Introduction
Gastric cancer (GC), a common malignancy of the
digestive tract worldwide, remains largely incurable (1,2). It is
one of the leading causes of tumor-associated mortality in a number
of developing Asian countries despite declining morbidity and
mortality among patients with GC in the majority of developed
countries (3,4). Tumor-induced immunosuppression, which
hinders the cytotoxic responses of T lymphocytes and natural killer
cells, is a principal problem to be solved in cancer immunotherapy
(5,6). There are various immunosuppressive
strategies employed by tumors in which regulatory T cells (Tregs)
play a pivotal role (7).
Tregs are a distinct lymphocyte lineage with
immunosuppressive potential in maintaining immunological tolerance.
The transcription factor Foxp3 is a unique marker for Tregs and is
indispensable for their development (8,9)
Mast cells (MCs) are a group of long-lived
heterogeneous cells originating from the bone marrow. They were
recognized by their modulating activities in inflammation and
angiogenesis until recent investigations revealed their roles in
shaping adaptive immune responses (10). MCs have been found to accumulate in
the tumor microenvironment through the SCF/c-kit signaling pathway,
leading to the aggravation of inflammation and immunosuppression in
the tumor microenvironment (11).
The complex reciprocal correlation between MCs and
Tregs determines the functions of both cell types. This interaction
of MCs with Tregs dictates the intensity of tumor-associated
inflammation and thus either promotes or inhibits tumor growth
(10).
In numerous experimental models and human specimens,
it has been observed that the increase in the number of MCs occurs
in a number of tumors (12).
However, there is little evidence of a clear correlation between
the number of MCs and Foxp3 expression in human GC. The purpose of
this study was to assess the infiltrating correlation between MCs
and Tregs in GC.
Patients and methods
Patients and specimens
Tumor samples were obtained from 60 patients with
pathologically confirmed gastric adenocarcinoma from the Department
of Gastrointestinal Surgery, Union Hospital (Wuhan, China). None of
the patients accepted anti-cancer therapy prior to surgical
resection. Clinical stages were classified according to the 7th
UICC TNM staging system.
Fresh GC tissues were used for the isolation of
tumor-infiltrating leukocytes, as previously described (13). The clinical characteristics of all
patients are summarized in Table I.
The research protocol was approved by the Institutional Review
Board of Tongji Medical College of Huazhong University of Science
and Technology (Wuhan, China). Written and oral consents were
obtained from the patients prior to the collection of samples.
 | Table I.Clinical characteristics and the
stages of the patients. |
Table I.
Clinical characteristics and the
stages of the patients.
|
Characteristics | Number (%) |
|---|
| Gender
(male/female) | 39/21 (65/35) |
| Age, years
(range) | 30–75 |
| TNM stage |
| I | 10 (16.7) |
| II | 15 (25) |
| III | 20 (33.3) |
| IV | 15 (25) |
Flow cytometry analysis
Tumor-infiltrating leukocytes were stained
extracellularly with specific antibodies against human CD45, CD3
and CD4 (BD Biosciences, Franklin Lakes, NJ, USA), fixed and
permeabilized with Perm/Fix solution (eBiosciences, San Diego, CA,
USA) and finally stained intracellularly with anti-Tryptase
(Millipore, Billerica, MA, USA) and anti-Foxp3 (eBiosciences).
Tryptase and Foxp3 were used as the markers for MCs and Tregs,
respectively. Samples were acquired on an LSR II (BD Biosciences)
and data were analyzed with DIVA software (BD Biosciences).
Statistical analysis
Results are expressed as mean ± SEM. Spearman’s rank
correlation coefficient test and ANOVA were carried out as
indicated. P<0.05 was considered to indicate a statistically
significant result. All analyses were performed using SPSS v12.0
software (Chicago, IL, USA).
