Introduction
Solid tumors are composed of a heterogeneous cell
population, including a variety of tumor stromal cells. These cells
create the tumor microenvironment, which is significant in tumor
progression, through cellular interactions (1). Mesenchymal stem cells (MSCs) are bone
marrow-derived multipotent cells, that are capable of
differentiating into a variety of tissues, including fat,
cartilage, bone and possibly muscle (2). Being part of the tumor
microenvironment, MSCs are considered to create niches and
facilitate tumor growth and metastasis (2). We have previously reported that MSCs
promoted engraftment and metastatic colonization in a rat
osteosarcoma (OS) model (3).
Pathway analysis from a gene expression profile identified that the
genes involved in adhesion, cytokinecytokine receptors and
extracellular matrix pathways were highly expressed in the MSCs
(3). In accordance with our data,
Bian et al have previously reported a critical role of
interleukin (IL)-6 in the interaction between OS cells and MSCs
(4).
β2-adrenergic receptors (β2ARs) mediate osteogenesis
(5). β2AR signaling induces
c-fos gene expression in OS cell lines (5), modulates bone turn-over in osteoblasts
(6) and affects the osteogenesis of
MSCs via the cyclic AMP (cAMP)/PKA pathway (7). In addition, β2AR activation leads to
the upregulation of hypoxia-inducible factor-1α (HIF-1α) via Akt
and ERK1/2 signaling (8), that may
be a mechanism by which β2AR signaling accelerates tumor growth in
several types of cancer (9–13). Conversely, hypoxia impairs β2AR
signaling in a variety of tissues (14–16).
Baloğlu et al demonstrated that in vitro hypoxia
increased the sensitivity of β2AR to desensitization, owing to an
increase in Gi/0 protein activity (14). Although hypoxia is a common feature
significant in the progression of solid tumors, little is known
about the effect of β2AR signaling in the tumor
microenvironment.
The present study investigated the effect of hypoxia
on β2AR signaling in OS cells and MSCs derived from the bone marrow
of syngeneic rats. Cellular interactions were examined using in
vitro and in vivo approaches. Co-culture experiments
revealed that hypoxia caused significant desensitization of β2AR on
OS cells but not MSCs and that MSCs affected the response of OS
cells to β2AR agonists. Systemic administration of MSCs resulted in
the enhanced growth of subcutaneously transplanted OS in response
to β2AR agonists.
Materials and methods
Cell lines
The COS1NR cell line was established from
4-(hydroxyamino)-quinoline 1-oxide-induced transplantable OS in
male Fischer 344 (F344) rats (17,18).
The cells were cultured in Eagle’s Modified Essential Medium (MEM)
supplemented with 10% FBS (JRH Biosciences, Lot no. 1E0666, Lenexa,
KS, USA). Rat MSCs were isolated and maintained in primary culture
as previously described (19,20).
Briefly, bone marrow cells were obtained from the femoral bone
shaft of 7-week-old male F344 rats and seeded into 75
cm2 flasks (T-75 flasks, Corning Costar, Cambridge, MA,
USA) containing 15 ml of standard medium consisting of MEM
supplemented with 15% FBS and a mixture of antibiotics (100 U/ml
penicillin and 100 μg/ml streptomycin; Sigma-Aldrich, St. Louis,
MO, USA). Cell cultures were maintained in a humidified atmosphere
of 95% air and 5% CO2 at 37°C. After reaching confluency
(∼10 days), the cells were released from the substratum using a
0.25% trypsin-EDTA solution and inoculated into 12- and 6-well
plates (Falcon, Franklin Lakes, NJ, USA) for biochemical analyses
and staining, respectively, at a density of 1×104
cells/cm2.
Gene expression profiling of COS1NR and
MSCs
The gene expression profiling was performed by
Agilent array analysis (Agilent Technologies, Böblingen, Germany).
Total RNA was isolated from COS1NR cells and MSCs and underwent a
quality assessment by Agilent 2100 Bioanalyzer. Total RNA (500 ng)
was processed by Agilent expression array analysis using a Quick
Amp Labeling kit and Gene Expression Hybridization kit. Data
analysis was carried out using Agilent Feature Extraction software,
analyzing pathways that possibly interact in OS cells and MSCs.
Hypoxic conditions
For hypoxic stimulation, the culture plates were
placed in a MCO-175M multi-gas incubator (Sanyo Electric Co., Ltd.,
Tokyo, Japan) at 37°C and flushed with a gas mixture of 5%
CO2/95% N2. The oxygen concentration was maintained at
2% in the chamber using an oxygen regulator (Sanyo Electric Co.,
Ltd.).
Cell viability assessment
Cell viability was assessed using the
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay. A single-cell suspension was plated in 96-well plates
at 2.0×103 cells/well and allowed to attach to the
plates at 37°C for 4 h. The cells were then cultured for 48 h under
hypoxic or normoxic conditions. The cells were left either
untreated or treated with neurotransmitters for 10 min, 8 h after
the start of culture. Cell viability was measured using the Cell
Titer 96 Aqueous One Solution Cell Proliferation Assay Reagent
(Promega Corp., Madison, WI, USA). Cell proliferation was measured
at an emission wavelength of 492 nm. All experiments were carried
out in quadruplicate and viability was expressed as the ratio of
the number of viable cells with treatment to the number of viable
cells without treatment.
cAMP enzyme immunoassay (EIA) and total
HIF-1α protein quantification
β2AR sensitivity was determined using a cAMP EIA kit
(Item no. 581001, Cayman Chemical Company, Ann Arbor, MI, USA),
according to the manufacturer’s instructions. Total HIF-1α protein
quantification was performed using the human/mouse total HIF-1α
immunoassay kit (Item no. KCB1935, R&D Systems, Minneapolis,
MN, USA) according to the manufacturer’s instructions. Cells were
cultured in 12-well plates for cAMP measurements and in 96-well
plates for HIF-1α protein quantification. Seeding density was
200,000–300,000 cells/cm2. Confluent cells were exposed
to hypoxia for 48 h. Following 8 h of hypoxia, the cells were
treated with 10 μM of isoproterenol for 10 min. Following 48 h of
hypoxia, cell culture extracts and formaldehyde-fixed cells were
used for the cAMP assay and HIF-1α immunoassay, respectively.
