition (TPN) (Table III). A dynamic ECG was performed
before starting and during the first cycle of chemotherapy in all
patients. In the three patients undergoing chemotherapy, dynamic
ECG and echocardiography were repeated at the end of treatment. No
disturbances in cardiac rhythm were noted and the heart ejection
fraction (EF) was not affected (Table
III). The patient on TPN succumbed to pancreatic cancer
progression after two months. For the other patients, no evidence
of infection or malfunction of the catheters was observed in the
following outpatient visits.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | No. of patients | % |
|---|
| Total | 600 | 100.0 |
| Age (years), mean
(range) | 58 (15–75) | |
| Gender |
| Male | 120 | 20.0 |
| Female | 480 | 80.0 |
| Treatment |
| Chemotherapy | 520 | 86.6 |
| Total parenteral
nutrition | 50 | 8.3 |
| Palliative
care | 25 | 4.1 |
| Transfusion | 5 | 0.8 |
 | Table II.Type of catheter implanted and side of
venipuncture. |
Table II.
Type of catheter implanted and side of
venipuncture.
| No. of patients | % |
|---|
| Totally implantable
catheter 7 Fr | 480 | 80 |
| Tunneled external
catheter 7 Fr | 120 | 20 |
| Internal jugular left
side | 510 | 85 |
| Internal jugular
right side | 90 | 15 |
 | Table III.Characteristics of 4 patients with
PLSVC. |
Table III.
Characteristics of 4 patients with
PLSVC.
| Characteristic | Case 1 | Case 2 | Case 3 | Case 4 |
|---|
| Age (years) | 66 | 74 | 52 | 54 |
| Gender | Male | Female | Female | Female |
| Solid tumor | Lung | Breast | Pancreas | Ovarian |
| Indication
(protocol) | CHT (Carboplatin +
etoposide for 6 cycles) | CHT (FEC 4 cycles +
taxotere 4 cycles) | TPN (2000
Kcal/day) | CHT (Carboplatin +
taxol for 6 cycles) |
| ECG findings | Normal | Normal | Normal | Normal |
| EF % pre-CHT | 60 | 55 | 66 | 60 |
| Diameter of PLSVC
(mm) | 14.2 | 15.3 | 16.1 | 13.5 |
| External diameter
of CVC (mm) | 2.31 | 2.31 | 2.31 | 2.31 |
| Absence of
RSVC | No | Yes | No | No |
| CS dilated | No | No | No | No |
| EF % post-CHT | 60 | 55 | Died after 2
months | 60 |
| Anomalies of
cardiac rhythm post-CHT | No | No | No | No |
| Thrombosis | No | No | No | No |
Discussion
The prevalence of PLSVC is 0.3–0.5% in the general
population. In 80–90% of individuals, PLSVC drains into the right
atrium directly or via the coronary sinus and is of no hemodynamic
consequence. In the remaining cases, it may drain into the left
atrium, resulting in a right to left sided shunt (1–4).
Almost 40% of patients with PLSVC have a variety of associated
cardiac anomalies, such as atrial septal defects, bicuspid aortic
valve, coarctation of the aorta, coronary sinus ostial atresia and
cor triatrium (9,10). The diagnosis of PLSVC is usually
made as an incidental finding during cardiovascular imaging or
placement of a central venous catheter via the left jugular or
subclavian vein (3,4). Chest X-ray demonstrates an unusual
course of the catheter in the left hemithorax. A CT scan or MRI may
be employed to establish the diagnosis. Echocardiography is useful
to verify the presence of a dilated coronary sinus and to rule out
variations in the typical anomalous venous course (2,8,10,11).
In our group of patients, the prevalence of PLSVC was more elevated
than in the general population, possibly owing to the choice of
inserting the catheter on the left side. In the four cases of
suspected PLSVC on chest radiograph, a cine MRI was carried out in
order to study both the path of the PLSVC and the blood vessels.
Three patients had both PLSVC and RSVC. Agenesia of RSVC and
presence of PLSVC were confirmed by cine MRI in only one patient.
Since echocardiography was performed before starting chemotherapy,
it was not repeated. PLSVC has various practical implications when
the left veins are used to access the right side of the heart or
pulmonary vasculature. Arrhythmia, cardiogenic shock, cardiac
tamponade and coronary sinus thrombosis have been reported when
catheters have been inserted via PLSVC. Fortunately, the incidence
of such complications is relatively low (3,10,12,13,14).