Results
Tryptase and Foxp3 expression were
positively correlated in human GC
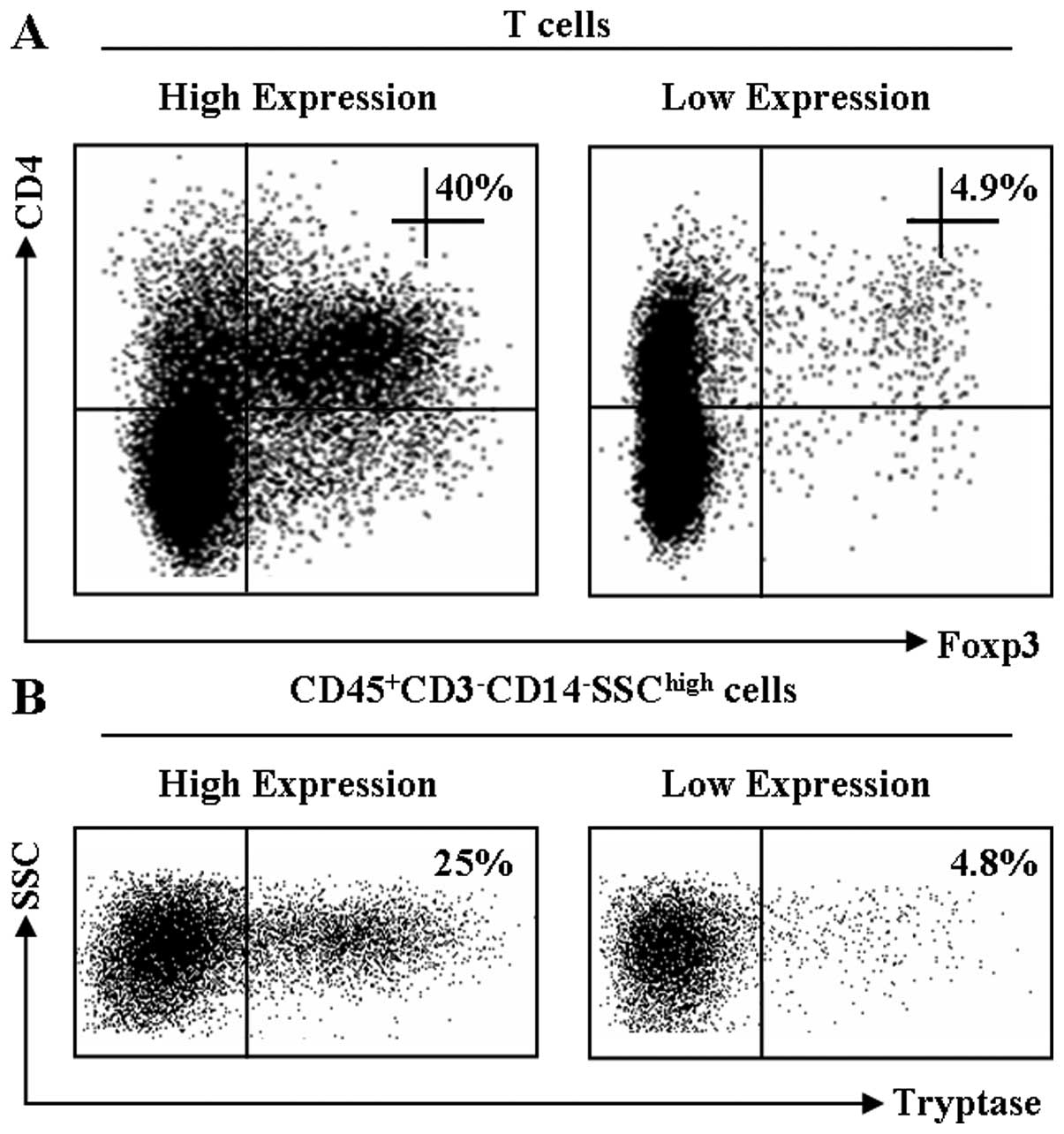
Representative dot plots for Foxp3 and tryptase
expression are shown in Fig. 1.
