Introduction
Male breast cancer (MBC) accounts for less than 1%
of all types of cancer in males (1). Due to its rarity, there have been no
prospective randomized clinical trials of MBC patients (2). Clinical management of MBC is guided by
research on the disease in females or by data from small case
studies. Due to its low incidence, a limited number of patients are
collected for this study.
Recent studies have been conducted with regard to
MBC survival in comparison with female breast cancer (FBC);
however, no MBC study has been reported in Chinese patients. It was
considered that MBC had a worse prognosis compared to FBC (3), but recent retrospective studies
revealed that MBC and FBC patients have a similar prognosis
(4,5). In view of this discrepancy, we
compared the disease-free survival (DFS) and overall survival (OS)
in a group of carefully matched MBC and FBC patients in a Chinese
population.
Patients and methods
Patients and methods
Data for our study were obtained retrospectively
from the breast cancer database at the Department of Breast
Oncology, The Central Hospital of Tai'an (Central Hospital of
Taishan Medical College), China. To protect patient
confidentiality, we collected only coded data without direct
patient identifiers. Each male with invasive breast cancer
diagnosed between September 1982 and December 2006 was recorded in
the database and carefully matched with two females. Inclusion
criteria of MBC for our study included availability of
well-documented clinical information and availability of accurate
TNM stage, stages I–III. Exclusion criteria for the study included
patients with carcinoma in situ or with history of other
tumors, presence of metastatic lesions at initial diagnosis and
patients who were operated in other institutions. Matching criteria
included pathology of invasive ductal carcinoma in MBC and FBC, age
at diagnosis (±5 years), year of diagnosis (±5 years) (6,7) and
identical stage of the primary cancer at diagnosis. The females
were selected from a total of 5,964 consecutive patients whose data
were recorded in the same database and who underwent treatment at
our institution during the same period. When more than two females
met the criteria for matching, the female patients whose date of
diagnosis was closer to the male patient's date of diagnosis were
selected (4). A total of 42 MBC
patients and 84 matched FBC patients were enrolled in the
study.
Potential prognostic factors included for each case
were: elements of American Joint Committee on Cancer (AJCC) stage
(TNM), Scarff-Bloom-Richardson (SBR) tumor grade, age at diagnosis,
year of diagnosis, histological type, estrogen receptor (ER) and
progesterone receptor (PR) status, laterality and tumor
location.
Statistical analysis
To compare the breast cancer characteristics between
males and females, the Chi-square test was used for categorical
data and the Student's t-test for continuous data. The median
length of follow-up was calculated using the inverse Kaplan-Meier
method (8), and DFS and OS were
calculated using Kaplan-Meier methods (9). A survival analysis was performed on
the data set including all cases diagnosed between January 1, 1982
and December 31, 2006 with follow-up until March 1, 2011.
Statistical analysis was performed using SPSS 16.0 for Windows and
P<0.05 was considered to indicate a statistically significant
difference.
Results
The 42 MBC patients were matched to 84 FBC patients
during the same period. Table I
demonstrates the matching information and demographic data of the
matched male and female breast cancer patients. The mean age at
diagnosis was similar for the two matched groups; 58.0±11.3 years
(range, 26–75 years) for males and 57.1±10.6 years (range, 22–76
years) for females. The mean follow-up time for the 25-year study
period was 74.2±49.3 months (range, 5–262 months) for males and
86.8±49.2 months (range, 29–283 months) for females. Invasive
ductal carcinoma was the pathological type of all cases.
 | Table I.Characteristics of patients with
breast carcinoma. |
Table I.
