Introduction
Colorectal cancer (CRC) is the second most common
cause of cancer-related mortality in Western societies (1). Despite recent advances in CRC
treatment, 5-fluorouracil (5-FU)-based regimens continue to be the
international standard chemotherapy for patients with advanced CRC
(2). However, drug resistance
profoundly limits the effectiveness of current CRC cancer
chemotherapies (3). Moreover,
application of 5-FU-based regimens is often coupled with serious
toxicity and side-effects such as anemia, leucopenia,
thrombocytopenia and peripheral neuropathy (4,5).
Therefore, it is essential to develop safer agents for the
chemotherapeutic treatment of CRC. Traditional Chinese medicine
(TCM), which has relatively few side-effects, plays an important
role in primary health care in China and has recently been
recognized by Western countries as a key source for revealing novel
lead molecules for modern drug discovery. Clinical practice has
also shown that many traditional Chinese medicines possess
antitumor activities, which provide insight into new therapeutic
strategies for cancer treatment (6–14).
Cancer cells are characterized by an uncontrolled
increase in cell proliferation (15). Eukaryotic cell proliferation is
primarily regulated by the cell cycle. G1/S transition is one of
the two main checkpoints of the cell cycle (16), which is responsible for initiation
and completion of DNA replication. G1/S progression is strongly
regulated by Cyclin D1, which exerts its function by forming an
active complex with its major catalytic partners, such as CDK4
(17). An unchecked or
hyperactivated Cyclin D1/CDK4 complex often leads to uncontrolled
cell division and malignancy (18–21).
Therefore, inhibiting excessive proliferation of tumor cells by
blocking Cyclin D1/CDK4-mediated G1/S progression is one of the key
approaches for the development of anticancer drugs.
Pien Tze Huang (PZH) is a well-known traditional
Chinese formula that was first prescribed 450 years ago in the Ming
Dynasty, with properties of heat-clearing and detoxification
(22). In the TCM system,
accumulation of toxic dampness and heat is a major pathogenic
factor of cancer, therefore clearing heat and detoxification is a
principle of anticancer treatment. For this reason, PZH has been
used in China and Southeast Asia for centuries as a folk remedy for
various types of cancer. Modern pharmacological studies have
proposed that PZH exhibits therapeutic effects in clinical trials
of tumors, such as hepatocellular carcinoma and colon cancer
(23,24). In addition, in experimental animals
PZH inhibits the growth of Ehrlich-Ascites tumor, gastric
carcinoma, and hepatoma (25).
Moreover, we recently reported that PZH is able to inhibit colon
cancer growth both in vivo and in vitro via the
promotion of apoptosis and inhibition of tumor angiogenesis
(26–28). To elucidate the mechanism of the
tumoricidal activity of PZH, we evaluated its effect on the
proliferation of the human colon carcinoma cell line Caco-2 and
investigated the underlying molecular mechanism.
Materials and methods
Materials and reagents
RPMI-1640, fetal bovine serum (FBS),
penicillin-streptomycin, Trypsin-EDTA, and TRIzol reagent were
purchased from Invitrogen (Carlsbad, CA, USA). SuperScript II
reverse transcriptase was obtained from Promega (Madison, WI, USA).
Cyclin D1, CDK4 and β-actin antibodies, and horseradish peroxidase
(HRP)-conjugated secondary antibodies were purchased from Cell
Signaling (Beverly, MA, USA). Any other chemicals, unless otherwise
stated, were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of PZH
PZH was obtained from and authenticated by the sole
manufacturer Zhangzhou Pien Tze Huang Pharmaceutical Co., Ltd.,
China (Chinese FDA approval no. Z35020242). Stock solution of PZH
was prepared imme diately prior to use by dissolving the PZH powder
in phosphate-buffered saline (PBS) to a concentration of 20 mg/ml.
The working concentrations of PZH were obtained by diluting the
stock solution in the culture medium.