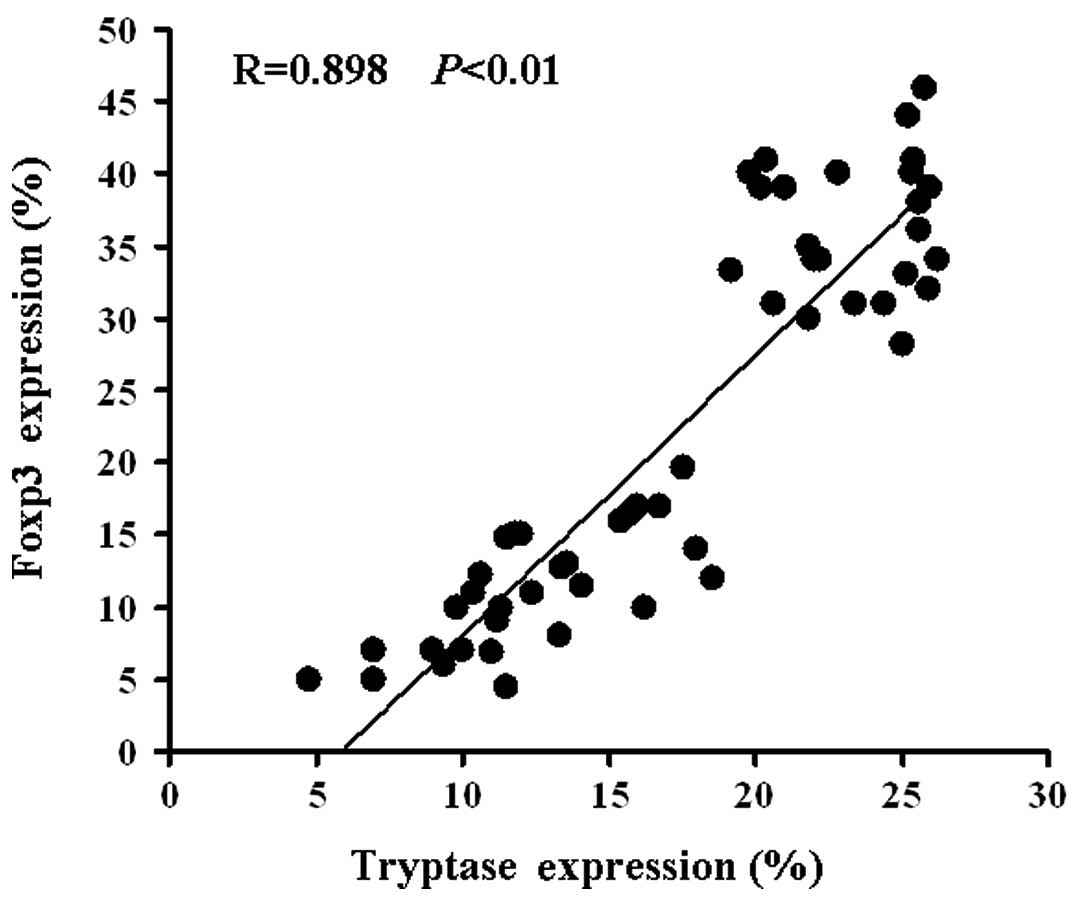
Tryptase and Foxp3 expression were positively correlated (R= 0.898,
P<0.01; Fig. 2). Correlations
were determined by Spearman’s rank correlation coefficients.
Tryptase and Foxp3 coexpression was
associated with TNM stage in human GC
The development and progression of cancer are known
to be regulated by various oncogenes and tumor suppressor genes. We
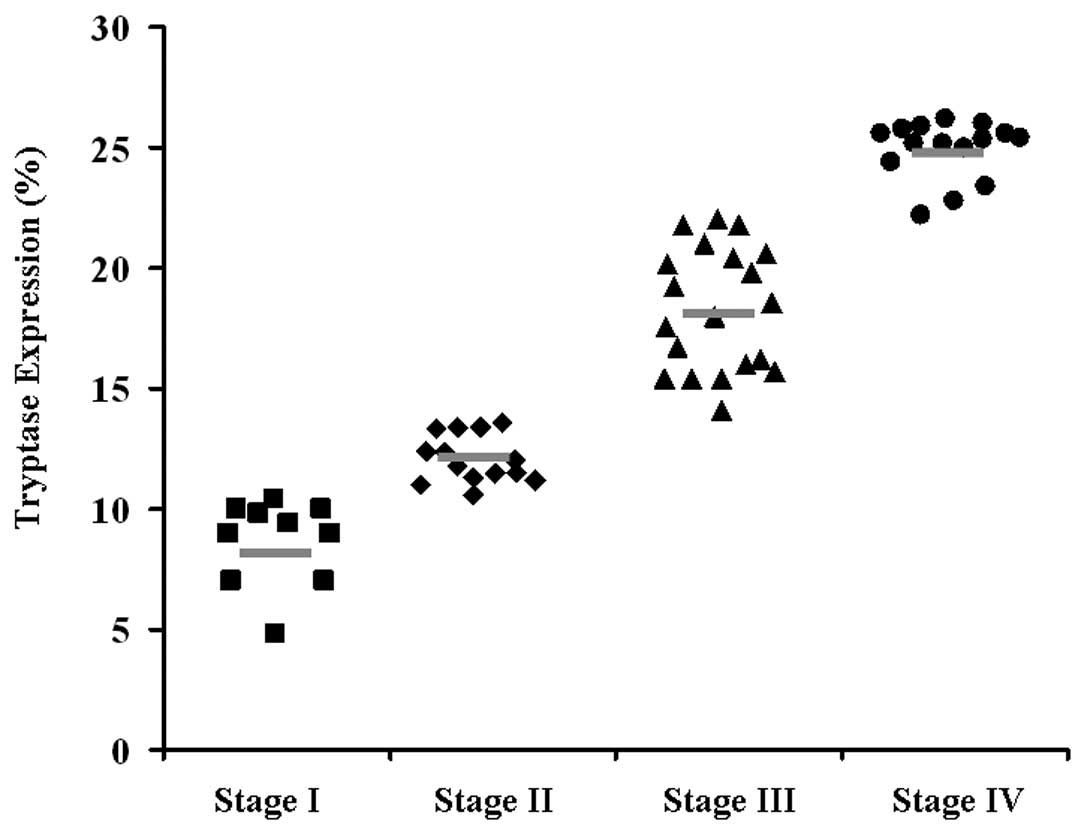
analyzed 60 primary GC patients for the expression of tryptase by
ANOVA. As shown in Fig. 3, a high
level of tryptase expression was associated with advanced tumor
stage, which is a marker of poor prognosis.
