Contents
Introduction
HL-60 cells
NB4 cells
MUTZ-3 cells
THP-1 cells
K562 cells
Concluding remarks
Introduction
Dendritic cells (DCs) are hematopoietic cells that
belong to the antigen-presenting cell (APC) family, which also
includes B cells and macrophages. They are responsible for the
induction of cellular immune responses, by recognizing, acquiring,
processing and presenting antigens to naïve, resting T cells for
the induction of an antigen-specific immune response. Of all the
antigen presenting cells, DCs are the most effective inducers of
T-cell-based immunity (1). DCs
originate from myeloid precursors that, during their development,
progress from the blood into the peripheral tissues. DCs reside in
the peripheral tissues in an immature state (iDCs) equipped with
specialized receptors, including toll-like receptors, C-type
lectins, the cytoplasmic NOD/NALP family, RIG-I/DDX58 and
MDA5/IFIH1 molecules and Fc receptors, where they capture and
process antigens for presentation in the context of MHC molecules
(2). iDCs undergo a maturation
process in response to pathogens, antigens and/or pro-inflammatory
signals, during which they acquire the morphological, phenotypical
and functional characteristics of mature DCs (mDCs). During
maturation, DCs upregulate the major histocompatibility complex
(MHC) and remodel their surfaces, typically expressing numerous
membrane-associated co-stimulatory molecules (including members of
the B7, TNF and notch families) (3). Subsequently, mDCs progress into the
secondary lymphoid tissues, where they present the processed
antigens to naïve T cells to generate effector T cells. Since DCs
are so well equipped for initiating adaptive immune responses, they
are considered to be prime targets for modulating immune responses
against cancer. In the overt absence of maturation stimuli, steady
state DCs induce tolerance when they capture self and environmental
antigens (4).
Worldwide, research is being conducted to explore
the therapeutic application of DCs; however, numerous aspects of
DC-biology remain poorly understood. Studies of human DCs have been
constrained by various factors. Firstly, differentiated DCs are
predominantly located in the tissue rather than the blood, making
isolation and analysis more difficult. Although DCs are isolated
from either the spleen (5) or blood
(6,7), their low and varying levels often
hamper studies by making it difficult to generate large amounts of
immunostimulatory DCs. Second, the life span of the culture is
limited since DCs are terminally differentiated and cannot divide.
Although larger numbers of DCs may be derived by the in
vitro differentiation of bone marrow stem cells (8), peripheral blood mononuclear cells
(9) or monocytes (10), the isolation of these DC-precursors
is laborintensive and the generation of typical DCs is dependent on
the differentiative stage of the primary culture. The subsequent
differentiation is also time consuming. Third, these cultures do
not sustain DC production for long periods of time and do not allow
the identification or study of the intermediate stages as the
majority of cytokine-supplemented cultures drive progenitors
quickly to mDCs. Moreover, the current methods of preparation ex
vivo or from primary cell culture are not only laborious, but
suffer from the inherent difficulties of reproducibility associated
with the study of primary human material. Finally, experiments
using these primary cells are often complicated by donor-donor
variations in cytokine expression levels, cell surface molecule
expression levels and the ability to stimulate T cells. Hence,
there is a significant requirement for model cell lines to aid in
the understanding of the developmental origins of DCs and the
aspects that are induced to undergo maturation in response to
pathogens or cytokine stimulation. It has been described that the
“dendritic-like” cells are established from the leukemia-derived
cell lines that are capable of differentiating into functional DCs
(37). This creates possibilities
for the development of highly reproducible DCs and providing in
vitro model systems for in-depth studies concerning DC
physiology.
This review outlines a number of human DC line
differentiation models, various leukemia cell lines, multiple
differentiation potentials and the plasticity to differentiate into
DCs. The evidence suggests that various leukemia cell lines retain
the potential for terminal differentiation into diverse peripheral
mature cell types, even DCs.
HL-60 cells
The HL-60 cell line, derived from a patient with
acute promyelocytic leukemia (APL), may be induced in vitro
to differentiate into numerous cell types. Studies using this
leukemic cell line have been invaluable in a variety of areas.