Co-culture experiment
The co-culture of COS1NR cells and MSCs was
performed using a 6-well format cell culture insert with a 1.0-μm
pore size polyethylene terephthalate track-etched membrane
(Becton-Dickinson, Franklin Lakes, NJ, USA) in 6-well flat-bottomed
multi-well tissue culture plates. MSCs were placed in the upper
chambers. The number of cells and the volume of culture medium in
the dividing chamber culture were identical to those in the
control. Co-cultured cells were subjected to the same treatment as
the isolated culture described above. For the neutralization of
IL-6 in bioassays, cells were cultured with a 1:400 dilution of an
IL-6 antibody for 3 days (ab6672, Abcam, Cambridge, UK).
In vivo tumor formation assay in
syngeneic rats Experiment I
Cells (5×106) of COS1NR in 100 μl of
phosphate-buffered saline (PBS) were inoculated into the
subcutaneous tissue in the posteriors of F344 rats (groups 1 and
2). Each group consisted of 4 rats and the growth rate of the tumor
was evaluated every week. The size of the tumors was calculated
using the formula volume = 0.2618 x L x W x (L + W) (21). For β2AR stimulation, the Alzet
osmotic minipumps were inserted (DURECT Corporation, Cupertino, CA,
USA) containing isoprotelenol (3 mg/kg/day, group 1) or PBS (group
2) in the nape of the neck at week 4. At week 7, the rats were
sacrificed and samples from subcutaneous tumors in each group were
fixed in 3.7% formaldehyde neutral-buffered solution and then
processed routinely for histology, stained with hematoxylin-eosin
and examined under light microscopy. On the day of minipump
implantation, intratumoral oxygen pressure was measured using the
needle probe technique (KIMOC-6650, Eppendorf, Germany). The
measurement was performed in quadruplicate at a depth of 5 mm from
the tumor surface.
Experiment II
cells (5×106) of COS1NR in 100 μl of PBS
were inoculated into the subcutaneous tissue in the posteriors of
F344 rats. Subsequently, the same number of MSCs in 100 μl of PBS
were directly injected into the circulation through the tail vein
twice at weeks 1 and 3 (groups 1 and 2). In group 2, the injected
MSCs were neutralized with anti-IL-6 antibodies as described above.
Each group consisted of 4 rats. The growth rate of the tumors was
evaluated every week and compared with that of the control group
without the injection of MSCs (group 3). For β2AR stimulation, the