Discussion
Our study revealed that an increased number of MCs
in GC patients is correlated with a higher frequency of Foxp3
expression. Numerous studies have documented an increased level of
Foxp3 during GC progression (14,15).
However, the role of MCs in GC remains unclear.
Ribatti et al (16) demonstrated that MC density
correlates with progression of patients with gastric carcinoma.
This means that the density of MCs is positively correlated with
the development of the disease from stage I to stage IV. However,
there have been reports of a protective role for MCs in human
cancer. For instance, in a multivariate analysis of colorectal
cancer patients, high counts of eosinophils and MCs predicted
longer survival (17). MC tryptases
activate the nuclear peroxisome proliferator-activated receptor-γ
(PPAR-γ); the expression of PPAR-γ is associated with improved
clinical outcome in colon cancer (18). Our findings imply a complex
correlation between the increased number of infiltrating MCs and
advanced stages of GC patients.
These results suggest that higher numbers of MCs are
associated with poor outcomes, which is similar to the results of
previous studies reported in GC and other tumors (19–23).
The present study not only provides support for the correlation
between MCs and the stages of GC, but also focused on mechanisms
other than angiogenesis, which has already been demonstrated
(16). We studied the correlation
between Tregs and MCs in order to provide an explanation of the
influence of MCs on the stage of GC. Several studies have
researched the immune suppression mechanism of MCs in tumors
(11). However, to date, no study
has directly demonstrated the correlation between the increased
frequency of MCs and higher levels of Foxp3 in human GC. Our data
demonstrate that MCs may affect the progression of GC, partially
via interaction with Tregs. Studies in liver cancer reported
similar results (32). Numerous
researchers consider IL-9 to be critical factor in this interaction
(24–26).
In several types of cancer, an increased level of
Foxp3+ Tregs has been detected in tumor tissues and
peripheral blood, consistent with their presumed function in
immuno-suppression (27,28). Much concern has also been attached
to the roles of Foxp3 in human GC. There is a link between the
concentration of Tregs and patient survival in GC (29,30).
Recently, a study partly explained the mechanisms of the weakened
immune reactions in GC based on the overexpression of Foxp3
(31). Yuan et al (32) demonstrated a mechanism by which
tumor-infiltrating Tregs with increased Foxp3 expression mediate
immune suppression via COX-2/PGE2 production in the GC
microenvironment. Furthermore, Tregs with higher levels of Foxp3
were able to suppress the proliferation of autologous
CD4+CD25−T cells. The suppression of the
effector T-cell response was reversed by COX inhibitors and PGE2
receptor-specific antagonists. In 2011, Yuan et al (33) performed further research on Tregs in
GC and found that GC cells induce the development of Tregs via the
production of TGF-β, by which the existence of cross-talk between
the tumor and immune cells may regulate antitumor immune responses.
Our research first confirmed that the expression of Foxp3 in
tumor-infiltrating T lymphocytes was higher in the GC tissues
compared with normal tissues. We also identified links between MCs
and Foxp3, providing a new strategy of targeting Tregs and Foxp3.
As mentioned, GC cells induce human
CD4+Foxp3+ Tregs through the production of
TGF-β (34), and as MCs secrete
TGF-β, we speculate that one of the ways in which MCs affect Tregs
is their secretion of TGF-β, thus explaining the correlation
between MCs and Foxp3 at the molecular level, and providing a new
support and research direction for the immunosuppressive effects of
MCs.