HL-60 is induced to differentiate into granulocyte-like cells by
incubation with a wide variety of compounds, including DMSO and
retinoic acid (11,12) or into monocyte (macrophage)-like
cells by incubation with 1,25-dihydroxyvitamin D3 (VD3) or phorbol
esters (13–15). Moreover, the HL-60 cell line is also
capable of differentiating into eosinophilic granulocytes when
cultured under mildly alkaline conditions (16). The HL-60 cell line expresses MHC
class I molecules (17), but lacks
the expression of MHC class II and costimulatory molecules
(18). The demonstrated capacity to
differentiate in vitro into cells exhibiting numerous
characteristics of various myeloid-lineage cell types proposed the
sensitivity of the HL-60 cell line for differentiation along the
myeloid DC pathway, whereas studies using cytokines to promote the
DC differentiation of the HL-60 cell line were not successful
(19,20). Treatment with cytokines did not
induce the enhanced expression of costimulatory nor MHC molecules.
Inclusion of calcium ionophore (CI) mobilization treatment resulted
in a more mature phenotype: CI induced the HL-60 cell line to
upregulate CD83 and CD86 expression and to acquire dendritic
processes, characteristics that are associated with the mature,
activated DC phenotype. Of note, CI treatment also resulted in a
marked increase in APC function, as determined by enhanced
allogeneic T-cell stimulation capacity. However, the T-cell
stimulatory capacity was low, as CI treatment failed to induce MHC
class II molecules and downregulate MHC class I molecules. HL-60
cells only upregulated the expression of MHC class II molecules
when induced with a combination of ionophore and inteferon-γ. The
mechanism by which CI induced the differentiation of HL-60 cells
into DCs was related to triggering a downstream signal transduction
pathway. Protein kinase C (PKC) plays a role in determining the
capacity of CI to induce leukemic cell differentiation and the
blockade of PKC with bisindolylmaleimide-I (Bis-1) inhibited the
differentiation of HL-60 myeloblasts into leukemic DCs with CI
(21,22).
NB4 cells
The NB4 cell line, a human promyelocytic leukemia
cell line, is the only permanent cell line with t(15;17)
established from the leukemic cells of a patient with APL. The
t(15;17) translocation produces a chimeric protein called PML-RARα
(23). NB4 cells were instrumental
in the molecular characterization of the PML-RARα fusion gene
(24). Although HL-60 was
previously known as “promyelocytic”, it has since been revealed
that it was derived from an acute promyelocytic leukemia with
maturation (M2). Moreover, it does not contain the typical t(15;17)
translocation (25). NB4 cells
undergo differentiation via the granulocytic pathway when exposed
to all-trans retinoic acid (ATRA) (23). ATRA mediates its effect via specific
nuclear retinoid receptors (26).
The further differentiation of leukemic promyelocytes into DC-like
cells following their differentiation into granulocytes by ATRA has
also been studied (24). The
differentiation of NB4 cells by ATRA causes the cells to express DC
markers that enable ATRA-differentiated NB4 cells to present
antigens to, and hence activate, T cells. NB4 cells upregulate the
markers identified in DCs, including HLA-DR, costimulatory
molecules (CD80 and CD86), adhesion molecules (CD40) and chemokine
receptors (CCR6) in the presence of ATRA. High levels of expression
of CD83, a specific surface marker of DC maturation (27), were also detected on the surface of
ATRA-treated NB4 cells. The mechanism by which ATRA-differentiated
NB4 cells are induced into DC-like cells involves the NF-κB pathway
(28,29). Previous research has demonstrated
that phosphatidic acid (PA) also differentiates the NB4 cells into
DC-like cells (30). The expression
of DC markers, including MHC-II, CD11c, CD80 CD86 and CD83, was
reported to be upregulated in PA-treated NB4 cells (30). Increased functional capacities were
also revealed in PA-differentiated NB4 cells with regard to changes
in T-cell proliferation, cytokine production, endocytic activity
and cytolytic capacity. The downregulation of PML-RARα or related
signaling pathways, including ERK, may mediate the differentiation
signals in the NB4 cells exposed to PA. However, further research
is required to identify the downstream target of ERK-1/2 that is
involved in the PA-induced differentiation of NB4 cells into
DC-like cells.
MUTZ-3 cells
The human acute myelomonocytic leukemic cell line
MUTZ-3 may provide an alternative model for DC studies. Several
previous publications have proposed that a proportion of MUTZ-3
cells exhibit the phenotypic and morphological characteristics that
resemble those of DCs and that these cells undergo further
maturation and acquire the ability to activate resting T cells
(31,32).
MUTZ-3 is a human myeloid leukemia cell line
established from a 29-year-old male, which carries the inv(3)(q21q26) and the t(12;22) (p13;qll)
chromosomal rearrangements (33).