Alzet osmotic minipumps containing isoprotelenol (3 mg/kg/day, all
groups) were inserted in the nape of neck at week 4. The growth
rate was evaluated as described above. Samples from subcutaneous
tumors in each group were fixed in 3.7% formal-dehyde
neutral-buffered solution and then processed routinely for
histology, stained with hematoxylin-eosin and examined under light
microscopy.
Statistical analysis
Statistical analyses of cellular proliferation,
intracellular cAMP and total HIF-1α expression were performed with
Student’s t-tests using Stata 8 (StataCorp, College City Station,
TX, USA).
Results
Effect of stress-related
neurotransmitters/hormones on COS1NR cells and MSCs
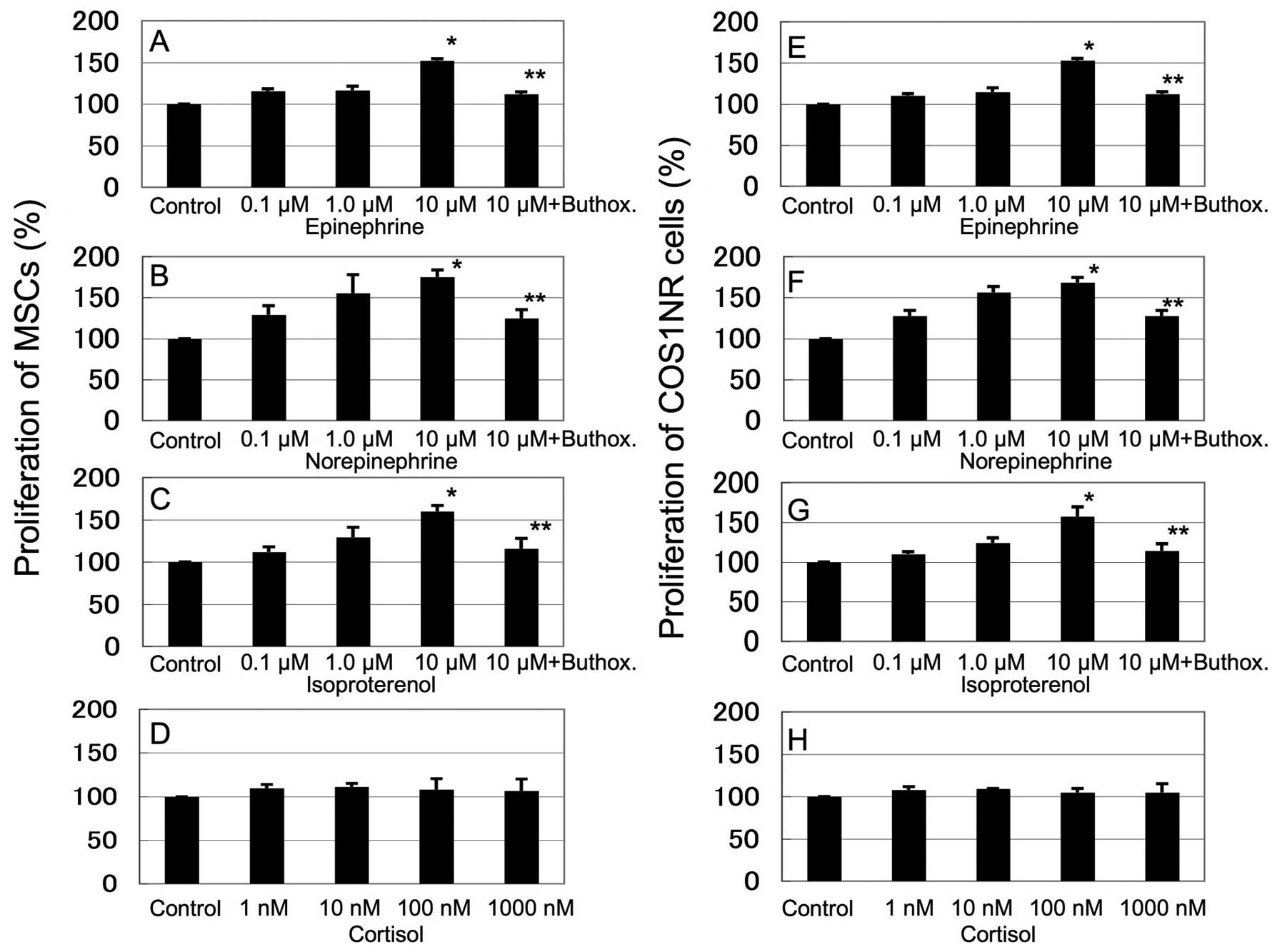
Fig. 1 shows the
in vitro response of COS1NR cells and MSCs treated with
epinephrine (0.1–10 μM), norepinephrine (0.1–10 μM), isoproterenol
(0.1–10 μM) and cortisol (1–1000 nM). Stimulation with epinephrine,
norepinephrine or isoproterenol increased the cellular
proliferation in a dose-dependent manner. Treatment with 10 μM
epinephrine, norepinephrine or isoproterenol resulted in
significantly increased proliferation in MSCs (136±3.9, 152±8.8 and
133±4.9% of the control, respectively, P<0.05) and in COS1NR
cells (131±9.1, 150±4.2 and 138±7.7% of the control, respectively,
P<0.05, Fig. 1A–C, E–G). These
effects were significantly inhibited by 100 μM buthoxamine, a
β2AR-selective inhibitor (P<0.05, Fig. 1A–C, E–G). Cortisol did not affect
cellular proliferation significantly. These results suggest that
β2AR signaling mediates the response to stress-related hormones and
neurotransmitters in the OS microenvironment (Fig. 1D and H).
Effect of hypoxic stimulation on COS1NR
cells and MSCs
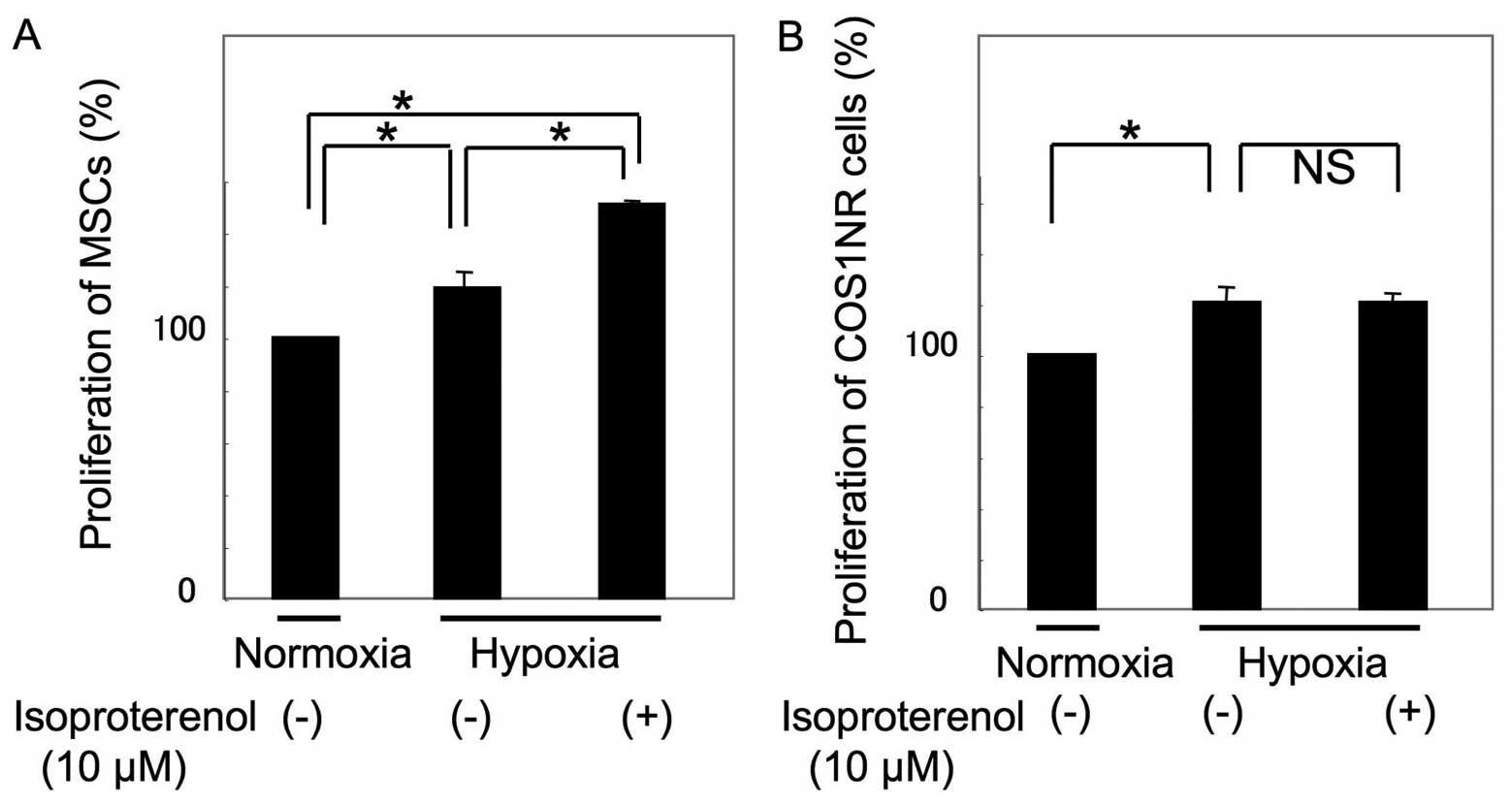
The cellular proliferation of COS1NR cells and MSCs
under hypoxic conditions was then examined. Following 48 h of
culturing under 2% O2 conditions, proliferation
increased to 118±7.8% in MSCs and 121±6.3% in COS1NR cells,
compared with the normoxic control (P<0.05, Fig. 2). Under hypoxic conditions, MSCs
treated with 10 μM isoproterenol demonstrated an increase in
proliferation to 127±2.1% of the hypoxic control (P<0.05),
whereas COS1NR cells demonstrated no significant effect, suggesting
that β2AR signaling-induced proliferation is impaired by hypoxic
stimulation in COS1NR cells.