In conclusion, our results reveal that the frequency
of MCs and the level of Foxp3 are increased in tumors compared with
normal tissues. The significant correlation between MCs and Foxp3
may be considered to support the hypothesis that MCs play a role in
immunosuppression in GC and may be, at least partially, responsible
for their prognosis. These results are significant and may provide
promising clinical treatments for cancer, in at least in three
aspects. First, these findings show the significance of MCs in GC
and provide a probable mechanism by which MCs affect GC
development, thus providing references for the application of
MC-regulating drugs. Second, there are few studies concerning the
correlation between MCs and Foxp3 and the present study linked them
and provided new insights into the mechanism of immune suppression.
Third, we confirmed the close correlation between MCs and Tregs,
making a foundation for the further study of the detailed
mechanisms. Therefore, further studies should be performed to
explore the mechanism of the correlation between MCs, Foxp3 and
GC.
Acknowledgements
This study was supported by the
Natural Science Foundation of Hubei Province and the Biological
Targeted Therapy Laboratory Foundation of Hubei Province
(02.03.2011-6).
References
|
1.
|
CJ MurrayAD LopezMortality by cause for
eight regions of the world: Global Burden of Disease
StudyLancet34912691276199710.1016/S0140-6736(96)07493-4
|
|
2.
|
A JemalR SiegelE WardY HaoJ XuMJ
ThunCancer statistics, 2009CA Cancer J
Clin59225249200910.3322/caac.20006
|
|
3.
|
KD CrewAI NeugutEpidemiology of gastric
cancerWorld J Gastroenterol123543622006
|
|
4.
|
DM ParkinF BrayJ FerlayP PisaniGlobal
cancer statistics, 2002CA Cancer J
Clin5574108200510.3322/canjclin.55.2.74
|
|
5.
|
M DouganG DranoffImmune therapy for
cancerAnnu Rev
Immunol2783117200910.1146/annurev.immunol.021908.132544
|
|
6.
|
GA RabinovichD GabrilovichEM
SotomayorImmunosuppressive strategies that are mediated by tumor
cellsAnnu Rev
Immunol25267296200710.1146/annurev.immunol.25.022106.14160917134371
|
|
7.
|
M BeyerJL SchultzeRegulatory T cells in
cancerBlood108804811200610.1182/blood-2006-02-00277416861339
|
|
8.
|
JD FontenotMA GavinAY RudenskyFoxp3
programs the development and function of CD4+CD25+ regulatory T
cellsNat Immunol43303362003
|
|
9.
|
I KryczekR LiuG WangK WuX ShuFOXP3 defines
regulatory T cells in human tumor and autoimmune diseaseCancer
Res6939954000200910.1158/0008-5472.CAN-08-380419383912
|
|
10.
|
K KhazaieNR BlatnerMW KhanThe significant
role of mast cells in cancerCancer Metastasis
Rev304560201110.1007/s10555-011-9286-z21287360
|
|
11.
|
B HuangZ LeiGM ZhangD LiC SongSCF-mediated
mast cell infiltration and activation exacerbate the inflammation
and immunosuppression in tumor
microenvironmentBlood11212691279200810.1182/blood-2008-03-14703318524989
|
|
12.
|
D RibattiE CrivellatoThe controversial
role of mast cells in tumor growthInt Rev Cell Mol
Biol27589131200910.1016/S1937-6448(09)75004-X19491054
|
|
13.
|
K WuI KryczekL ChenW ZouTH WellingKupffer
cell suppression of CD8+ T cells in human hepatocellular carcinoma
is mediated by B7-H1/programmed death-1 interactionsCancer
Res69806780752009
|
|
14.
|
LS ShenJ WangDF ShenXL YuanP DongCD4(+)
CD25(+)CD127(low/−)regulatory T cells express Foxp3 and suppress
effector T cell proliferation and contribute to gastric cancers
progressionClin Immunol1311091182009
|
|
15.
|
K KonoH KawaidaA TakahashiH SugaiK
MimuraCD4(+)CD25high regulatory T cells increase with tumor stage
in patients with gastric and esophageal cancersCancer Immunol
Immunother55106410712006
|
|
16.
|
D RibattiD GuidolinA MarzulloMast cells
and angiogenesis in gastric carcinomaInt J Exp
Pathol91350356201010.1111/j.1365-2613.2010.00714.x20412338
|
|
17.