Cell culture
Human colon carcinoma Caco-2 cells were obtained
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). Caco-2 cells were grown in RPMI-1640 containing 10% (v/v)
FBS, and 100 U/ml penicillin and 100 μg/ ml streptomycin. Cells
were cultured at 37°C, in a 5% CO2 humidified
environment.
Assessment of cell viability
Cell viability was assessed by MTT colorimetric
assay. Caco-2 cells were seeded into 96-well plates at a density of
5×103 cells/well in 0.1 ml medium. The cells were
treated with various concentrations of PZH for different periods of
time. At the end of the treatment, 100 μl MTT (0.5 mg/ml in PBS)
were added to each well, and the samples were incubated for an
additional 4 h at 37°C. The purple-blue MTT formazan precipitate
was dissolved in 100 μl DMSO. The absorbance was measured at 570 nm
using an ELISA reader (BioTek, Model ELX800, USA).
Colony formation
Caco-2 cells were seeded into 6-well plates at a
density of 1×105 cells/well in 2 ml medium. After
treatment with various concentrations of PZH for 24 h, the cells
were collected and diluted in fresh medium in the absence of PZH
and then reseeded into 6-well plates at a density of
1×103 cells/well. Following incubation for 8 days in a
37°C humidified incubator with 5% CO2, the formed
colonies were fixed with 10% formaldehyde, stained with 0.01%
crystal violet and counted. Cell survival was calculated by
normalizing the survival of the control cells as 100%.
Cell cycle analysis
The cell cycle analysis was carried out by flow
cytometry using a fluorescence-activated cell sorting (FACS)
Calibur (Becton-Dickinson, San Jose, CA, USA) and propidium iodide
(PI) staining. Subsequent to treatment with various concentrations
of PZH for 24 h, Caco-2 cells were collected and adjusted to a
concentration of 1x106 cells/ml, and fixed in 70%
ethanol at 4°C overnight. The fixed cells were washed twice with
cold PBS, and then incubated for 30 min with RNase (8 μg/ml) and PI
(10 μg/ml). The fluorescent signal was detected through the FL2
channel and the proportion of DNA in different phases was analyzed
using ModfitLT version 3.0 (Verity Software House, Topsham).
RT-PCR analysis
Caco-2 cells were seeded into 6-well plates at a
density of 1×105 cells/well in 2 ml medium and treated
with various concentrations of PZH for 24 h. Total RNA was isolated
with TRIzol reagent. Oligo(dT)-primed RNA (1 μg) was
reverse-transcribed with SuperScript II reverse transcriptase
(Promega) according to the manufacturer’s instructions. The
obtained cDNA was used to determine the mRNA amount of Cyclin D1
and CDK4 by PCR. GAPDH was used as an internal control. The primer
sequences used for the amplification of Cyclin D1, CDK4 and GAPDH
transcripts were: Cyclin D1, forward: 5′-TGG ATG CTG GAG GTC TGC
GAG GAA -3′ and reverse: 5′-GGC TTC GAT CTG CTC CTG GCA GGC-3′;
CDK4, forward: 5′-CAT GTA GAC CAG GAC CTA AGC-3′ and reverse:
5′-AAC TGG CGC ATC AGA TCC TAG-3′; GAPDH, forward: 5′-CG ACC ACT
TTG TCA AGC TCA-3′ and reverse: 5′-AG GGG TCT ACA TGG CAA CTG-3′.
Samples were analyzed by gel electrophoresis (1.5% agarose). The
DNA bands were examined using a Gel Documentation System (BioRad,
Model Gel Doc 2000, USA).
Western blotting
Caco-2 cells (2.5×105 cells/well) were
seeded into 25 cm2 flasks in 5 ml medium. Cells were
treated with various concentrations of PZH for 24 h and then lysed
with mammalian cell lysis buffer containing protease and
phosphatase inhibitor cocktails. The lysates were resolved in 12%
SDS-PAGE gels and electroblotted. The PVDF membranes were blocked
with 5% skimmed milk and probed with primary antibodies against
CyclinD1, CDK4 and β-actin (1:1,000) overnight at 4°C and then with
appropriate HRP-conjugated secondary antibody followed by enhanced
chemiluminescence detection.