It exhibits the morphological and phenotypical characteristics of
monocytes, as suggested by its expression of monocyte-specific
esterase and myeloperoxidase enzymes and the expression of the
monocytic marker CD14. The MUTZ-3 cell line consists of three
distinct subpopulations, a proliferating pool of small
CD34+CD14−CD11b− progenitors,
which differentiate through an intermediate
CD34−CD14−CD11b+ stage to
ultimately give rise to a morphologically large, more
differentiated, nonproliferating CD14+CD11bhi
precursor population during cytokine-dependent culture. This
maintained capacity has been described to be cytokine-dependent for
its proliferation and survival (33). Masterson et al have
demonstrated that MUTZ-3 cells have the potential to differentiate
into Langerhans-like cells upon the addition of a cocktail of
differentiating cytokines (34).
Over the course of MUTZ-3 differentiation, cytokine receptors that
are associated with DC differentiation, including GM-CSF-R and
TNF-R, are upregulated (35). The
MUTZ-3 cell line has been shown to downregulate CD14 in response to
GM-CSF and IL-4 and to exhibit the characteristics of CD34-derived
DC precursors (34), showing a
unique potential for the phenotypical differentiation into
functional DCs with discrete immature and mature stages. Culturing
MUTZ-3 cells in GM-CSF, IL-4 resulted in proliferation arrest, cell
differentiation and the neoexpression of CD1a. The culture of
MUTZ-3 iDCs with high TNFα induced the neoexpression of the DC
maturation marker CD83 with further upregulation of CD1a.
MUTZ-3-derived DC (MuDC) maturation was also induced by
CD40-mediated stimulation and demonstrated a high level of
expression of HLA class II molecules, CD80 and CD86 (36). It has been further demonstrated that
MuDCs possess the capacity to acquire a functional cytokine-induced
DC phenotype, which exhibits the full range of functional
antigen-processing and presentation pathways and exhibits
functional properties that are essential for the in vivo
generation of cytotoxic T lymphocyte (CTL)-mediated immunity
(37,38). Signals that promote DC maturation
and survival may follow various pathways. It has been shown that
the maturation of DCs is mediated by the activation of
p38-mitogen-activated protein kinase (MAPK) (39).
Furthermore, on the basis of the comparative
functional and transcriptional profiles of MuDCs and
monocyte-derived DCs used as a standard source of DCs, MUTZ-3
exhibited a gene induction similar to that of monocyte-derived DCs
(31).
The ability to differentiate into DCs has led
scholars to investigate the MUTZ-3 cell line as a potential in
vitro model for the identification of skin allergens (40,41).
Indeed, the immortalized human MUTZ-3 cell line constitutes an
unlimited supply of DC precursors and is a potentially useful tool
for the generation of stable transfectants for the further
elucidation of DC differentiation pathways.
THP-1 cells
THP-1 is a human monocytic leukemia cell line that
was cultured from the blood of a 1-year-old male with acute
monocytic leukemia (42). THP-1 has
been used not only as a clinical model of a leukemic cell, but also
as a scientific model of differentiation in response to various
stimuli. On stimulation with phorbol 12-myristate 13-acetate (PMA)
or VD3, which activates PKC, THP-1 cells cease proliferation,
become adherent and differentiate into macrophage-like cells
(43). IL-32 also induces the
differentiation of the THP-1 cells into macrophage-like cells, the
expression of CD1a, a DC marker, and amplifies the effects of
GM-CSF/IL-4 on CD83 expression. These observations demonstrate that
IL-32 induces the differentiation of monocytes into a phenotype
that exhibits an increase in certain DC markers. The effect of
IL-32 on monocyte differentiation appears to be dependent on
caspase-3 activity (44). Moreover,
previous studies have identified that the THP-1 cell line is
differentiated rapidly into mature DCs when cultured in serum-free
medium containing GM-CSF, TNF-α and ionomycin (45).
Generally, only the use of a serum-free medium
complemented with GM-CSF and TNF-α results in the differentiation
into iDCs. The culturing of THP-1 cells in a serum-free medium
supplemented with ionomycin resulted in a complete differentiation
of the cells into mDCs (45). These
THP-1 cell line-derived highly pure DCs exhibit the morphological,
phenotypical, molecular and functional properties, including
characteristic DC morphology and cell-surface molecule expression
profiles, as determined by the cell-surface expression of CD83,
CD80, CD86, CD40, CD206, CD209, CD120 (46,47),
endocytotic activity (48,49) and strong T-cell stimulatory capacity
(50,51). Ionomycin is a CI that mediates the
intracellular calcium flux by increasing the cell membrane
permeability to Ca2+. Mobilization of intracellular
calcium has been shown to activate the signaling pathways known to
be induced in response to cytokine stimulation and finally
converges on the activation and nuclear translocation of
transcription factors of the NF-jB/Rel family, leading to DC
maturation (52,53).