Characteristics of patients with
breast carcinoma.
|
Characteristics | MBC, n=42 (%) | FBC, n=84 (%) |
|---|
| Age at diagnosis
(years) | | |
| Mean ± SD | 58.0±11.3 | 57.1±10.6 |
| Median
(range) | 60 (26–75) | 58 (22–76) |
| Year of
diagnosis | | |
| 1982–1989 | 7 (16.7) | 14 (16.7) |
| 1990–1999 | 18 (42.8) | 36 (42.8) |
| 2000–2006 | 17 (40.5) | 34 (40.5) |
| Follow-up time
(months) | | |
| Mean ± SD | 74.2±49.3 | 86.8±49.2 |
| Median
(range) | 64 (5-262) | 71 (29–283) |
| AJCC stage | | |
| I | 6 (14.3) | 12 (14.3) |
| II | 24 (57.1) | 48 (57.1) |
| III | 12 (28.6) | 24 (28.6) |
| Recurrence during
follow-up | | |
| No | 22 (52.4) | 49 (58.3) |
| Yes | 20 (47.6) | 35 (41.7) |
| Status at end of
follow-up | | |
| Alive | 20 (47.6) | 48 (57.1) |
| Dead | 22 (52.4) | 36 (42.9) |
| Cause of
mortality | | |
| Breast
cancer | 12 (54.6) | 26 (72.2) |
| Complication of
treatment | 1 (4.5) | 0 (0) |
| Contralateral
breast cancer | 1 (4.5) | 1 (2.8) |
| Another
cancer | 5 (22.7) | 2 (5.6) |
| Another
disease | 3 (13.7) | 7 (19.4) |
Significant differences were identified for tumor
location (P<0.01), hormone receptor status (P=0.018), adjuvant
chemotherapy (P=0.041) and hormone therapy (P=0.010) between male
and female patients (Table II).
Males were more likely to have an unknown tumor receptor status
(26.2%) compared to females (14.3%). Stage-specific treatment and
use of multi-modality therapy (surgery and radiotherapy) did not
differ between the two matched groups.
 | Table II.Comparison of characteristics between
MBC and FBC patients. |
Table II.
Comparison of characteristics between
MBC and FBC patients.
|
Characteristics | MBC n=42 (%) | FBC n=84 (%) |
χ2 | P-value |
|---|
| Laterality | | | | |
| Right | 20 (47.6) | 43 (51.2) | | |
| Left | 22 (52.4) | 41 (48.8) | 0.143 | 0.705 |
| Tumor location | | | | |
| Central | 14 (33.3) | 3 (3.6) | | |
| Peripheral
quadrant | 28 (66.7) | 81 (96.4) | 21.249 | 0.000a |
| SBR tumor
grade | | | | |
| 1 | 9 (21.4) | 11 (13.1) | | |
| 2 | 27 (64.3) | 63 (75.0) | | |
| 3 | 6 (14.3) | 10 (11.9) | 1.800 | 0.407 |
| Tumor size | | | | |
| ≤5 cm | 35 (83.3) | 69 (82.1) | | |
| >5 cm | 7 (16.7) | 15 (17.9) | 0.028 | 0.868 |
| Lymph node
status | | | | |
| Negative | 23 (54.8) | 39 (46.4) | | |
| Positive | 19 (45.2) | 45 (53.6) | 0.778 | 0.378 |
| Hormone
receptor | | | | |
| ER- and/or
PR-positive | 28 (66.7) | 49 (58.3) | | |
| ER- and/or
PR-negative | 3 (7.1) | 23 (27.4) | | |
| Unknown | 11 (26.2) | 12 (14.3) | 8.050 | 0.018a |
| Surgical
procedure | | | | |
| Radical
mastectomy | 40 (95.2) | 82 (97.6) | | |
| Modified radical
mastectomy | 2 (1.3) | 2 (2.7) | 0.032 | 0.857 |
| Adjuvant
chemotherapy | | | | |
| No | 18 (42.9) | 21 (25.0) | | |
| Yes | 24 (57.1) | 63 (75.0) | 4.178 | 0.041a |
| Radiotherapy | | | | |
| No | 25 (59.5) | 46 (54.8) | | |
| Yes | 17 (40.5) | 38 (45.2) | 0.258 | 0.611 |
| Hormone
therapy | | | | |
| No | 32 (76.2) | 44 (52.4) | | |
| Yes | 10 (23.8) | 40 (47.6) | 6.632 | 0.010a |
Univariate analysis revealed that tumor size, lymph
node state and AJCC stage were significant prognostic factors of
MBC (P<0.05) (Table III).