Statistical analysis
Data were analyzed using the SPSS package for
Windows (Version 11.5). Statistical analysis of the data was
performed using the Student’s t-test and one-way ANOVA. Differences
with P<0.05 were considered statistically significant.
Results
PZH inhibits the proliferation of Caco-2
cells
The viability of Caco-2 cells was determined by MTT
assay to compare the relative number of cells in PZH-treated
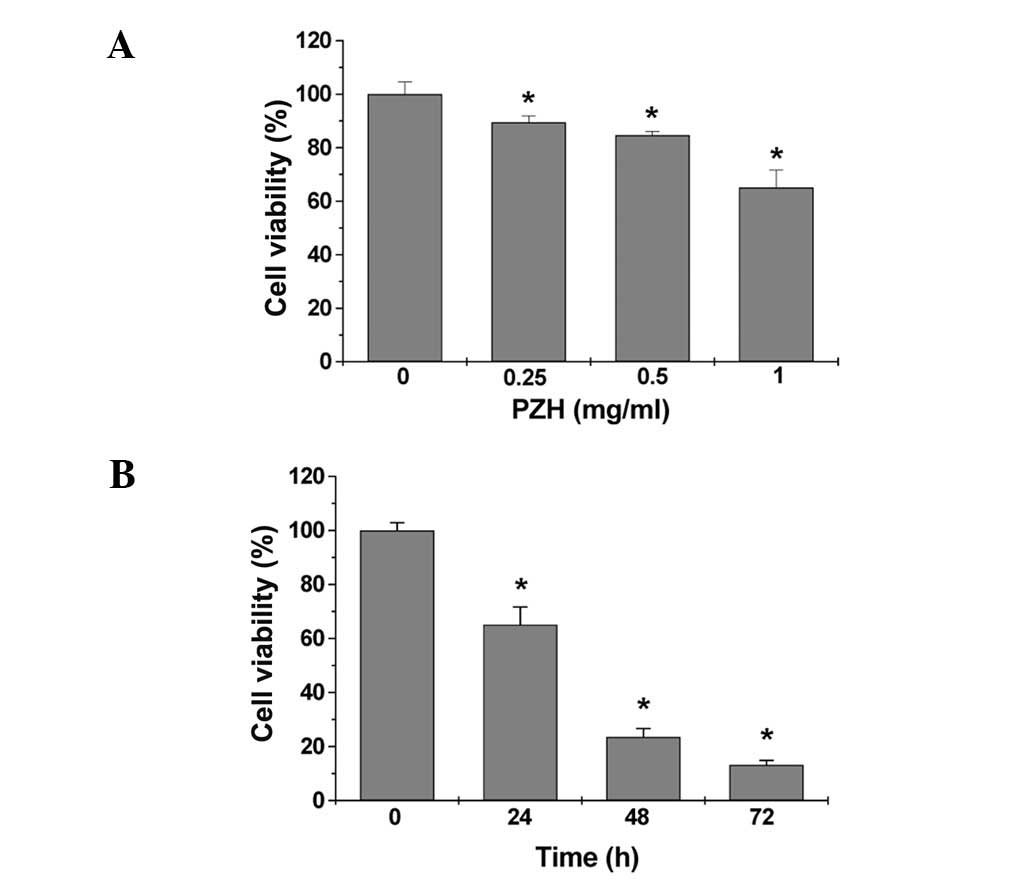
monolayers to untreated controls. As shown in Fig. 1A, treatment with 0.25–1 mg/ml of PZH
for 24 h dose-dependently reduced cell viability by 11–35% compared
to the untreated control cells (P<0.05). We also evaluated the