K562 cells
The K562 cell line was originally established from a
pleural effusion of a patient with chronic myelogenous leukemia in
terminal blast crisis (54), which
exhibits the Philadelphia (Ph) chromosome, an aberration involving
a 9:22 chromosomal translocation identified in >90% of chronic
myelogenous leukemia cases (55).
These cells exhibit erythroid, granulocytic, monocytic or
megakaryocytic markers (56), which
suggests that they may result from the transformation of a
multipotential hematopoietic precursor. K562 cells have been
extensively used as an in vitro model system for studying
the differentiation along the erythroid lineage. Following
treatment with hemin, 5-azacytidine,
l-/3-D-arabinofuranosylcytosine, daunomycin or erbimycin, the cell
line is induced to differentiate into an erythroid lineage
(57–59). By contrast, phorbol dibutyrate and
phorbol 12-myristate 13-acetate (TPA) induce differentiation into
monocytes (60).
In addition, upon stimulation with PMA plus TNF,
K562 cells develop DC-like cytoplasmic projections, but the
expression of typical DC markers, including CD86, CD40 and CD83,
remains low. However, PMA plus A23187 was found to induce
differentiation into cells with typical DC morphology,
characteristic surface markers (MHC class I, MHC class II, CD86,
CD40, CD83), chemokine and transcription factor expression and the
ability to stimulate T-cell proliferation. The mechanisms
underlying this differentiation process concerned with the
downregulation of BCR-ABL gene expression specifically involve
downstream signaling through the MAPK pathway (61).
Concluding remarks
DCs play a fundamental role in modulating the innate
and adaptive immune responses. Thus, they have evoked considerable
interest as potential tools for the development of cell therapies
to induce antitumor immune responses. There has therefore been
enormous interest in understanding the function of these cells and
in applying this to immunotherapy. DCs may be isolated as
terminally differentiated, post-mitotic cells from all tissues and
in the blood, but they comprise only a small proportion (less than
0.1% of circulating leukocytes). DCs may also be obtained from
adherent peripheral blood monocytes and CD34+ stem cells
in vitro. The relatively low numbers of cells that may thus
be obtained, compared with the difficulties: laborintensity and
reproducibility associated with the study of primary human material
in vitro and in vivo, have seriously hampered studies
aimed at exploring the cell biology of DCs. Studies of human DCs
have been constrained by such difficulties, therefore the majority
of studies have been carried out using in vitro model
systems. These model systems have proved extremely powerful,
generating a large body of fundamental research describing numerous
aspects of DC function, alongside a body of translational research
using DCs for the cellular adoptive immunotherapy of infection and
cancer.
A number of cell lines exhibiting the