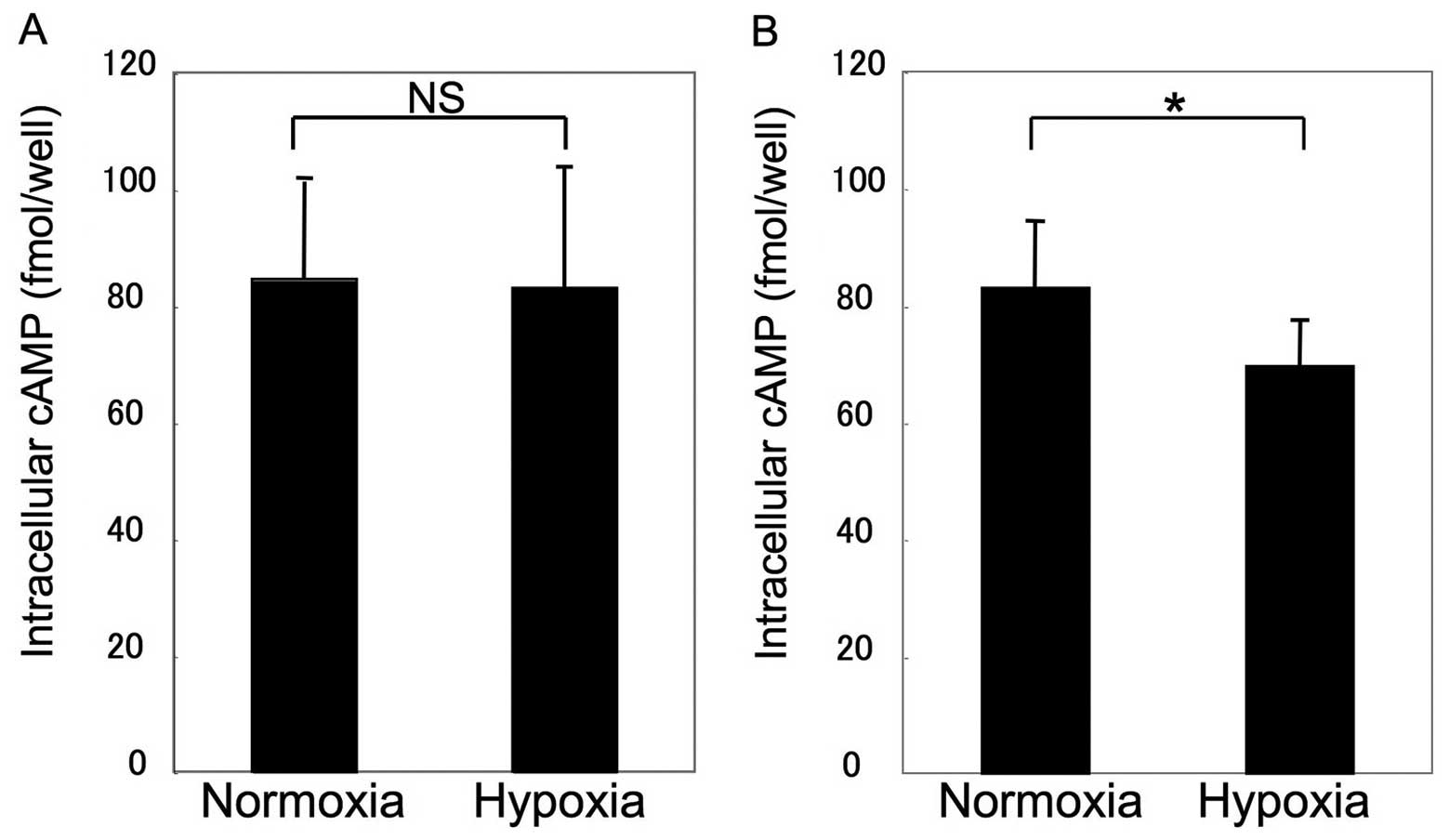
β2AR sensitivity and HIF-1α
expression
Assays for β2AR sensitivity and HIF-1α expression
were performed to clarify the mechanism of impairment. Fig. 3 shows that β2AR sensitivity was
unchanged in MSCs under hypoxic conditions, whereas β2AR
sensitivity demonstrated a significant decrease to 78±11.5% of the
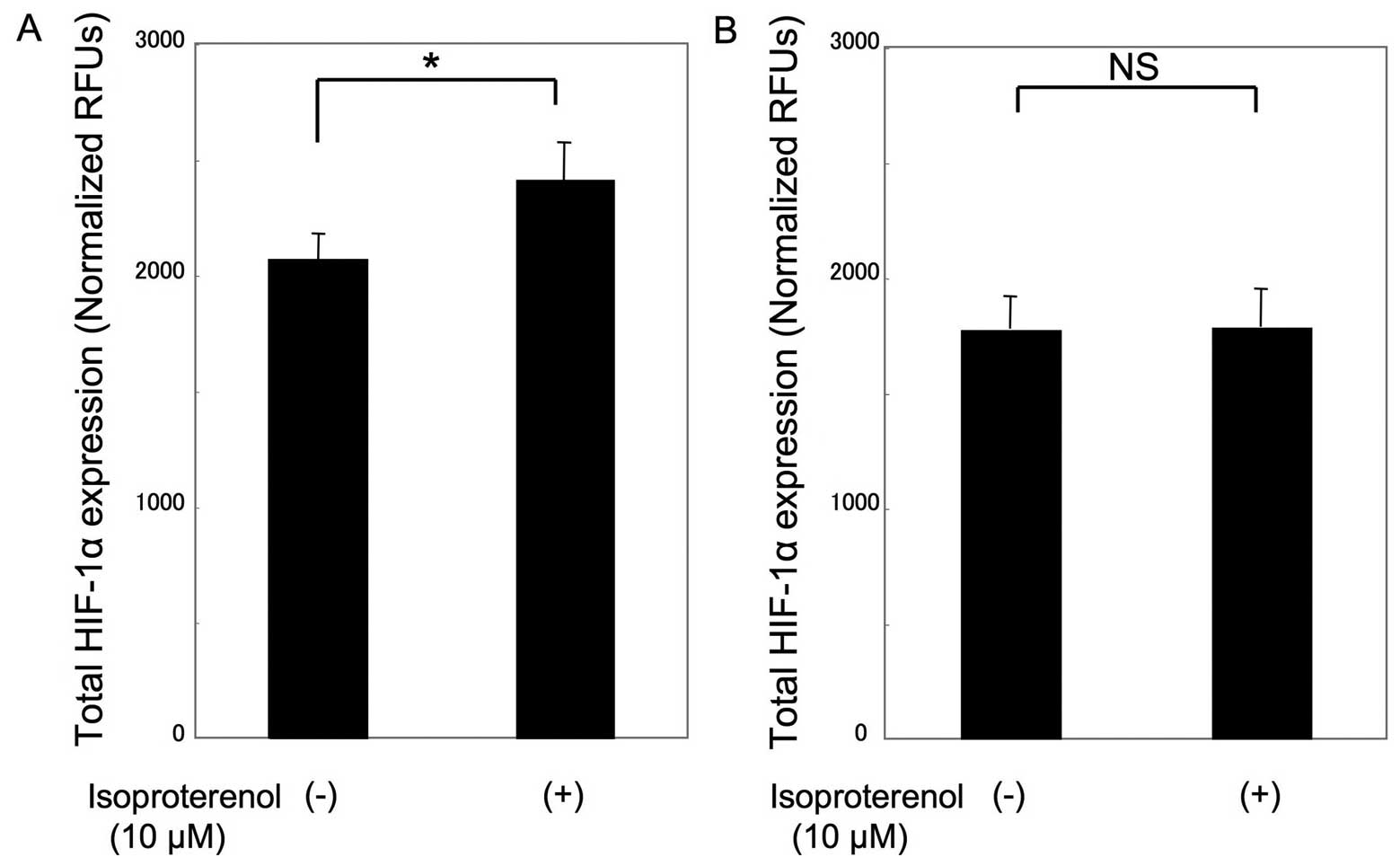
control in COS1NR cells (P<0.05). Consistent with this
observation, HIF-1α expression in isoproterenol-treated MSCs was
115±8.3% of the expression in the untreated control cells under
hypoxic conditions (P<0.05), whereas there was no significant