effect of 1 mg/ml of PZH on cell viability with incubation for
different periods of time. As shown in Fig. 1B, PZH treatment led to a gradual
decrease in cell viability with the increase of exposure time. To
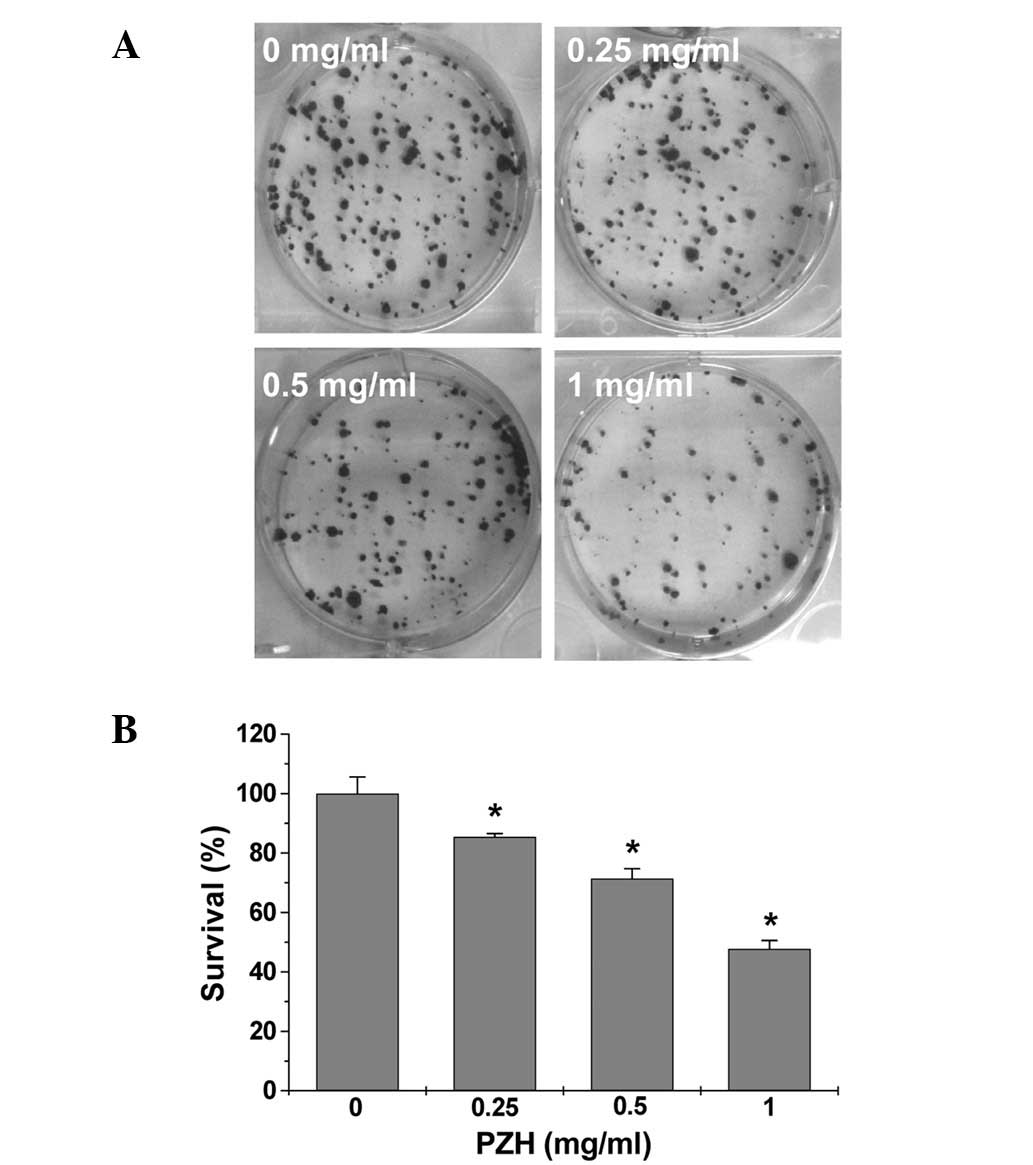
verify these results, we examined the effect of PZH on Caco-2 cell
survival using a colony formation assay. As shown in Fig. 2A and B, PZH treatment
dose-dependently reduced the cell survival rate by 15–52% compared
to the untreated control cells (P<0.05). Taken together, these
data suggest that PZH inhibits Caco-2 cell proliferation in a dose-
and time-dependent manner.