characteristics of DCs would allow for the detailed study of DC
differentiation without the associated problems. Leukemic cell
lines retain a degree of lineage plasticity and a number
differentiate further in response to defined stimuli. Moreover,
trans-lineage differentiation among erythroid, myeloid,
megakaryocytic and lymphoid compartments have been also reported
in vitro in the presence of selected cytokines or chemicals
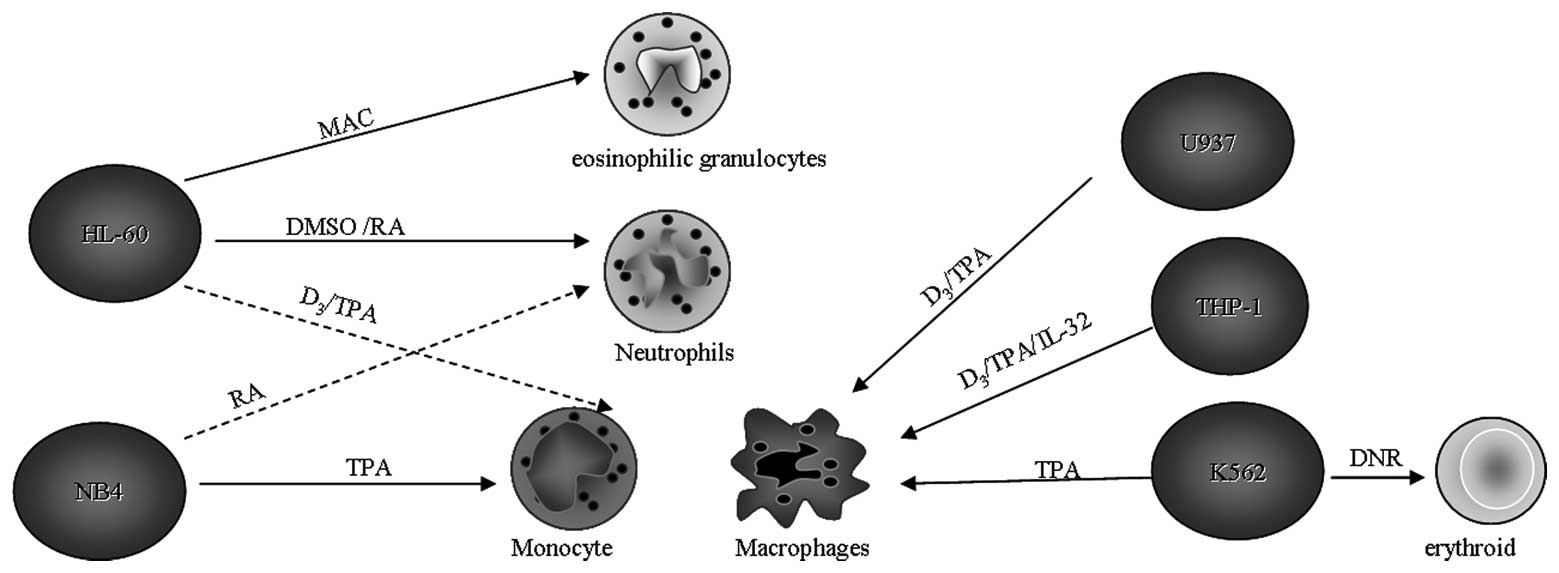
(Fig. 1) (62). This suggests that myeloid leukemias
retain the potential for terminal differentiation into various
peripheral mature cell types. Therefore, the flexible ability
allows the leukemic cells to share a potential for differentiation
into DCs.
The potential to differentiate into DCs has already
been studied for several cell lines. Using diverse combinations of
cytokines and hematopoietic differentiation agents, various human
hematopoietic cell lines have the ability to differentiate into
iDCs and mDCs with the phenotypic, molecular and functional
properties of DCs. These model systems have proven extremely
powerful, generating a large body of fundamental research
describing numerous aspects of DC function, alongside a body of
translational research using DCs for the cellular adoptive
immunotherapy of infection and cancer.
Leukemia cell lines were predicted to be good
candidates for differentiation into DCs, although MUTZ-3 is unique
in comparison with other myeloid leukemic cell lines (45,63) in
that it allows the study of discrete transitional stages in myeloid
DC development. No single cell line is likely to provide a perfect
model for a normal cell phenotype. In spite of their limitations, a
number of DC model cell lines may be successfully used to study
significant aspects of DC biology in vitro. The MUTZ-3 cell
line thus represents a valuable and sustainable model system for
the further elucidation of the molecular mechanisms regulating the
early and late stages of human myeloid DC differentiation.
We expect that the proper use of DC model cell lines
may aid in the revelation of significant new insights into DC
development, maturation, physiology and pathology that may
subsequently be applied to improve the application of DCs in the
clinic.
References
|
1.
|
J BanchereauRM SteinmanDendritic cells and
the control of immunityNature392245252199810.1038/32588
|
|
2.
|
J BanchereauF BriereC CauxImmunobiology of
dendritic cellsAnnu Rev
Immunol18767811200010.1146/annurev.immunol.18.1.767
|
|
3.
|
R SpörriC Reise e SousaInflammatory
mediators are insufficient for full dendritic cell activation and
promote expansion of CD4+ T cell populations lacking helper
functionNat Immunol6163170200515654341
|
|
4.
|
D HawigerK InabaY DorsettDendritic cells
induce peripheral T cell unresponsiveness under steady state
conditions in vivoJ Exp
Med194769779200110.1084/jem.194.6.76911560993
|
|
5.
|
RM SteinmanZA CohnIdentification of a
novel cell type in peripheral lymphoid organs of mice. I.