change in isoproterenol-treated COS1NR cells (Fig. 4).
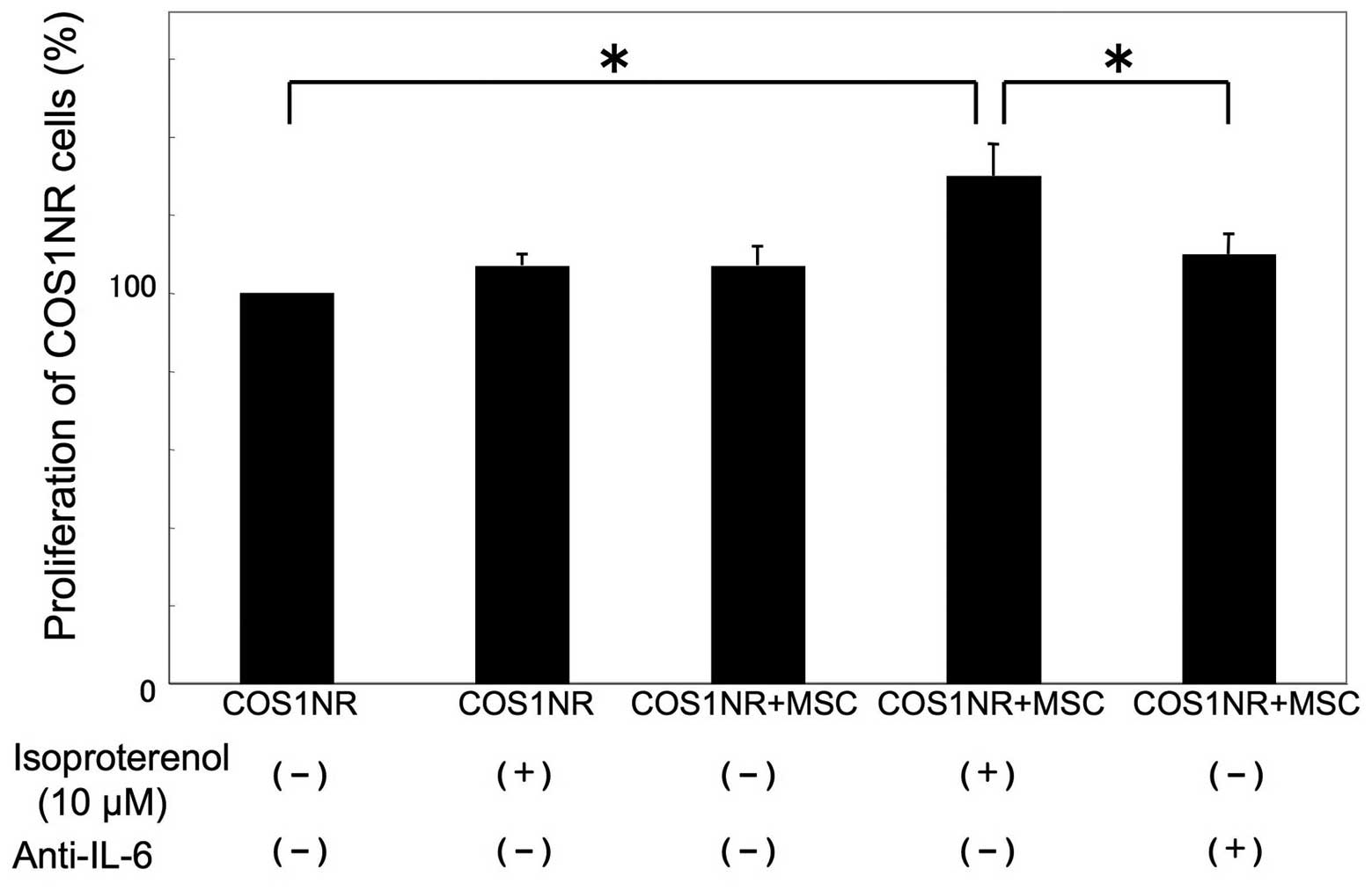
Co-culture of COS1NR cells and MSCs under
hypoxic conditions
To examine whether MSCs may affect the in
vitro response of COS1NR cells, a co-culture experiment was
performed. Co-cultured cells were treated with 10 μM isoproterenol
under hypoxic conditions for 48 h as described previously. In the
presence of co-cultured MSCs, COS1NR cells demonstrated a
significant increase in cellular proliferation following
isoproterenol treatment (131±8.5%, P<0.05). Based on our gene
profiling assay (Table I), IL-6 was
then neutralized in co-culture medium. The neutralization of IL-6