In cancer patients, the placement of a CVC in PLSVC may have
practical implications as chemotherapic drugs and hyperosmolar
solutions are injected into a smaller vein. In our patients,
endothelial damage and risk of thrombosis or anomalies of the
cardiac rhythm were feared. Indeed, these complications are more
likely when the diameter of the vein is small, the blood flow is
slowed or the catheter is large. In our four patients, no
clinically significant complications were recorded. The mean
diameter ± SD of the PLSVC was 14.77±1.15 mm and the external
diameter of the catheter was 7 Fr (2.31 mm). Cardiac rhythm
disturbances were not observed on dynamic ECG, and structural heart
damage was ruled out by echocardiography. We are unable to draw
conclusions as to what would occur in the case of PLSVC draining
into the coronary sinus. In conclusion, although PLSVC may be a
risky condition, no complications were recorded in the present
study when the CVC was positioned in PLSVC for administering
chemotherapeutic drugs or hyperosmolar solutions. However, further
research is needed to confirm our data.
References
|
1.
|
SK GoyalSR PunnamG VermaFL
RubergPersistent left superior vena cava: a case report and review
of literatureCardiovasc
Ultrasound650200810.1186/1476-7120-6-5018847480
|
|
2.
|
T SaranteasC MandilaJ PoularasJ
PapanicolaouA PatriankosD KarakitsosA KarabinisTransesophageal
echocardiography and vascular ultrasound in the diagnosis of
catheter-related persistent left superior vena cava thrombosisEur J
Echocardiogr10452455200910.1093/ejechocard/jen33419252187
|
|
3.
|
LF ParreiraCC LucasCC GilJD
BarataCatheterization of a persistent left superior vena cavaJ Vasc
Access10214215200919670178
|
|
4.
|
R PahwaA KumarPersistent left superior
vena cava: an intensivist’s experience and review of the
literatureSouth Med J9652852920038363033
|
|
5.
|
L CavannaG CivardiD VallisaC Di NunzioL
CapucciatiUltrasound-guided central venous catheterization in
cancer patients improves the success rate of cannulation and
reduces mechanical complications: A prospective observational study
of 1,978 consecutive catheterizationsWorld J Surg
Oncol89198201010.1186/1477-7819-8-91
|
|
6.
|
C Gonzales-JuanateyA TestaJ
VidanPersistent left superior vena cava draining into the coronary
sinus: Report of 10 cases and literature reviewClin
Cardiol27515518200410.1002/clc.496027090915471164
|
|
7.
|
F IovinoM PittirutiM BuononatoF Lo
SchiavoCentral venous catheterization: complications of different
placementsAnn Chir12610011016200111803622
|
|
8.
|
P VociG LuziL AgatiDiagnosis of persistent
left superior vena cava by multiplane transesophageal
echocardiographyCardiologia4027327519957553698
|
|
9.
|
B SarodiaJ StollerPersistent left superior
vena cava: case report and literature reviewRespir
Care45411416200010780037
|
|
10.
|
A RecuperoP PugliattiF RizzoS CarerjG
CavalliPersistent left-sided superior vena cava: integrated
noninvasive
diagnosisEchocardiography24982986200710.1111/j.1540-8175.2007.00509.x17894578
|
|
11.
|
VB KuteAV VanikarMR GumberPR ShahKR
GoplaniHL TrivediHemodialysis through persistent left superior vena
cavaIndian J Crit Care
Med154042201110.4103/0972-5229.7822321633545
|
|
12.
|
E WissnerR TilzM KonstantinidouA MetznerB
SchmidtKR ChunKH KuckF OuyangCatheter ablation of atrial
fibrillation in patients with persistent left superior vena cava is
associated with major intraprocedural complicationsHeart
Rhythm717551760201010.1016/j.hrthm.2010.08.005
|
|
13.
|
L LaurenziS NatoliL PelagalliME MarcelliD
AbbattistaL CarpaneseE ArcuriLong-term central venous
catheterization via persistent left superior vena cava: a case
reportSupport Care Cancer11190192200312618930
|
|
14.
|
RG PaiEchocardiographic features of
persistent left superior vena
cavaEchocardiography16435436199910.1111/j.1540-8175.1999.tb00088.x11175173
|