Morphology, quantitation, tissue distributionJ Exp
Med13711421162197310.1084/jem.137.5.1142
|
|
6.
|
WC Van VoorhisLS HairRM SteinmanG
KaplanHuman dendritic cells. Enrichment and characterization from
peripheral bloodJ Exp Med1551172118719826460832
|
|
7.
|
MK CrowHG KunkelHuman dendritic cells:
major stimulators of the autologous and allogeneic mixed leucocyte
reactionsClin Exp Immunol493383461982
|
|
8.
|
K InabaM InabaN RomaniGeneration of large
numbers of dendritic cells from mouse bone marrow cultures
supplemented with granulocyte/macrophage colony-stimulating factorJ
Exp Med17616931702199210.1084/jem.176.6.16931460426
|
|
9.
|
N RomaniS GrunerD BrangProliferating
dendritic cell progenitors in human bloodJ Exp
Med1808393199410.1084/jem.180.1.838006603
|
|
10.
|
SM KiertscherMD RothHuman CD14+ leukocytes
acquire the phenotype and function of antigen-presenting dendritic
cells when cultured in GM-CSF and IL-4J Leukoc Biol592082181996
|
|
11.
|
SJ CollinsFW RuscettiRE GallagherRC
GalloNormal functional characteristics of cultured human
promyelocytic leukemia cells (HL-60) after induction of
differentiation by dimethylsulfoxideJ Exp
Med149969974197910.1084/jem.149.4.969
|
|
12.
|
TR BreitmanSE SelonickSJ CollinsInduction
of differentiation of the human promyelocytic leukemia cell line
(HL-60) by retinoic acidProc Natl Acad Sci
USA7729362940198010.1073/pnas.77.5.2936
|
|
13.
|
C MiyauraE AbeT
Kuribayashi1,25-Dihydroxyvitamin D3 induces differentiation of
human myeloid leukemia cellsBiochem Biophys Res
Commun102937943198110.1016/0006-291X(81)91628-46946774
|
|
14.
|
ED BallPM GuyreL ShenInterferon induces
monocytoid differentiation in the HL-60 cell lineJ Clin
Invest7310721077198410.1172/JCI1112926231309
|
|
15.
|
G RoveraD SantoliC DamskyHuman
promyelocytic leukemia cells in culture differentiate into
macrophage-like cells when treated with a phorbol diesterProc Natl
Acad Sci USA7627792783197910.1073/pnas.76.6.2779288066
|
|
16.
|
SA FischkoffA PollakGJ GleichEosinophilic
differentiation of the human promyelocytic leukemia cell line,
HL-60J Exp Med160179196198410.1084/jem.160.1.1796588134
|
|
17.
|
B ChorváthJ SedlákN FuchsbergerInterferon
α-induced modulation of leukocyte cell surface antigens:
immunocytofluorometric study with human leukaemia/lymphoma cell
linesActa Virol357181991
|
|
18.
|
JJ YunisH BandF BonnevilleEJ
YunisDifferential expression of MHC class II antigens in
myelomonocytic leukemia cell linesBlood7393193719892465791
|
|
19.
|
F Santiago-SchwarzDL CoppockAA HindenburgJ
KernIdentification of a malignant counterpart of the
monocyte-dendritic cell progenitor in an acute myeloid
leukemiaBlood843054306219947949177
|
|
20.
|
GK KoskiGN SchwartzDE WengCalcium
ionophore-treated myeloid cells acquire many dendritic cell
characteristics independent of prior differentiation state,
transformation status, or sensitivity to biologic
agentsBlood94135913711999
|
|
21.
|
Q LiH OzerI LindnerProtein kinase C
blockade inhibits differentiation of myeloid blasts into dendritic
cells by calcium ionophore A23187Int J
Hematol81131137200510.1532/IJH97.NA0405
|
|
22.
|
TN NguyenBH ChoiHK KangOptimization and
limitation of calcium ionophore to generate DCs from acute myeloid
leukemic cellsCancer Res
Treat39175180200710.4143/crt.2007.39.4.17519746185
|
|
23.
|
M LanotteTV MartinS NajmanNB4, a
maturation inducible cell line with t(15;17) marker isolated from a
human acute promyelocytic leukemia
(M3)Blood771080108619911995093
|
|
24.
|
HY ParkJY ParkJW KimDifferential
expression of dendritic cell markers by all-trans retinoic acid on
human acute promyelocytic leukemic cell lineInt
Immunopharmacol415871601200410.1016/j.intimp.2004.07.01015454112
|
|
25.
|
WT DaltonMJ AhearnKB McCredieHMO cell line
was derived from a patient with FAB-M2 and not
FAB-M3Blood7124224719883422031
|
|
26.