significantly inhibited the effect of co-cultured MSCs (P<0.05,
Fig. 5).
 | Table I.Expression of IL-6 and IL-6R in
COS1NR cells and MSCs. |
Table I.
Expression of IL-6 and IL-6R in
COS1NR cells and MSCs.
| Gene | MSC | COS1NR |
|---|
| IL6 | 1078.80584 | 3100.5327 |
| IL6R | 400.46093 | 908.59117 |
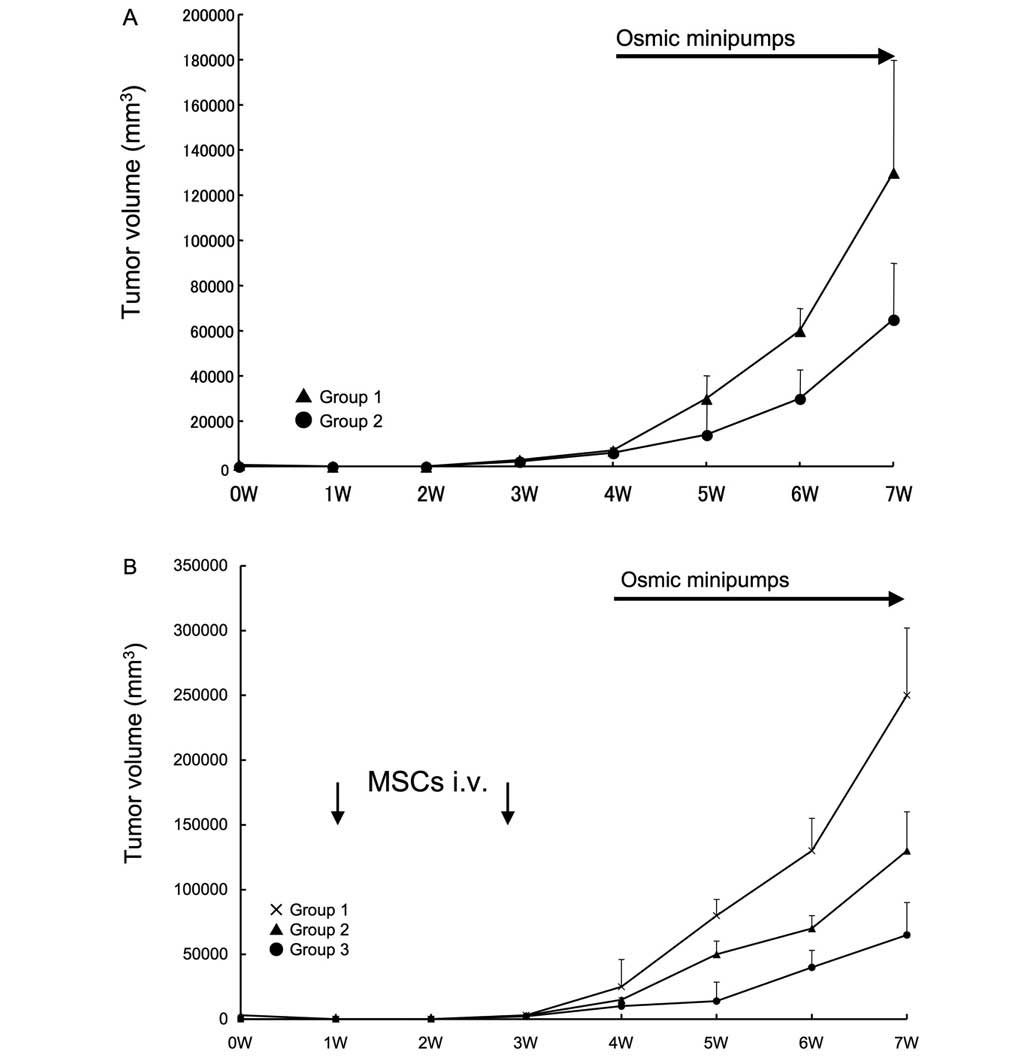
Effect of intravenous injection of MSCs
on tumor growth in syngeneic rats
Subsequently, an in vivo tumor formation
assay in syngeneic rats was performed. An hypoxic condition was
confirmed in the rat model, with a tumoral oxygen pressure of
14±2.5 mmHg. No significant inter-group variation was revealed.