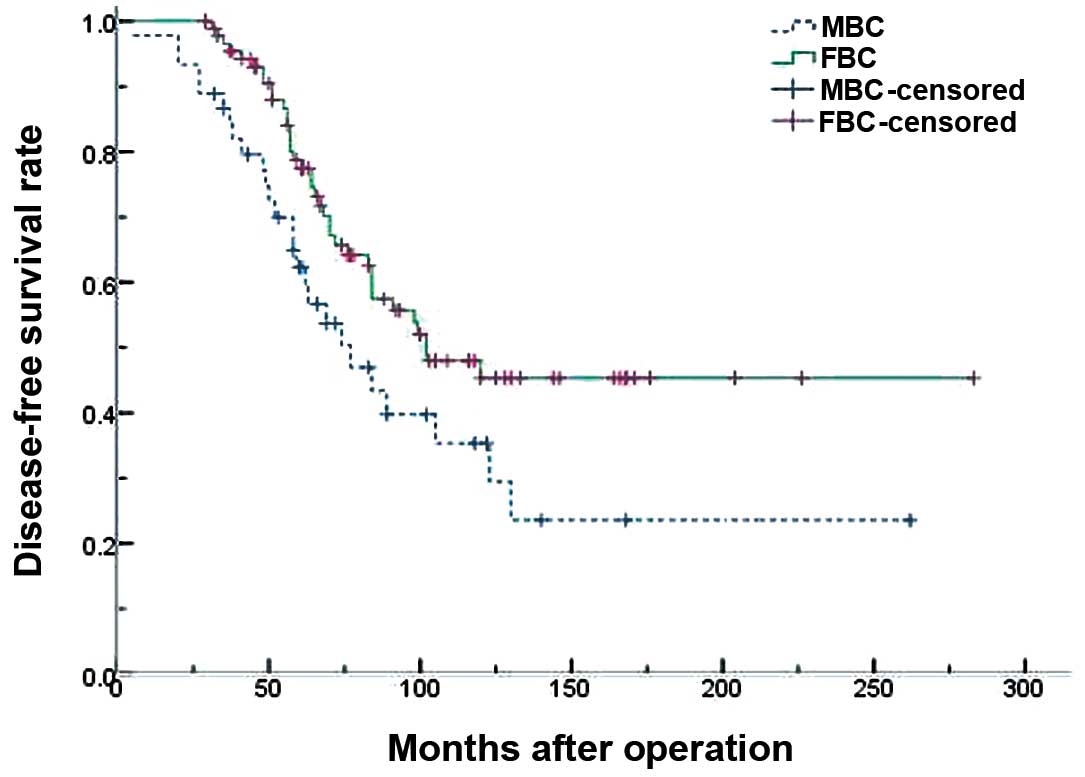
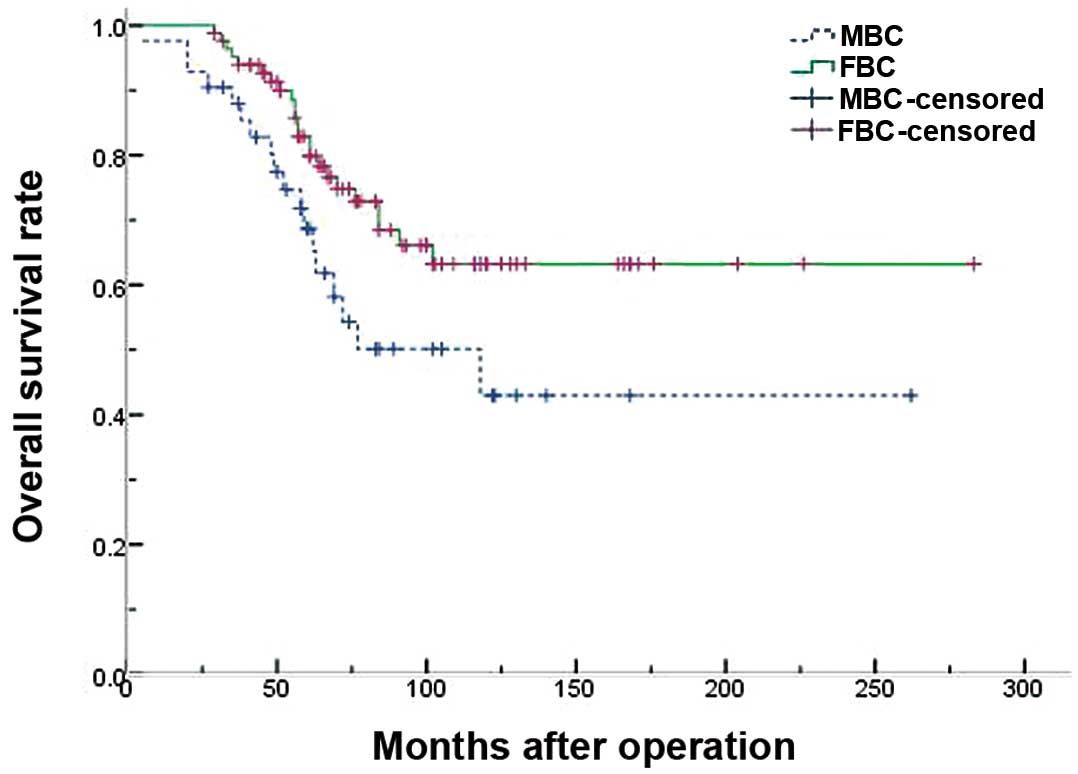
Figs. 1 and 2 demonstrate the survival results of the
two groups. The 5- and 10-year DFS rates were 61.2 and 40.7% for
males, and 75.3 and 52.3% for females, respectively, which was
significantly different (P=0.043) (Table IV). The 5- and 10-year OS rates were
68.7 and 43.0% for males, and 82.9 and 63.2% for females,
respectively, which was also significantly different (P=0.037).
 | Table III.Monofactorial analysis of prognosis
in MBC patients. |
Table III.
Monofactorial analysis of prognosis
in MBC patients.
|
Characteristics | N | DFS
| OS
|
|---|
| 5-year % | χ2 | P-value | 5-year % | χ2 | P-value |
|---|
| Age (years) | | | | | | | |
| <60 | 20 | 62.7 | | | 76.1 | | |
| ≥60 | 22 | 58.7 | 2.058 | 0.151 | 71.9 | 3.309 | 0.129 |
| Tumor size | | | | | | | |