PZH blocks G1/S progression of Caco-2
cells
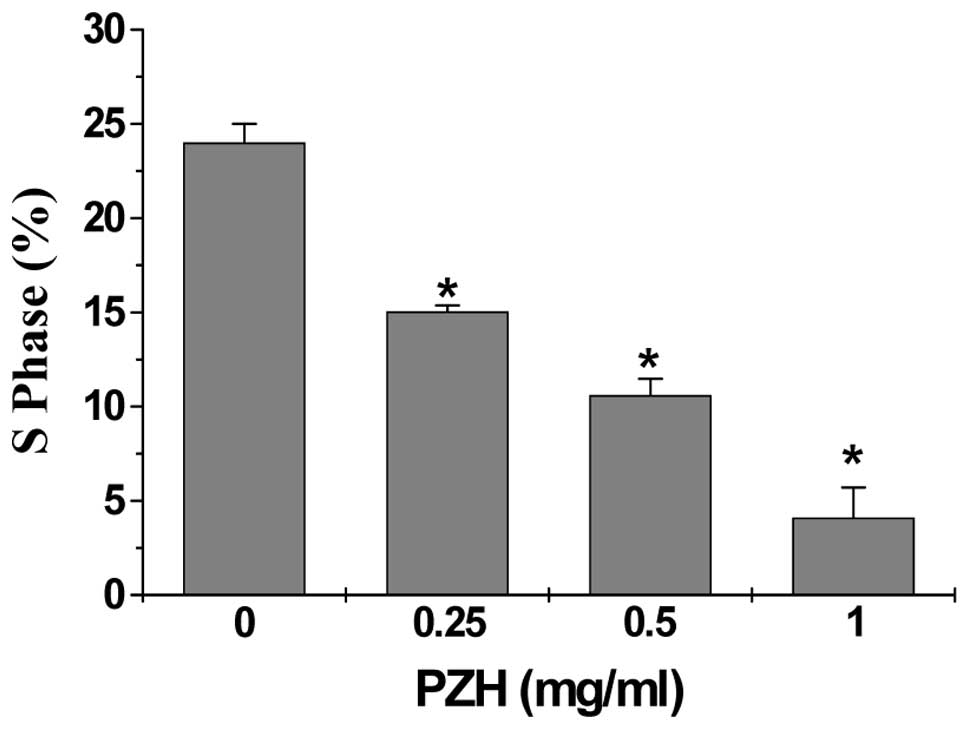
The effect of PZH on the G1 to S progression in
Caco-2 cells by PI staining, followed by FACS analysis. Subsequent
to treatment with 0, 0.25, 0.5 and 1 mg/ml of PZH the percentage of
S-phase cells was found to be 24.0, 15.0, 10.59 and 4.11%,
respectively (P<0.05) (Fig. 3),
indicating that the inhibitory effect of PZH on Caco-2 cell
proliferation is correlated with the arrest of G1/S cell cycle
progression.