|
I Pitha-RoweWJ PettyS KitareewanE
DmitrovskyRetinoid target genes in acute promyelocytic
leukemiaLeukemia1717231730200310.1038/sj.leu.240306512970771
|
|
27.
|
LJ ZhouTF TedderHuman blood dendritic
cells selectively express CD83, a member of the immunoglobulin
superfamilyJ Immunol1543821383519957706722
|
|
28.
|
S YoshimuraJ BondesonBM FoxwellEffective
antigen presentation by dendritic cells is NF-κB dependent:
coordinate regulation of MHC, costimulatory molecules and
cytokinesInt Immunol136756832001
|
|
29.
|
S BerchtoldP Mühl-ZürbesE MaczekCloning
and characterization of the promoter region of the human CD83
geneImmunobiology205231246200210.1078/0171-2985-0012812182451
|
|
30.
|
JO JinHY ParkJW KimPhosphatidic acid
induces the differentiation of human acute promyelocytic leukemic
cells into dendritic cell-likeJ Cell
Biochem100191203200710.1002/jcb.2105416924673
|
|
31.
|
K LarssonM LindstedtCA
BorrebaeckFunctional and transcriptional profiling of MUTZ-3, a
myeloid cell line acting as a model for dendritic
cellsImmunology117156166200610.1111/j.1365-2567.2005.02274.x16423051
|
|
32.
|
SJ SantegoetsMW SchreursAJ MastersonIn
vitro priming of tumor-specific cytotoxic T lymphocytes using
allogeneic dendritic cells derived from the human MUTZ-3 cell
lineCancer Immunol
Immunother5514801490200610.1007/s00262-006-0142-x16468034
|
|
33.
|
ZB HuW MaM ZaborskiEstablishment and
characterization of two novel cytokine responsive acute myeloid and
monocytic leukemia cell lines, MUTZ-2 and
MUTZ-3Leukemia101025104019968667638
|
|
34.
|
AJ MastersonCC SombroekTD De GruijlMUTZ-3,
a human cell line model for the cytokine induced differentiation of
dendritic cells from CD34+ precursorsBlood1007017032002
|
|
35.
|
SJ SantegoetsAJ MastersonPC van der SluisA
CD34(+) human cell line model of myeloid dendritic cell
differentiation: evidence for a CD14(+)CD11b(+) Langerhans cell
precursorJ Leukoc Biol80133713442006
|
|
36.
|
KD KimSC ChoiYW NohImpaired responses of
leukemic dendritic cells derived from a human myeloid cell line to
LPS stimulationExp Mol Med387284200610.1038/emm.2006.916520555
|
|
37.
|
SJ SantegoetsAJ van den EertweghAA van de
LoosdrechtHuman dendritic cell line models for DC differentiation
and clinical DC vaccination studiesJ Leukoc
Biol8413641373200810.1189/jlb.020809218664532
|
|
38.
|
I NelissenI SelderslaghsRV
HeuvelMUTZ-3-derived dendritic cells as an in vitro alternative
model to CD34+ progenitor-derived dendritic cells for testing of
chemical sensitizersToxicol In Vitro2314771481200919732821
|
|
39.
|
JF ArrighiM RebsamenF RoussetA critical
role for p38 mitogen-activated protein kinase in the maturation of
human blood-derived dendritic cells induced by lipopolysaccharide,
TNF-α and contact sensitizersJ Immunol16638373845200111238627
|
|
40.
|
BC HuletteCA RyanGF GerberickElucidating
changes in surface marker expression of dendritic cells following
chemical allergen treatmentToxicol Appl
Pharmacol182226233200210.1006/taap.2002.944712183102
|
|
41.
|
P AzamJL PeifferD ChamoussetThe
cytokine-dependent MUTZ-3 cell line as an in vitro model for the
screening of contact sensitizersToxicol Appl
Pharmacol2121423200610.1016/j.taap.2005.06.01816039684
|
|
42.
|
S TsuchiyaM YamabeY YamaguchiEstablishment
and characterization of a human acute monocytic leukemia cell line
(THP-1)Int J Cancer26171176198010.1002/ijc.29102602086970727
|
|
43.
|
H SchwendeE FitzkeP AmbsP
DieterDifferences in the state of differentiation of THP-1 cells
induced by phorbol ester and 1,25-dihydroxyvitamin D3J Leukoc
Biol5955556119968613704
|
|
44.
|
MG NeteaEC LewisT AzamInterleukin-32
induces the differentiation of monocytes into macrophage-like
cellsProc Natl Acad Sci
USA10535153520200810.1073/pnas.071238110518296636
|
|
45.