| ≤5 cm | 35 | 64.4 | | | 81.1 | | |
| >5 cm | 7 | 14.3 | 9.033 | 0.003a | 38.1 | 8.006 | 0.005a |
| Lymph node
status | | | | | | | |
| Negative | 23 | 69.3 | | | 86.5 | | |
| Positive | 19 | 28.9 | 4.639 | 0.031a | 56.5 | 7.256 | 0.007a |
| AJCC stage | | | | | | | |
| I+II | 30 | 71.1 | | | 92.6 | | |
| III | 12 | 8.3 | 30.189 | 0.000a | 22.9 | 25.967 | 0.000a |
| Adjuvant
chemotherapy | | | | | | | |
| No | 18 | 48.5 | | | 57.9 | | |
| Yes | 24 | 56.1 | 2.126 | 0.145 | 86.8 | 2.251 | 0.134 |
| Radiotherapy | | | | | | | |
| No | 25 | 51.7 | | | 70.6 | | |
| Yes | 17 | 53.6 | 0.012 | 0.912 | 80.9 | 1.434 | 0.231 |
| Hormone
therapy | | | | | | | |
| No | 32 | 49.0 | | | 75.8 | | |
| Yes | 10 | 63.6 | 0.004 | 0.947 | 68.2 | 0.103 | 0.748 |
 | Table IV.Comparison of survival between MBC
and FBC patients. |
Table IV.