PZH regulates the expression of Cyclin D1
and CDK4 in Caco-2 cells
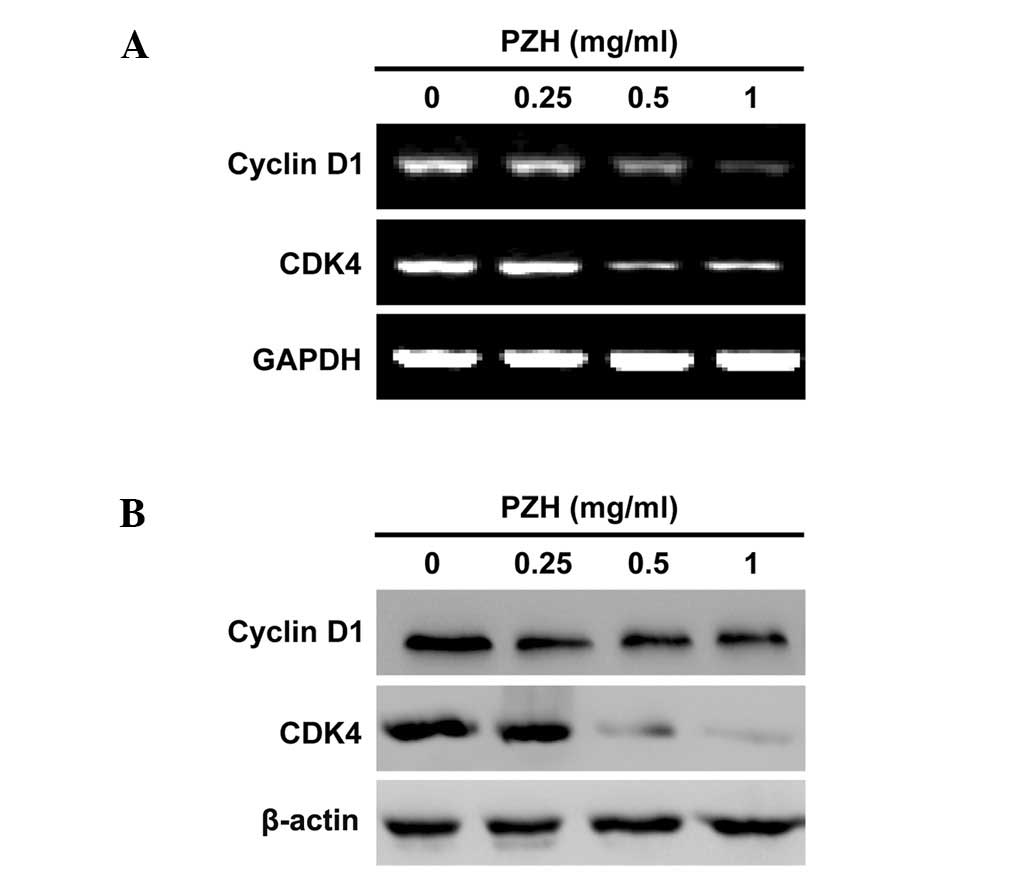
To explore the mechanism of the anti-proliferative
activity of PZH, we performed RT-PCR and western blotting to
respectively examine the mRNA and protein expression of Cyclin D1
and CDK4 in Caco-2 cells. As shown in Fig. 4, PZH treatment profoundly and
dose-dependently reduced the expression of Cyclin D1 and CDK4, at
both the transcriptional and translational levels.
Discussion
Drug resistance and toxicity against normal cells
limit the effectiveness of current cancer chemotherapies, including
those used to treat colorectal cancer (3–5),
emphasizing the need for the development of novel cancer
chemotherapies. Natural products have received a lot of attention
due to their relatively few side-effects compared to modern
chemotherapeutics and have been used clinically for thousands of
years as important alternative remedies for various diseases,
including cancer (6,7). PZH a well-known traditional Chinese
formula, first prescribed 450 years ago in the Ming Dynasty, has
long been used in China for cancer treatment (22–24).
Although it has been shown that PZH inhibits colon cancer growth
via the induction of apoptosis and inhibition of tumor angiogenesis
(26–28), the precise mechanism of its
anticancer effect remains largely unclear. Therefore, in order for
PZH to be developed further as an anticancer agent, its underlying
molecular mechanism of action should be elucidated.