|
C BergesC NaujokatS TinappA cell line
model for the differentiation of human dendritic cellsBiochem
Biophys Res
Commun333896907200510.1016/j.bbrc.2005.05.17115963458
|
|
46.
|
M RosenzwajgB CanqueJC GluckmanHuman
dendritic cell differentiation pathway from CD34+ hematopoietic
precursor cellsBlood875355441996
|
|
47.
|
LJ ZhouTF TedderCD14+ blood monocytes can
differentiate into functionally mature CD83+ dendritic cellsProc
Natl Acad Sci USA93258825921996
|
|
48.
|
F SallustoM CellaC DanieliA
LanzavecchiaDendritic cells use macropinocytosis and the mannose
receptor to concentrate macromolecules in the major
histocompatibility complex class II compartment: downregulation by
cytokines and bacterial productsJ Exp
Med182389400199510.1084/jem.182.2.389
|
|
49.
|
P MontiA MercalliBE LeoneRapamycin impairs
antigen uptake of human dendritic
cellsTransplantation75137145200310.1097/00007890-200301150-0002512544886
|
|
50.
|
F SallustoA LanzavecchiaEfficient
presentation of soluble antigen by cultured human dendritic cells
is maintained by granulocyte/macrophage colony-stimulating factor
plus interleukin 4 and downregulated by tumor necrosis factor αJ
Exp Med1791109111819948145033
|
|
51.
|
G FerlazzoA WesaWZ WeiA GalyDendritic
cells generated either from CD34+ progenitor cells or from
monocytes differ in their ability to activate antigen-specific CD8+
T cellsJ Immunol163359736041999
|
|
52.
|
LA LyakhGK KoskiW TelfordBacterial
lipopolysaccharide, TNF-α, and calcium ionophore under serum-free
conditions promote rapid dendritic cell-like differentiation in
CD14+ monocytes through distinct pathways that activate NK-jBJ
Immunol165364736552000
|
|
53.
|
MB FariesI BedrosianS XuCalcium signaling
inhibits interleukin-12 production and activates CD83+ dendritic
cells that induce Th2 cell
developmentBlood9824892497200111588047
|
|
54.
|
CB LozzioBB LozzioHuman chronic
myelogenous leukemia cell line with positive Philadelphia
chromosomeBlood453213341975163658
|
|
55.
|
PJ FialkowClonal origin of human
tumorsAnnu Rev
Med30135143197910.1146/annurev.me.30.020179.001031
|
|
56.
|
AR GreenS RockmanE DeLucaCG BegleyInduced
myeloid differentiation of K562 cells with downregulation of
erythroid and megakaryocytic transcription factors: a novel
experimental model for hemopoietic lineage restrictionExp
Hematol215255311993
|
|
57.
|
GL Bianchi ScarráM RomaniDA
CovielloTerminal erythroid differentiation in the K-562 cell line
by 1-beta-D-arabinofuranosylcytosine: accompaniment by c-myc
messenger RNA decreaseCancer Res466327633219863536078
|
|
58.
|
GP ToniniD RadziochA GronbergErythroid
differentiation and modulation of c-myc expression induced by
antineoplastic drugs in the human leukemic cell line K562Cancer
Res474544454719873476195
|
|
59.
|
Y HonmaJ Okabe-KadoM HozumiInduction of
erythroid differentiation of K562 human leukemic cells by
herbimycin A. an inhibitor of tyrosine kinase activityCancer
Res4933133419892910452
|
|
60.
|
JA SutherlandAR TurnerP
MannoniDifferentiation of K562 leukemia cells along erythroid,
macrophage, and megakaryocyte lineagesJ Biol Response
Mod525026219862425057
|
|
61.
|
I LindnerMA Kharfan-DabajaE AyalaInduced
dendritic cell differentiation of chronic myeloid leukemia blasts
is associated with down-regulation of BCR-ABLJ
Immunol17117801791200310.4049/jimmunol.171.4.178012902478
|
|
62.
|
M MontanariC GemelliE TenediniCorrelation
between differentiation plasticity and mRNA expression profiling of
CD34+-derived CD14− and CD14+ human normal myeloid precursorsCell
Death Differ1215881600200515947790
|
|
63.
|
B PlatzerA JörglS TaschnerRelB regulates
human dendritic cell subset development by promoting monocyte
intermediatesBlood10436553663200410.1182/blood-2004-02-041215315978
|