Comparison of survival between MBC
and FBC patients.
| Survival rate
(years) | DFS
| OS
|
|---|
| MBC % | FBC % | χ2 | P-value | MBC % | FBC % | χ2 | P-value |
|---|
| 5 | 61.2 | 75.3 | | | 68.7 | 82.9 | | |
| 10 | 40.7 | 52.3 | 4.113 | 0.043a | 43.0 | 63.2 | 4.331 | 0.037a |
Discussion
MBC is a rare disease, but the incidence is
increasing, with a reported 26% increase in the USA between 1973
and 1998 (10). The poor prognosis
for MBC has been attributed to the advanced stage at presentation
due to the lack of awareness and high incidence of lymph node
metastases (11,12). However, recent studies have
demonstrated that if MBC patients are matched for prognostic
factors, including age and TNM stage, the clinical outcome is
similar (4,13).
Following successful matching, we compared the
survival of MBC and FBC patients. We analyzed potential prognostic
factors prior to prognosis analysis and identified that the two
groups were comparable. The matching criteria of this study
included similar age at diagnosis, similar time of diagnosis,
identical stage and identical pathology. Upon investigation of the
characteristics of all patients, we revealed that the tumor
location was different. Compared to female patients, more male
patients had centrally located tumors (33.3 vs. 3.6%), and the
difference was significant (P<0.001). We also compared the
estrogen receptor and progesterone receptor (ER/PR) status between
the two groups. Expression levels of ER/PR were higher in MBC
patients compared to FBC patients (14). Due to the long time span of this
study and the late development of c-erbB-2 detection methods in
2003, the patients with c-erbB-2 detection were few (11 MBC
patients, 36 FBC patients). Among the patients examined for
c-erbB-2 detection, 3 male patients (3/11, 27.3%) and 14 female
patients (14/36, 38.9%) had c-erbB-2 expression. Our data
corresponds with a previous study demonstrating that c-erbB-2
expression levels are lower in MBC patients than in FBC patients
(15). Since ER/PR and c-erbB-2
predict prognosis in breast cancer (16,17),
we suggest that this may play a role in the difference in prognosis
between our two study groups. ER/PR and c-erbB-2 status have been
identified as predictive factors of response to endocrine therapy
and trastuzumab treatment, respectively (16). In this study, a lower number of male
patients underwent endocrine therapy (10 cases, 23.8%) in
comparison to female patients (40 cases, 47.6%) (P=0.010),
resulting in a lost opportunity to achieve a good outcome. We
revealed that MBC patients had a significantly lower 5-year
survival rate than FBC patients (P<0.05), which may be due to
the different endocrine therapies used in the two groups. We
suggest that this phenomenon could be overcome if doctors and
patients were educated on the advantages of treating MBC and FBC
patients using similar endocrine therapeutic strategies.
According to the univariate survival analysis, tumor
size (P<0.05), lymph node involvement (P<0.05) and AJCC stage
(P<0.05) were statistically significant in the 5-year DFS and OS
in MBC patients. The 5- and 10-year DFS and OS rates in MBC
patients were significantly lower compared to those in FBC patients
(P<0.05). Additionally, in this study we revealed that MBC
survivors have an increased risk of developing a second primary
cancers other than contralateral breast cancer (10% vs. 2.1%).
Previous studies have demonstrated that males with a history of
breast cancer had a 30- to 93-fold greater risk of contralateral
breast cancer, while FBC patients only had a 2- to 4-fold greater
risk (18,19). The risk of other cancers, including
melanoma and prostate cancer, may also be elevated in MBC survivors
(18–22). Male breast carcinoma patients should
be monitored carefully for the occurrence of second primary
cancers, particularly a second primary breast cancer (23).