|
HJ NielsenU HansenIJ
ChristensenIndependent prognostic value of eosinophil and mast cell
infiltration in colorectal cancer tissueJ
Pathol189487495199910.1002/(SICI)1096-9896(199912)189:4%3C487::AID-PATH484%3E3.0.CO;2-I10629548
|
|
18.
|
S OginoK ShimaY BabaK NoshoN
IraharaColorectal cancer expression of peroxisome proliferator
activated receptor gamma (PPARG, PPARgamma) is associated with good
prognosisGastroenterology13612421250200910.1053/j.gastro.2008.12.04819186181
|
|
19.
|
GO ElpekT GelenNH AksoyThe prognostic
relevance of angiogenesis and mast cells in squamous cell carcinoma
of the oesophagusJ Clin
Pathol54940944200110.1136/jcp.54.12.94011729214
|
|
20.
|
I TakanamiK TakeuchiM NarukeMast cell
density is associated with angiogenesis and poor prognosis in
pulmonary
adenocarcinomaCancer8826862692200010.1002/1097-0142(20000615)88:12%3C2686::AID-CNCR6%3E3.0.CO;2-610870050
|
|
21.
|
D MolinA EdströmI GlimeliusMast cell
infiltration correlates with poor prognosis in Hodgkin’s lymphomaBr
J Haematol119122124200212358914
|
|
22.
|
MF AcikalinU OnerI TopçuB YaşarH KiperE
ColakTumour angiogenesis and mast cell density in the prognostic
assessment of colorectal carcinomasDig Liver
Dis37162169200510.1016/j.dld.2004.09.02815888280
|
|
23.
|
E StoyanovM UddinD MankutaMast cells and
histamine enhance the proliferation of non-small cell lung cancer
cellsLung Cancer753844201210.1016/j.lungcan.2011.05.02921733595
|
|
24.
|
MJ JuSJ QiuQ GaoJ FanMY CaiCombination of
peritumoral mast cells and T-regulatory cells predicts prognosis of
hepatocellular carcinomaCancer
Sci10012671274200910.1111/j.1349-7006.2009.01182.x19432885
|
|
25.
|
LF LuEF LindDC GondekMast cells are
essential intermediaries in regulatory T-cell
toleranceNature319971002200616921386
|
|
26.
|
K EllerD WolfJM HuberIL-9 production by
regulatory T cells recruits mast cells that are essential for
regulatory T cell-induced immune suppressionJ
Immunol1868391201110.4049/jimmunol.100118321115728
|
|
27.
|
Z YangB ZhangD LiM LvC HuangGX ShenB
HuangMast cells mobilize myeloid-derived suppressor cells and Treg
cells in tumor microenvironment via IL-17 pathway in murine
hepatocarcinoma modelPLoS
One5e8922201010.1371/journal.pone.000892220111717
|
|
28.
|
N KobayashiN HiraokaW YamagamiH OjimaY
KanaiFOXP3+ regulatory T cells affect the development and
progression of hepatocarcinogenesisClin Cancer Res139029112007
|
|
29.
|
RW GriffithsE ElkordDE GilhamV RamaniN
ClarkePL SternRE HawkinsFrequency of regulatory T cells in renal
cell carcinoma patients and investigation of correlation with
survivalCancer Immunol
Immunother5617431753200710.1007/s00262-007-0318-z17487490
|
|
30.
|
F IchiharaK KonoA TakahashiH KawaidaH
SugaiH FujiiIncreased populations of regulatory T cells in
peripheral blood and tumor-infiltrating lymphocytes in patients
with gastric and esophageal cancersClin Cancer
Res944044408200314555512
|
|
31.
|
H KawaidaK KonoA TakahashiH SugaiK MimuraN
MiyagawaH OmataA OoiH FujiiDistribution of CD4+CD25high regulatory
T-cells in tumor-draining lymph nodes in patients with gastric
cancerJ Surg Res1241511572005
|
|
32.
|
XL YuanL ChenMX LiElevated expression of
Foxp3 in tumor-infiltrating Treg cells suppresses T-cell
proliferation and contributes to gastric cancer progression in a
COX-2-dependent mannerClin
Immunol134277288201010.1016/j.clim.2009.10.00519900843
|
|
33.
|
XL YuanL ChenTT ZhangGastric cancer cells
induce human CD4+Foxp3+ regulatory T cells through the production
of TGF-β1World J Gastroenterol17201920272011
|
|
34.
|
BA SayedA ChristyMR QuirionMA BrownThe
master switch: the role of mast cells in autoimmunity and
toleranceAnnu Rev
Immunol26705739200810.1146/annurev.immunol.26.021607.09032018370925
|