Cancer cells are characterized by an uncontrolled
proliferation (15). Therefore,
inhibiting excessive proliferation of tumor cells is one of the key
approaches for the development of anticancer drugs. Using MTT and
colony formation analyses, we demonstrated that PZH inhibited the
proliferation of human colon carcinoma Caco-2 cells, in a dose- and
time-dependent manner. Eukaryotic cell proliferation is primarily
regulated by the cell cycle. G1/S transition is one of the two main
checkpoints of the cell cycle, responsible for initiation and
completion of DNA replication (16). By using FACS analysis with PI
staining, we found that PZH dose-dependently repressed the G1 to S
transition in Caco-2 cells. G1/S progression is tightly regulated
by the pro-proliferative Cyclin D1 and CDK4 (17). Overexpression of Cyclin D1 and CDK4
is commonly detected in various types of cancer (18–21).
Consistent with the inhibitory effect of PZH on G1/S transition,
our data indicated that PZH treatment suppressed the mRNA and
protein expression of Cyclin D1 and CDK4 in Caco-2 cells. In
conclusion, the present study has demonstrated for the first time
that PZH inhibited cancer cell proliferation by blocking G1 to S
progression, which may be one of the mechanisms mediating its
antitumor activity.
Abbreviations:
|
CRC
|
colorectal cancer;
|
|
PZH
|
Pien Tze Huang;
|
|
TCM
|
traditional Chinese medicine;
|
|
MTT
|
3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
Acknowledgements
This study was sponsored by the
National Natural Science Foundation of China (no. 81073097), the
Developmental Fund of Chen Keji Integrative Medicine (no. CKJ
2011001), and the China Postdoctoral Science Foundation (no.
2012M511437).
References
|
1.
|
A JemalF BrayMM CenterJ FerlayE WardD
FormanGlobal cancer statisticsCA Cancer J
Clin616990201110.3322/caac.20107
|
|
2.
|
DM GustinDE BrennerChemoprevention of
colon cancer: current status and future prospectsCancer Metast
Rev21323348200210.1023/A:102127122947612549770
|
|
3.
|
DB LongleyWL AllenPG JohnstonDrug
resistance, predictive markers and pharmacogenomics in colorectal
cancerBiochim Biophys Acta1766184196200616973289
|
|
4.
|
Y SunH ZhaoY GuoF LinL TangY YaoClinical
study of combining chemotherapy of oxaliplatin or
5-fluorouracil/leucovorin with hydroxycamptothecine for advanced
colorectal cancerClin Oncol Cancer
Res6117123200910.1007/s11805-009-0117-8
|
|
5.
|
G BooseH StopperGenotoxicity of several
clinically used topoisomerase II inhibitorsToxicol
Lett116716200010.1016/S0378-4274(00)00192-210906417
|
|
6.
|
DJ NewmanGM CraggKM SnaderThe influence of
natural products upon drug discoveryNat Prod
Rep17215234200010.1039/a902202c10888010
|
|
7.
|
M GordalizaNatural products as leads to
anticancer drugsClin Transl
Oncol9767776200710.1007/s12094-007-0138-918158980
|
|
8.
|
JM LinYQ ChenLH WeiXZ ChenW XuZF HongTJ
SferraJ PengHedyotis Diffusa Willd extract induces apoptosis
via activation of the mitochondrion-dependent pathway in human
colon carcinoma cellsInt J Oncol37133113382010
|
|
9.
|
J PengYQ ChenJM LinQC ZhuangW XuZF HongTJ
SferraPatrinia scabiosaefolia extract suppresses
prolife-ration and promotes apoptosis by inhibiting STAT3 pathway
in human multiple myeloma cellsMol Med Rep43133182011
|
|
10.
|
JM LinLH WeiW XuZF HongXX LiuJ PengEffect
of Hedyotis Diffusa Willd extract on tumor angiogenesisMol
Med Rep4128312882011
|
|
11.
|
QY CaiJM LinLH WeiL ZhangLL WangYZ ZhanJW
ZengW XuAL ShenZF HongJ PengHedyotis diffusa Willd inhibits
colorectal cancer growth in vivo via inhibition of STAT3 signaling
pathwayInt J Mol Sci1361176128201210.3390/ijms13056117
|
|
12.