In general, breast cancer in males should be treated
using the same strategy as used with females (24). Although the benefits of adjuvant
chemotherapy and hormonal therapy have been demonstrated in a
subgroup of FBC patients, the role of adjuvant chemotherapy in MBC
is unclear (25). However, recent
studies reported that adjuvant chemotherapy was beneficial for MBC
patients (26), and may be decided
by assessing the risks and benefits in the same manner as in FBC
(26–29). In the present study, the proportion
of MBC patients who received chemotherapy (57.1%) was lower than
FBC patients (75.0%) (P=0.041). We suggest that the differences in
treatment strategies may be the most significant factors
determining prognostic differences between MBC and FBC patients. We
demonstrated that fewer MBC patients underwent endocrine therapy
(P=0.010) and fewer accepted adjuvant chemotherapy (P=0.041)
following surgery. Our results suggest that poor survival rates in
MBC may be associated with the lack of adjuvant therapy in these
patients. Evidence-based guidelines for adjuvant systemic therapy
in FBC are rapidly implemented into community practice (30), but the same may not be true for MBC.
The smaller improvement for male hazard ratios suggests a delay
and/or underutilization of adjuvant therapy in males compared to
females, in particular tamoxifen for hormone-positive MBC (31). Use of tamoxifen for MBC may also be
limited by poor compliance due to its association with a high rate
of treatment-limiting side effects in males (32), including decreased libido, weight
gain, hot flashes and deep vein thrombosis.
To the best of our knowledge, this is one of the
first studies of matched male and female breast cancer patients in
China. It allowed the comparison of breast cancer in a homogeneous
population of patients, where each male was carefully matched with
two females. Limitations include the retrospective design of the
study, the lack of detailed information of c-erbB-2 overexpression
and the inclusion of a number of patients with unknown hormone
status.
In conclusion, MBC patients had a decreased 5- and
10-year survival when basic characteristics were matched. We
propose that the difference in prognosis between MBC and FBC
patients in this study was closely associated with the lack of
adjuvant treatment strategies in the two groups.
References
|
1.
|
A JemalR SiegelE WardCancer statistics,
2008CA Cancer J Clin587196200810.3322/CA.2007.0010
|
|
2.
|
SH GiordanoMale breast cancer: it's time
for evidence instead of extrapolationOnkologie315055062008
|
|
3.
|
KA RosenblattDB ThomasA McTiernanBreast
cancer in men: aspects of familial aggregationJ Natl Cancer
Inst83849854199110.1093/jnci/83.12.8492061945
|
|
4.
|
F MarchalM SalouC MarchalMen with breast
cancer have same disease-specific and event-free survival as
womenAnn Surg
Oncol16972978200910.1245/s10434-009-0327-619184227
|
|
5.
|
K LynnRare male breast cancer has
similarities to female diseaseMLO Med Lab Obs423436201021213581
|
|
6.
|
L NeumayerTL SchifftnerWG HendersonBreast
cancer surgery in Veterans Affairs and selected university medical
centers: results of the patient safety in surgery studyJ Am Coll
Surg20412351241200710.1016/j.jamcollsurg.2007.03.018
|
|
7.
|
K AnanS MitsuyamaK NishiharaBreast cancer
in Japanese men: does sex affect prognosis?Breast
Cancer11180186200410.1007/BF0296829915550865
|
|
8.
|
JJ ShusterMedian follow-up in clinical
trialsJ Clin Oncol919119219911985169
|
|
9.
|
EL KaplanP MeierNonparametric estimation
from incomplete observationsJ Am Statist
Assoc53457481195810.1080/01621459.1958.10501452
|
|
10.
|
SH GiordanoDS CohenAU BuzdarBreast
carcinoma in men: a population-based
studyCancer1015157200410.1002/cncr.2031215221988
|
|
11.
|
MG JoshiAK LeeM LodaMale breast carcinoma:
an evaluation of prognostic factors contributing to a poorer
outcomeCancer77490498199610.1002/(SICI)1097-0142(19960201)77:3%3C490::AID-CNCR10%3E3.0.CO;2-%238630956
|
|
12.
|
PC WillsherIH LeachIO EllisA comparison
outcome of male breast cancer with female breast cancerAm J
Surg173185188199710.1016/S0002-9610(97)89592-X9124623
|
|
13.
|
MB El-TamerIK KomenakaA TroxelMen with
breast cancer have better disease-specific survival than womenArch
Surg13910791082200410.1001/archsurg.139.10.107915492147
|
|
14.