Rats receiving isoprotelenol exhibited increased growth of tumors
(Fig. 6A), however, the systemic
administration of MSCs enhanced the growth of tumors in response to
isoproterenol injection (Fig. 6B).
Notably, this effect was inhibited by the IL-6 neutralization of
MSCs prior to administration.
Discussion
Neurotransmitters are significant in cancer
progression (22). Immune cells are
involved in the hypothalamic-pituitary-adrenal axis and the
sympathetic nervous system, through their expression of receptors
for glucocorticoids and catecholamines, respectively (22). In addition, the direct effects of
stress-related neurotransmitters have been reported in various
types of cancer through β2AR signaling (9–13).
However, under hypoxic conditions in vitro, β2AR signaling
does not appear to simply be a facilitator of cancer expansion.
Baloğlu et al (14) reported
that hypoxia caused the desensitization of the β2AR, owing to an
increase in Gi/0 protein activity, or possibly,
heterologous receptor desensitization by the activation of other
receptors. The authors suggested that the effects are likely to
depend on tissue type as well as the degree and duration of hypoxia
(14).
The current study focused on the hypoxia-induced
desensitization of β2AR on OS cells. It was observed that the
sensitivity of COS1NR cells was significantly decreased under
hypoxia, while that of MSCs was not. HIF-1α expression was
unchanged in COS1NR cells while expression increased in MSCs. These
observations may explain why the cellular proliferation of COS1NR
cells was not increased by β2AR stimulation under hypoxia. In terms
of cancer growth, this desensitization would be unprofitable for
the neoplasms themselves, however, IL-6 stimulation by MSCs may
compensate for this. In the present in vivo experiment, the
systemic administration of MSCs resulted in increased tumor growth,
while native MSCs were also recruited to tumors.
The correlation between OS- and bone marrow-derived
MSCs remains uncertain. Mohseny et al (23) reported that OS originates from MSCs
as a consequence of aneuploidization and the genomic loss of
cyclin-dependent kinase inhibitor 2, which suggests an obstacle to
the clinical use of MSCs. Shimizu et al (24) reported that the overexpression of
c-myc with a loss of Ink4a/Arf transforms bone
marrow-derived cells into OS. These two groups focused on early
genetic events in the pathogenesis of OS, where MSCs are candidates
for tumor origin. Conversely, several groups have emphasized a
supportive role of MSCs, a heterogeneous stromal cellular
population, as a member of the tumor microenvironment. Johann et
al (25) reported that tumor
stromal cells derived from 11 cases of pediatric malignancies,
including two cases of OS, exhibited MSC-like properties and
impaired NK cell cytotoxicity. Brune et al (26) examined six cases of OS and reported
that non-malignant MSCs were isolated from human primary OS
samples, stressing the hypothesis that bone-marrow MSCs may be
related to tumor stromal MSCs. Previously, Bian et al
(4) reported that human MSCs
promoted OS growth through a positive feedback loop involving IL-6.
Although a number of previous researchers have studied the
secretion of cytokines, including IL-6, IL-10, CCL5 and vascular
endothelial growth factor (2) by
MSCs, the importance of IL-6 in the enhancement of MSC
proliferation by OS cells has been stressed and vice versa. The
gene expression profile analysis of the current study confirmed the
expression of IL-6 and IL-6 receptors on MSCs and COS1NR cells and
successfully demonstrated that the IL-6 antibody inhibited the
effect of MSCs on COS1NR cells in a co-culture experiment and
tumor-forming assays in rats.
MSCs may compensate for the desensitization of β2AR
signaling on OS cells. The inhibition of the β2AR pathway in MSCs
recruited to the tumor may be a therapeutic target in the field of
musculoskeletal oncology.
Acknowledgements
This study was supported by a
Grant-in-aid to K. H. (no. 20591765) and A. K. (no. 24592241) from
the Ministry of Education, Culture, Sports, Science and Technology,
Japan.
References
|
1.
|
J MarxCancer biology. All in the stroma:
cancer’s Cosa NostraScience32038412008
|
|
2.
|
SA BergfeldYA DeClerckBone marrow-derived
mesenchymal stem cells and the tumor microenvironmentCancer
Metastasis Rev29249261201010.1007/s10555-010-9222-720411303
|
|
3.
|
S TsukamotoK HonokiH FujiiY TohmaA KidoT
MoriT TsujiuchiY TanakaMesenchymal stem cells promote tumor
engraftment and metastatic colonization in rat osteosarcoma
modelInt J Oncol40163169201221971610
|
|
4.
|
ZY BianQM FanG LiWT XuTT TangHuman
mesenchymal stem cells promote growth of osteosarcoma: involvement
of interleukin-6 in the interaction between human mesenchymal stem
cells and Saos-2Cancer
Sci10125542560201010.1111/j.1349-7006.2010.01731.x20874851
|
|
5.
|
S KellenbergerK MullerH RichenerG
BilbeFormoterol and isoproterenol induce c-fos gene expression in
osteoblast-like cells by activating β2-adrenergic
receptorsBone2247147819989600780
|
|
6.