|
LH WeiYQ ChenJM LinJY ZhaoXZ ChenW XuXX
LiuTJ SferraJ PengScutellaria Barbata D. Don induces
apoptosis of human colon carcinoma cell via activation of the
mitochondrion-dependent pathwayJ Med Plants Res5196219702011
|
|
13.
|
LH WeiJM LinW XuZF HongXX LiuTJ SferraJ
PengInhibition of tumor angiogenesis by Scutellaria Barbata
D. Don via suppressing proliferation, migration and tube formation
of endo thelial cells and downregulation of the expression of
VEGF-A in cancer cellsJ Med Plants Res5326032682011
|
|
14.
|
LP ZhengYQ ChenW LinQC ZhuangXZ ChenW XuXX
LiuJ PengTJ SferraSpica Prunellae extract promotes
mitochondrion-dependent apoptosis in a human colon carcinoma cell
lineAfr J Pharm Pharmacol5327335201110.5897/AJPP10.354
|
|
15.
|
GI EvanKH VousdenProliferation, cell cycle
and apoptosis in
cancerNature411342348200110.1038/3507721311357141
|
|
16.
|
P NurseOrdering S phase and M phase in the
cell cycleCell79547550199410.1016/0092-8674(94)90539-87954820
|
|
17.
|
DO MorganPrinciples of CDK
regulationNature374131134199510.1038/374131a07877684
|
|
18.
|
S HarakehK Abu-El-ArdatM Diab-AssafA
NiedzwieckiM El-SabbanM RathEpigallocatechin-3-gallate induces
apoptosis and cell cycle arrest in HTLV-1-positive and-negative
leukemia cellsMed
Oncol253039200810.1007/s12032-007-0036-618188712
|
|
19.
|
D KesselY LuoCells in cryptophycin-induced
cell-cycle arrest are susceptible to apoptosisCancer
Lett1512529200010.1016/S0304-3835(99)00409-710766419
|
|
20.
|
A PurohitHAM HejazL WaldenL
MacCarthy-MorroghG PackhamBVL PotterMJ ReedThe effect of
2-methoxyoestrone-3-O-sulphamate on the growth of breast
cancer cells and induced mammary tumoursInt J
Cancer855845892000
|
|
21.
|
BT ZafonteJ HulitDF AmanatullahC AlbaneseC
WangE RosenA ReutensJA SparanoMP LisantiRG PestellCell-cycle
dysregulation in breast cancer: breast cancer therapies targeting
the cell cycleFront Biosci5D938D961200010.2741/zafonte11102317
|
|
22.
|
Chinese Pharmacopoeia
CommissionPharmacopoeia of the People’s Republic of China1Chinese
Medical Science and Technology PressBeijing5735752010
|
|
23.
|
YY XuEX YuClinical analysis of the effect
of Pien Tze Huang in treatment of 42 patients with moderate or
advanced liver cancerShanghai J Tradit Chin Med12451994
|
|
24.
|
ZX GuTherapeutical observation of advanced
colon cancerChin Tradit Patent Med15231993
|
|
25.
|
CS LiuReview of Pharmacology and clinical
application of Pien Tze HuangMed Pharm World764662006
|
|
26.
|
JM LinLH WeiYQ ChenXX LiuZF HongTJ SferraJ
PengPien Tze Huang-induced apoptosis in human colon cancer HT-29
cells is associated with regulation of the Bcl-2 family and
activation of caspase 3Chin J Integr
Med17685690201110.1007/s11655-011-0846-421910070
|
|
27.
|
QC ZhuangF HongAL ShenLP ZhengJW ZengW
LinYQ ChenTJ SferraZF HongJ PengPien Tze Huang inhibits tumor cell
proliferation and promotes apoptosis via suppressing the STAT3
pathway in colorectal cancer mouseInt J
Oncol4015691574201222218594
|
|
28.
|
AL ShenF HongLY LiuJM LinQC ZhuangZF
HongTJ SferraJ PengEffects of Pien Tze Huang on angiogenesis in
vivo and in vitroChin J Integr
Med18431436201210.1007/s11655-012-1121-z22821655
|