|
WF AndersonMD AlthuisLA BrintonSS DevesaIs
male breast cancer similar or different than female breast
cancer?Breast Cancer Res
Treat837786200410.1023/B:BREA.0000010701.08825.2d14997057
|
|
15.
|
C RudlowskiN FriedrichsA FaridiHer-2/neu
gene amplification and protein expression in primary male breast
cancerBreast Cancer Res
Treat84215223200410.1023/B:BREA.0000019953.92921.7e15026619
|
|
16.
|
DG TiezziJM AndradeA Ribeiro-SilvaHER-2,
p53, p21 and hormonal receptors proteins expression as predictive
factors of response and prognosis in locally advanced breast cancer
treated with neoadjuvant docetaxel plus epirubicin combinationBMC
Cancer736200710.1186/1471-2407-7-36
|
|
17.
|
JM BentelSN BirrellMA PicheringAndrogen
receptor agonist activity of the synthetic progestin,
medroxyprogesterone acetate, in human breast cancer cellsMol Cell
Endocrinol1541120199910.1016/S0303-7207(99)00109-4
|
|
18.
|
A AuvinenRE CurtisE RonRisk of subsequent
cancer following breast cancer in menJ Natl Cancer
Inst9413301332200210.1093/jnci/94.17.133012208898
|
|
19.
|
C DongK HemminkiSecond primary breast
cancer in menBreast Cancer Res
Treat66171172200110.1023/A:101063942920711437104
|
|
20.
|
NC HodgsonJH ButtonD FranceschiMale breast
cancer: is the incidence increasing?Ann Surg
Oncol11751755200410.1245/ASO.2004.01.00115289238
|
|
21.
|
K HemminkiG SceloP BoffettaSencond primary
malignancies in patients with male breast cancerBr J
Cancer9212881292200510.1038/sj.bjc.660250515798766
|
|
22.
|
SB LeibowitzJE GarberEA FoxMale patients
with diagnoses of both breast cancer and prostate cancerBreast
J9208212200310.1046/j.1524-4741.2003.09312.x12752629
|
|
23.
|
S Satram-HoangA ZiogasH Anton-CulverRisk
of second primary cancer in men with breast cancerBreast Cancer
Res9R10200710.1186/bcr164317254323
|
|
24.
|
National Comprehensive Cancer NetworkNCCN
practice guidelines in oncology 2009Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
Accessed 30 May, 2010
|
|
25.
|
SH GiordanoAU BuzdarGN HortobagyiBreast
cancer in menAnn Intern
Med137678687200210.7326/0003-4819-137-8-200210150-0001312379069
|
|
26.
|
SH GiordanoGH PerkinsK BroglioAdjuvant
systemic therapy for male breast
carcinomaCancer10423592364200510.1002/cncr.2152616270318
|
|
27.
|
A AgrawalAA AyantundeR RampaulJF
RobertsonMale breast cancer: a review of clinical managementBreast
Cancer Res Treat1031121200710.1007/s10549-006-9356-z
|
|
28.
|
Z NahlehS GirniusMale breast cancer: a
gender issueNat Clin Pract Oncol3428437200610.1038/ncponc0564
|
|
29.
|
IS FentimanA FourquetGN HortobagyiMale
breast cancerLancet367595604200610.1016/S0140-6736(06)68226-3
|
|
30.
|
A MariottoEJ FeuerLC HarlanTrends in use
of adjuvant multi-agent chemotherapy and tamoxifen for breast
cancer in the United States: 1975–1999J Natl Cancer
Inst94162616342003
|
|
31.
|
G RibeiroR SwindellAdjuvant tamoxifen for
male breast cancer (MBC)Br J
Cancer65252254199210.1038/bjc.1992.501739625
|
|
32.
|
TF AnelliA AnelliKN TranTamoxifen
administration is associated with a high rate of treatment-limiting
symptoms in male breast cancer
patientsCancer747477199410.1002/1097-0142(19940701)74:1%3C74::AID-CNCR2820740113%3E3.0.CO;2-%238004585
|