|
HH HuangTC BrennanMM MuirRS
MasonFunctional α1- and β2-adrenergic receptors in human
osteoblastsJ Cell Physiol2202672752009
|
|
7.
|
H LiC FongY ChenG CaiM Yangβ2- and β3-,
but not β1-adrenergic receptors are involved in osteogenesis of
mouse mesenchymal stem cells via cAMP/PKA signalingArch Biochem
Biophys49677832010
|
|
8.
|
PH ThakerSK LutgendorfAK SoodThe
neuroendocrine impact of chronic stress on cancerCell
Cycle6430433200710.4161/cc.6.4.382917312398
|
|
9.
|
GR BadinoA NovelliC GirardiF Di
CarloEvidence for functional β-adrenoceptor subtypes in CG-5 breast
cancer cellPharmacol Res332552601996
|
|
10.
|
SK LutgendorfS ColeE CostanzoS BradleyJ
CoffinS JabbariK RainwaterJM RitchieM YangAK SoodStress-related
mediators stimulate vascular endothelial growth factor secretion by
two ovarian cancer cell linesClin Cancer
Res945144521200314555525
|
|
11.
|
PH ThakerLY HanAA KamatJM ArevaloR
TakahashiC LuNB JenningsG Armaiz-PenaJA BanksonM RavooriChronic
stress promotes tumor growth and angiogenesis in a mouse model of
ovarian carcinomaNat Med12939944200610.1038/nm144716862152
|
|
12.
|
HM SchullerHA Al-WadeiNeurotransmitter
receptors as central regulators of pancreatic cancerFuture
Oncol6221228201010.2217/fon.09.17120146581
|
|
13.
|
EA KasbohmR GuoCW YowellG BagchiP KellyP
AroraPJ DaakaAndrogen receptor activation by G(s) signaling in
prostate cancer cellsJ Biol
Chem2801158311589200510.1074/jbc.M41442320015653681
|
|
14.
|
E BaloğluA KeIH Abu-TahaP BärtschH
MairbäurlIn vitro hypoxia impairs β2-adrenergic receptor signaling
in primary rat alveolar epithelial cellsAm J Physiol Lung Cell Mol
Physiol296L500L5092009
|
|
15.
|
K MardonP MerletA SyrotaB MazièreEffects
of 5-day hypoxia on cardiac adrenergic neurotransmission in ratsJ
Appl Physiol8589089719989729562
|
|
16.
|
JM PeiXC YuML FungJJ ZhouCS CheungNS
WongMP LeungTM WongImpaired G(s)α and adenylyl cyclase cause
β-adrenoceptor desensitization in chronically hypoxic rat heartsAm
J Physiol Cell Physiol279C1455C14632000
|
|
17.
|
K HonokiT MoriM TsutsumiT TsujiuchiA KidoT
MorishitaY MiyauchiY DohiY MiiS TamaiY KonishiHeterogeneous pattern
of gene expression in cloned cell lines established from a rat
transplantable osteosarcoma lung meta-static noduleCancer
Lett127221228199810.1016/S0304-3835(98)00048-29619880
|
|
18.
|
K HonokiT TsujiuchiT MoriA KidoK
YoshitaniT MoriY TakakuraExpression of the p16INK4a gene and
methylation pattern of CpG sites in the promoter region in rat
tumor cell linesMol Carcinog391014200410.1002/mc.1016514694443
|
|
19.
|
H OhgushiY DohiT KatudaS TamaiS TabataY
SuwaIn vitro bone formation by rat marrow cell cultureJ Biomed
Mater
Res32333340199610.1002/(SICI)1097-4636(199611)32:3%3C333::AID-JBM5%3E3.0.CO;2-T8897137
|
|
20.
|
M AkahaneA NakamuraH OhgushiH ShigematsuY
DohiY TakakuraOsteogenic matrix sheet-cell transplantation using
osteoblastic cell sheet resulted in bone formation without scaffold
at an ectopic siteJ Tissue Eng Regen
Med2196201200810.1002/term.81
|
|
21.
|
HH LuuQ KangJK ParkW SiQ LuoW JiangH YinAG
MontagMA SimonTD PeabodyAn orthotopic model of human osteosarcoma
growth and spontaneous pulmonary metastasisClin Exp
Metastasis22319329200510.1007/s10585-005-0365-916170668
|
|
22.
|
HT HuQY MaD ZhangSG ShenL HanYD MaRF LiKP
XieHIF-1α links β-adrenoceptor agonists and pancreatic cancer cells
under normoxic conditionActa Pharmacol Sin311021102010
|
|
23.
|
AB MohsenyK SzuhaiS RomeoEP BuddinghI
Briaire-de BruijnD de JongM van PelAM Cleton-JansenPC
HogendoornOsteosarcoma originates from mesenchymal stem cells in
consequence of aneuploidization and genomic loss of Cdkn2J
Pathol219294305200910.1002/path.260319718709
|
|
24.
|
T ShimizuT IshikawaE SugiharaS KuninakaT
MiyamotoY MabuchiY MatsuzakiT TsunodaF MiyaH Moriokac-MYC
overexpression with loss of Ink4a/Arf transforms bone marrow
stromal cells into osteosarcoma accompanied by loss of
adipogenesisOncogene2956875699201010.1038/onc.2010.31220676132
|
|
25.
|
PD JohannM VaeglerF GiesekeP MangS
Armeanu-EbingerT KlubaR HandgretingerI MüllerTumour stromal cells
derived from paediatric malignancies display MSC-like properties
and impair NK cell cytotoxicityBMC
Cancer10501201010.1186/1471-2407-10-50120858262
|
|
26.
|
JC BruneA TorminMC JohanssonP RisslerO
BrosjöR LöfvenbergFV von SteyernF MertensA RydholmS
SchedingMesenchymal stromal cells from primary osteosarcoma are
non-malignant and strikingly similar to their bone marrow
counterpartsInt J Cancer129313330201110.1002/ijc.2569720878957
|